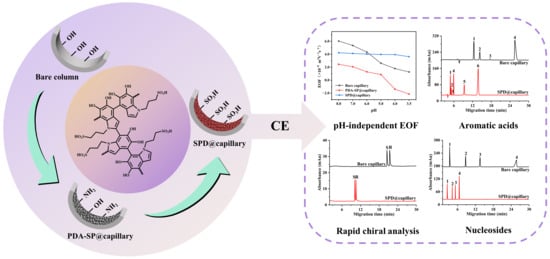
Sulfonic Functionalized Polydopamine Coatings with pH-Independent Surface Charge for Optimizing Capillary Electrophoretic Separations
Abstract
:1. Introduction
2. Results
2.1. Characterization of PDA and SPD Coatings
2.1.1. FESEM
2.1.2. ATR-FT-IR and XPS
2.2. Influence of Number of PDA-Tris and PDA-SP Coatings on EOF Mobility
2.3. EOF Stability and Reproducibility of PDA-Tris@capillary and PDA-SP@capillary
2.4. EOF Mobilities of SPD@capillary
2.5. Applications of SPD@capillary
2.5.1. High-Efficiency Separation of Aromatic Acids
2.5.2. Rapid CDs-Based Chiral Analysis
2.5.3. High-Efficiency Separation of Nucleosides
2.6. Repeatability and Stability of SPD@capillary
3. Materials and Methods
3.1. Reagents and Chemicals
3.2. Instrumentation
3.3. Preparation of PDA Coated Columns
3.4. Sample and Buffer Solutions Preparation
3.5. CE Procedures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, J.; Bose, S.; Hage, D.S. Improved reproducibility in capillary electrophoresis through the use of mobility and migration time ratios. J. Chromatogr. A 1996, 735, 209–220. [Google Scholar] [CrossRef]
- Stine, J.J.; Palmer, C.P. Covalent modification of fused silica capillaries with quaternized polyamines to achieve robust and stable anodic electroosmotic flow. J. Sep. Sci. 2009, 32, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Szabó, Z.-I.; Benkő, B.-M.; Bartalis-Fábián, Á.; Iványi, R.; Varga, E.; Szőcs, L.; Tóth, G. Chiral Separation of Apremilast by Capillary Electrophoresis Using Succinyl-β-Cyclodextrin—Reversal of Enantiomer Elution Order by Cationic Capillary Coating. Molecules 2023, 28, 3310. [Google Scholar] [CrossRef] [PubMed]
- Sola, L.; Chiari, M. Tuning capillary surface properties by charged polymeric coatings. J. Chromatogr. A 2015, 1414, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Zhang, W.; Liu, Y.; Yu, X.; Chen, Z. Polydopamine-functionalized poly(ether ether ketone) tube for capillary electrophoresis-mass spectrometry. Anal. Chim. Acta 2017, 987, 64–71. [Google Scholar] [CrossRef]
- Li, L.; Xue, X.; Zhang, H.; Lv, W.; Qi, S.; Du, H.; Manyande, A.; Chen, H. In-situ and one-step preparation of protein film in capillary column for open tubular capillary electrochromatography enantioseparation. Chin. Chem. Lett. 2021, 32, 2139–2142. [Google Scholar] [CrossRef]
- Melanson, J.E.; Baryla, N.E.; Lucy, C.A. Dynamic capillary coatings for electroosmotic flow control in capillary electrophoresis. Trac. Trends Anal. Chem. 2001, 20, 365–374. [Google Scholar] [CrossRef]
- Vitali, L.; Della Betta, F.; Costa, A.C.O.; Vaz, F.A.S.; Oliveira, M.A.L.; Pereira Vistuba, J.; Fávere, V.T.; Micke, G.A. New multilayer coating using quaternary ammonium chitosan and κ-carrageenan in capillary electrophoresis: Application in fast analysis of betaine and methionine. Talanta 2014, 123, 45–53. [Google Scholar] [CrossRef]
- Konášová, R.; Butnariu, M.; Šolínová, V.; Kašička, V.; Koval, D. Covalent cationic copolymer coatings allowing tunable electroosmotic flow for optimization of capillary electrophoretic separations. Anal. Chim. Acta 2021, 1178, 338789. [Google Scholar] [CrossRef]
