Tetramethylpyrazine Antagonizes the Subchronic Cadmium Exposure-Induced Oxidative Damage in Mouse Livers via the Nrf2/HO-1 Pathway
Abstract
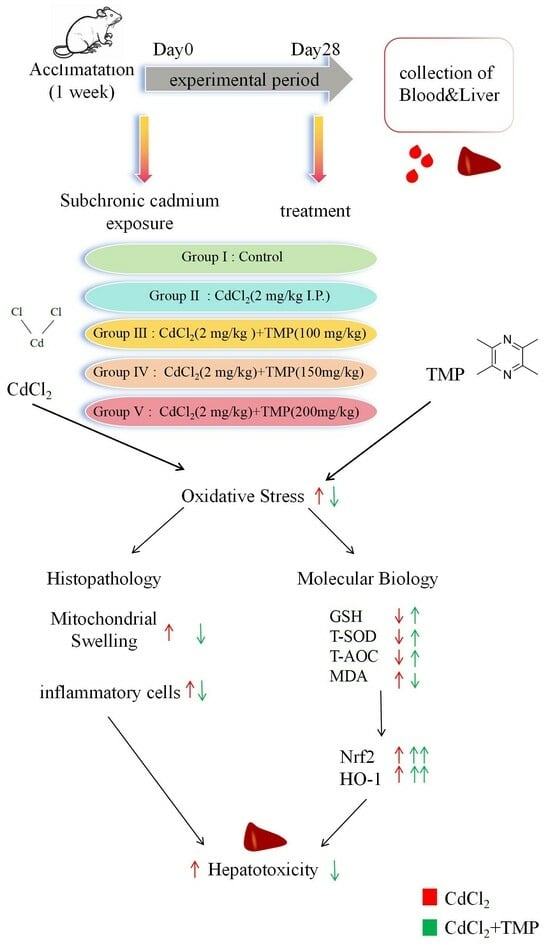
:1. Introduction
2. Results
2.1. TMP Inhibits Cd-Induced Weight Loss
2.2. TMP Decreases Cd Burden in the Liver
2.3. TMP Attenuated Cd-Induced Hepatic and Serum Oxidative Stress
2.3.1. Liver Antioxidant Biomarkers
2.3.2. Serum Antioxidant Biomarkers
2.4. TMP Alleviates Cd-Induced Liver Histopathological Lesions
2.5. TMP Reduces Liver Ultrastructural Damage in Subchronic Cd Poisoning
2.6. TMP Enhances mRNA Expression of Nrf2 and HO-1 Pathways
3. Discussion
4. Materials and Methods
4.1. Animals and Treatments
4.2. Body Weight and Organ Index
4.3. Measurement of Cd Accumulation in the Liver
4.4. Detection of Antioxidant Biomarkers in the Serum and Liver
4.5. Histopathological Examination of the Liver
4.6. Ultrastructural Observations
4.7. Quantitative Real-Time PCR (qRT-PCR) Analysis
4.7.1. RNA Extraction and Reverse Transcription
4.7.2. Real-Time Quantitative PCR
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, H.; Reynolds, M. Cadmium exposure in living organisms: A short review. Sci. Total Environ. 2019, 678, 761–767. [Google Scholar] [CrossRef]
- Li, W.; Tan, M.; Wang, H. METTL3-mediated m6A mRNA modification was involved in cadmium-induced liver injury. Environ. Pollut. 2023, 331, 121887. [Google Scholar] [CrossRef]
- Luo, T.; Wu, Y.; Wang, S.J.; Song, H.H. Mechanism of cadmium-induced liver injury and research progress of selenium antagonism of cadmium hepatotoxicity. Chin. J. Anim. Vet. Sci. 2024, 1–12. [Google Scholar]
- Yuan, G.P.; Dai, S.J.; Yin, Z.Q. Toxicological assessment of combined lead and cadmium:acute and sub-ehronic toxicity study in rats. Food Chem. Toxicol. 2014, 65, 260–268. [Google Scholar] [CrossRef]
- Song, W.E.; Chen, S.B.; Liu, J.F.; Chen, L.; Song, N.N.; Li, N.; Liu, B. Variation of Cd concentration in various rice cultivars and derivation of cadmium toxicity thresholds for paddy soil by species-sensitivity distribution. J. Integr. Agric. 2015, 14, 1845–1854. [Google Scholar] [CrossRef]
- Li, P.X.; Zhong, L.; Guo, R. Potential mechanism and treatment of cardiovascular disease induced by heavy metal cadmium. Sci. China Life Sci. 2021, 51, 1241–1253. [Google Scholar] [CrossRef]
- Ma, S.S.; Ma, T.H.; Liu, Z.P.; Gu, J.H. Effect of cadmium on differentiation and apoptosis of chicken chondrocytes cultured in vitro. Chin. Poult. 2022, 44, 43–49. [Google Scholar]
- Wang, J.; Zhu, H.L.; Lin, L.; Zhang, C.; Wang, H.W.; Liu, F.J.; Yang, Z.J. Cadmium-induced toxic damage in blood, liver, kidney and testis of rats. J. Toxicol. 2017, 31, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Kayama, F.; Yoshida, T.; Elwell, M.R.; Luster, M.I. Role of tumor necrosis factor-α in cadmium-induced hepatotoxicity. Toxicol. Appl. Pharmacol. 1995, 131, 224–234. [Google Scholar] [CrossRef]
- Xie, D.N.; Zhang, H.L.; Yang, J. Protective effects of curcumin on liver function in cadmium-contaminated rats. J. Southwest Med. Univ. 2024, 1, 34–38. [Google Scholar]
- Wang, Y.Y. Protective Effects of Aloe-Emodin and Paeoniflorin on Cadmium-Induced Hepatic and Renal Injury in Mice. Master’s Thesis, Southwest University of China, Chongqing, China, 2023. [Google Scholar]
- Xu, Y.L.; Feng, Y.S.; Ge, M.N.; Hu, J.T.; Xu, Q.W.; Jiang, J.P. Effects of pyrroloquinoline quinone on liver function and lipid peroxidation in mice acutely exposed to cadmium. Anhui Agric. Bull. 2022, 28, 23–25. [Google Scholar]
- Zhang, F. Investigation of Cadmium Pollution in Pig Farms around Harbin and the Role of Cadmium Poisoning on Ileal Damage in Pigs. Master’s Thesis, Northeast Agricultural University, Harbin, China, 2022. [Google Scholar]
- Tan, M.Y. Study on the Protective Effect of Vitamin E against Oxidative Damage in Liver of Rats with Subchronic Cadmium Poisoning. Master’s Thesis, Sichuan Agricultural University, Yaan, China, 2022. [Google Scholar]
- Li, G.X. Study on the Effect of GMDTC on Chronic Cadmium Poisoning in Mice and Rats and Its Toxic Side Effects. Master’s Thesis, Guangdong Pharmaceutical University, Ghuangzhou, China, 2015. [Google Scholar]
- Zheng, Y.Q.; Zeng, J.X.; Lin, J.X.; Xia, Y.F.; He, G.H. Herbal textual research on Chuanxiong Rhizoma in Chinese classical prescriptions. J. Chin. Mater. Medica 2021, 46, 4293–4299. [Google Scholar]
- Tang, W.C.; He, Q.; Li, J.F.; Wu, S.L.; Wang, J.S. Tetramethylpyrazine attenuates acetaminophen-induced liver injury by inhibiting inflammatory response and iron death. World Tradit. Chin. Med. 2023, 18, 3512–3517. [Google Scholar]
- Qian, J.; Xu, Z.; Zhu, P.; Meng, C.; Liu, Y.; Shan, W.; He, A.; Gu, Y.; Ran, F.; Zhang, Y.; et al. A derivative of piperlongu-mine and ligustrazine as a potential thioredoxin reductase inhibitor in drug -resistant hepatocellular carcinoma. Nat. Prod. 2021, 84, 3161–3168. [Google Scholar] [CrossRef]
- Zhou, X.; Cao, H.L.; Sun, X.Y.; Jia, L.Y.; Guo, M.; Jin, J.; Wang, Y.D.; Huang, L.; Li, Y.H.; He, Z.J. Research progress on application and mechanism of traditional Chinese medicine tetramethylpyrazine in disease treatment. Shaanxi J. Tradit. Chin. Med. 2022, 43, 541–544. [Google Scholar]
- Zhao, J.L.; Cheng, J.; Chen, J.J.; Xie, X.D. Research progress on pharmacological activities of ligustrazine derivatives. W. China J. Pharm. 2023, 38, 340–344. [Google Scholar]
- Feng, L.; Zhao, J. Effects of Ligustrazine Hydrochloride Injection on hepatic ischemia-reperfusion injury through Nrf2/HO-1 pathway. Drugs Clin. 2023, 38, 22–28. [Google Scholar]
- Renugadevi J, Prabu S M Cadmium-induced hepatotoxicity in rats and the protective effect of naringenin. Exp. Toxicol. Pathol. 2010, 62, 171–181. [CrossRef] [PubMed]
- Lan, T.F.; Ai, C.X.; Yi, S.; Gao, W.; Yuan, B.; Chen, J.; Hu, J.P.; Du, X.Y.; Ren, W.Z. The establishment of cadmium subchronic poisoning moled in C57BL/6J mice. Chin. J. Vet. 2013, 33, 730–733. [Google Scholar]