- Tůma, P.; Koval, D.; Sommerová, B.; Vaculín, Š. Separation of anaesthetic ketamine and its derivates in PAMAPTAC coated capillaries with tuneable counter-current electroosmotic flow. Talanta 2020, 217, 121094. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, D.-D.; Yang, F.-Q.; Qian, Z.-M.; Li, C.-H.; Li, W.-J.; Wang, S.-P.; Wang, Y.-T. Modulation of electroosmotic flow in capillary electrophoresis by plant polyphenol-inspired gallic acid/polyethyleneimine coatings: Analysis of small molecules. J. Chromatogr. B 2019, 1124, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Štěpánová, S.; Kašička, V. Recent applications of capillary electromigration methods to separation and analysis of proteins. Anal. Chim. Acta 2016, 933, 23–42. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Landman, A.; Barker, G.E.; Hartwick, R.A. Synthesis and evaluation of anionic polymer-coated capillaries with pH-independent electroosmotic flows for capillary electrophoresis. J. Chromatogr. A 1994, 685, 303–312. [Google Scholar] [CrossRef]
- Xu, L.; Feng, Y.-Q.; Shi, Z.-G.; Da, S.-L.; Wei, F. Preparation of a sulfonated fused-silica capillary and its application in capillary electrophoresis and electrochromatography. J. Chromatogr. A 2004, 1033, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Katayama, H.; Ishihama, Y.; Asakawa, N. Stable Capillary Coating with Successive Multiple Ionic Polymer Layers. Anal. Chem. 1998, 70, 2254–2260. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-Inspired Surface Chemistry for Multifunctional Coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Tu, W.; Liu, Y.; Chen, M.; Ma, L.; Li, L.; Yang, B. A mussel-induced approach to secondary functional cross-linking 3-aminopropytriethoxysilane to modify the graphene oxide membrane for wastewater purification. Chin. Chem. Lett. 2023, 34, 107322. [Google Scholar] [CrossRef]
- Li, A.; Huang, M.; Hu, D.; Tang, Z.; Xu, J.; Li, Y.; Zhang, X.; Chen, X.; Wang, G. Polydopamine-coated metal-organic framework-based composite phase change materials for photothermal conversion and storage. Chin. Chem. Lett. 2023, 34, 107916. [Google Scholar] [CrossRef]
- Cui, X.; Xu, S.; Jin, C.; Ji, Y. Recent advances in the preparation and application of mussel-inspired polydopamine-coated capillary tubes in microextraction and miniaturized chromatography systems. Anal. Chim. Acta 2018, 1033, 35–48. [Google Scholar] [CrossRef]
- Yi, G.; Ji, B.a.; Xia, Z.; Fu, Q. Advances in polydopamine surface modification for capillary electrochromatography. Chin. J. Chromatogra. 2020, 38, 1057–1068. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, F.-Q. Applications of polydopamine modifications in capillary electrophoretic analysis. J. Sep. Sci. 2019, 42, 342–359. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yi, G.; Ji, B.; Gao, D.; Bai, Y.; Liu, Y.; Wang, L.; Xia, Z.; Fu, Q. In situ one-pot synthesis of polydopamine/octadecylamine co-deposited coating in capillary for open-tubular capillary electrochromatography. J. Chromatogr. A 2020, 1610, 460559. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Mao, Z.; Hu, C.; Li, Q.; Chen, Z. Fluoro-functionalized stationary phases for electrochromatographic separation of organic fluorides. J. Chromatogr. A 2020, 1625, 461269. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Mao, Z.; Chen, Z. Polydopamine-assisted immobilization of a zinc(II)-derived metal-organic cage as a stationary phase for open-tubular capillary electrochromatography. Microchim. Acta 2019, 186, 449. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.-B.; Liu, D.-Y. Polydopamine-based permanent coating capillary electrochromatography for auxin determination. J. Chromatogr. A 2008, 1212, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, W.; Ma, X.; Jia, L. Polydopamine assisted fabrication of titanium oxide nanoparticles modified column for proteins separation by capillary electrochromatography. Anal. Biochem. 2016, 512, 103–109. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, L.; Zhang, C.; Liu, S.; Zhu, H.; Wang, Y. Polydopamine-assisted partial hydrolyzed poly(2-methyl-2-oxazolinze) as coating for determination of melamine in milk by capillary electrophoresis. Talanta 2016, 150, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Guan, J.; Huang, Z.; Huo, H.; Shi, S.; Zhang, D.; Yan, F. β-Cyclodextrin covalent organic framework supported by polydopamine as stationary phases for electrochromatographic enantioseparation. Electrophoresis 2022, 43, 1446–1454. [Google Scholar] [CrossRef] [PubMed]
- Yi, G.; Ji, B.; Du, J.; Zhou, J.; Chen, Z.; Mao, Y.; Wei, Y.; Xia, Z.; Fu, Q. Enhanced enantioseparation performance in cyclodextrin-electrokinetic chromatography using quinine modified polydopamine coated capillary column. Microchem. J. 2021, 167, 106315. [Google Scholar] [CrossRef]
- Gui, Y.; Ji, B.; Yi, G.; Li, X.; Zhang, K.; Fu, Q. Polydopamine-Assisted Rapid One-Step Immobilization of L-Arginine in Capillary as Immobilized Chiral Ligands for Enantioseparation of Dansyl Amino Acids by Chiral Ligand Exchange Capillary Electrochromatography. Molecules 2021, 26, 1800. [Google Scholar] [CrossRef]
- Li, Z.; Mao, Z.; Zhou, W.; Chen, Z. γ-Cyclodextrin metal-organic framework supported by polydopamine as stationary phases for electrochromatographic enantioseparation. Talanta 2020, 218, 121160. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Li, X.; Zhang, Q.; Yang, F.; Wei, W.; Xia, Z. A facile and versatile approach for controlling electroosmotic flow in capillary electrophoresis via mussel inspired polydopamine/polyethyleneimine co-deposition. J. Chromatogr. A 2015, 1416, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Ball, V. Impedance spectroscopy and zeta potential titration of dopa-melanin films produced by oxidation of dopamine. Colloids Surf. A 2010, 363, 92–97. [Google Scholar] [CrossRef]
- Zeng, R.; Luo, Z.; Zhou, D.; Cao, F.; Wang, Y. A novel PEG coating immobilized onto capillary through polydopamine coating for separation of proteins in CE. Electrophoresis 2010, 31, 3334–3341. [Google Scholar] [CrossRef] [PubMed]
- Ponzio, F.; Barthès, J.; Bour, J.; Michel, M.; Bertani, P.; Hemmerlé, J.; d’Ischia, M.; Ball, V. Oxidant Control of Polydopamine Surface Chemistry in Acids: A Mechanism-Based Entry to Superhydrophilic-Superoleophobic Coatings. Chem. Mater. 2016, 28, 4697–4705. [Google Scholar] [CrossRef]
- Xia, L.; Yuan, L.; Zhou, K.; Zeng, J.; Zhang, K.; Zheng, G.; Fu, Q.; Xia, Z.; Fu, Q. Mixed-Solvent-Mediated Strategy for Enhancing Light Absorption of Polydopamine and Adhesion Persistence of Dopamine Solutions. ACS Appl. Mater. Interfaces 2023, 15, 22493–22505. [Google Scholar] [CrossRef] [PubMed]
- Baig, N.; Khan, N.A.; Salhi, B.; Abdulazeez, I.; Abu-Zahra, N.; Abdelazem, S.; Aljundi, I.H. Highly Permeable Sulfonated Polydopamine Integrated MXene Membranes for Efficient Surfactant-Stabilized Oil-in-Water Separation. Langmuir 2023, 39, 13953–13967. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, K.; Qiu, Y.; Xia, L.; Xia, Z.; Zhang, K.; Fu, Q. Strongly emissive formamide-derived N-doped carbon dots embedded Eu(III)-based metal-organic frameworks as a ratiometric fluorescent probe for ultrasensitive and visual quantitative detection of Ag+. Sens. Actuators B Chem. 2021, 339, 129922. [Google Scholar] [CrossRef]
- Yi, G.; He, J.; Ji, B.; Gao, D.; Zhang, K.; Wang, L.; Zeng, J.; Xia, Z.; Fu, Q. Solvothermal-assisted in situ rapid growth of octadecylamine functionalized polydopamine-based permanent coating as stationary phase for open-tubular capillary electrochromatography. J. Chromatogr. A 2020, 1628, 461436. [Google Scholar] [CrossRef]
- Legan, L.; Retko, K.; Ropret, P. Vibrational spectroscopic study on degradation of alizarin carmine. Microchem. J. 2016, 127, 36–45. [Google Scholar] [CrossRef]
- Ruan, H.; Zheng, Z.; Pan, J.; Gao, C.; Van der Bruggen, B.; Shen, J. Mussel-inspired sulfonated polydopamine coating on anion exchange membrane for improving permselectivity and anti-fouling property. J. Membr. Sci. 2018, 550, 427–435. [Google Scholar] [CrossRef]
- Tinh, V.D.C.; Bhandari, S.C.; Bose, A. A sulfonated polydopamine coated-PVDF membrane development for oil/water separation via eco-friendly methodology. J. Environ. Chem. Eng. 2023, 11, 110560. [Google Scholar] [CrossRef]







| Analytes | Migration Time (RSD%) | ||
|---|---|---|---|
| Intra-Day (n = 5) | Inter-Day (n = 5) | Column-to-Column (n = 3) | |
| 4-hydroxybenzoic acide | 1.74 | 1.35 | 1.22 |
| 2-phenylpropionic acid | 1.89 | 1.58 | 1.37 |
| ascorbic acid | 1.97 | 0.67 | 1.47 |
| benzoic acid | 2.59 | 1.95 | 1.84 |
| 4-nitrobenzoic acid | 2.49 | 2.06 | 2.48 |
| salicylic acid | 3.54 | 2.39 | 3.46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, W.; You, M.; Li, J.; Wang, Y.; Wang, D.; Tao, X.; Rao, L.; Xia, Z.; Fu, Q. Sulfonic Functionalized Polydopamine Coatings with pH-Independent Surface Charge for Optimizing Capillary Electrophoretic Separations. Molecules 2024, 29, 1600. https://doi.org/10.3390/molecules29071600
Long W, You M, Li J, Wang Y, Wang D, Tao X, Rao L, Xia Z, Fu Q. Sulfonic Functionalized Polydopamine Coatings with pH-Independent Surface Charge for Optimizing Capillary Electrophoretic Separations. Molecules. 2024; 29(7):1600. https://doi.org/10.3390/molecules29071600
Chicago/Turabian StyleLong, Wenwen, Mingyue You, Jieli Li, Yan Wang, Dan Wang, Xueping Tao, Li Rao, Zhining Xia, and Qifeng Fu. 2024. "Sulfonic Functionalized Polydopamine Coatings with pH-Independent Surface Charge for Optimizing Capillary Electrophoretic Separations" Molecules 29, no. 7: 1600. https://doi.org/10.3390/molecules29071600