- Goodarzi, Z.; Karami, E.; Yousefi, S.; Dehdashti, A.; Bandegi, A.R.; Ghanbari, A. Hepatoprotective effect of atorvastatin on Cadmium chloride induced hepatotoxicity in rats. Life Sci. 2020, 254, 117770. [Google Scholar] [CrossRef]
- Matovic, V.; Buha, A.; Ðukic-Cosic, D.; Bulat, Z. Insight into the oxidative stress induced by lead and/or cadmium in blood, liver and kidneys. Food Chem. Toxicol. 2015, 78, 130–140. [Google Scholar] [CrossRef]
- Yang, H.; Shu, Y. Cadmium transporters in the kidney and cadmium-induced nephrotoxicity. Int. J. Mol. Sci. 2015, 16, 1484–1494. [Google Scholar] [CrossRef]
- Đukić-Ćosić, D.; Baralić, K.; Javorac, D.; Djordjevic, A.B.; Bulat, Z. An overview of molecular mechanisms in cadmium toxicity. Curr. Opin. Toxicol. 2020, 19, 56–62. [Google Scholar] [CrossRef]
- Renu, K.; Chakraborty, R.; Myakala, H.; Koti, R.; Famurewa, A.C.; Madhyastha, H.; Vellingiri, B.; George, A.; Gopalakrishnan, A.V. Molecular mechanism of heavy metals (Lead, Chromium, Arsenic, Mercury, Nickel and Cadmium)—Induced hepatotoxicity—A review. Chemosphere 2021, 271, 129735. [Google Scholar] [CrossRef]
- Jiang, F.; Qian, J.; Chen, S.; Zhang, W.; Liu, C. Ligustrazine improves atherosclerosis in rat via attenuation of oxidative stress. Pharm. Biol. 2011, 49, 856–863. [Google Scholar] [CrossRef]
- Liu, X.H.; Li, J.; Li, Q.X.; Ai, Y.X.; Zhang, L. Protective effects of ligustrazine on cisplatin-induced oxidative stress, apoptosis and nephrotoxicity in rats. Environ. Toxicol. Pharmacol. 2008, 26, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Policar, C.; Bouvet, J.; Bertrand, H.C.; Delsuc, N. SOD mimics: From the tool box of the chemists to cellular studies. Curr. Opin. Chem. Biol. 2022, 67, 102109. [Google Scholar] [CrossRef]
- Zhu, X.Q.; Lei, S.S. Experimental study on the protective effect of chuanxiongxizine on chemical liver injury in mice. Liaoning J. Tradit. Chin. Med. 2008, 35, 3. [Google Scholar]
- Fang, J.; Yin, H.; Yang, Z.; Tan, M.; Wang, F.; Chen, K.; Zuo, Z.; Shu, G.; Cui, H.; Ping, O.; et al. Vitamin E protects against cadmium-induced sub-chronic liver injury associated with the inhibition of oxidative stress and activation of Nrf2 pathway. Ecotoxicol. Environ. Saf. 2021, 208, 111610. [Google Scholar] [CrossRef]
- Pang, Y.Q.; Zhou, M.; Zheng, Z.M. Protective effect of tomato juice on liver and kidney damage in mice with cadmium poisoning. Chin. J. Public Health 2010, 26, 1552–1553. [Google Scholar]
- Bian, X.L.; Chen, X.M.; Liu, Y.X.; Pan, C. Scavenging effect of tetramethylpyrazine and its derivatives on hydroxyl radicals. Chin. J. Hosp. Pharm. 2003, 23, 2. [Google Scholar]
- Oyinloye, B.E.; Adenowo, A.F.; Osunsanmi, F.O.; Ogunyinka, B.I.; Nwozo, S.O.; Kappo, A.P. Aqueous extract of Monodora myristica ameliorates cadmium-induced hepatotoxicity in male rats. Springerplus 2016, 5, 641. [Google Scholar] [CrossRef]
- Jiang, Y.M.; Wang, D.Q. Advances in the pharmacological effects of chuanxiongxizine. Mod. Chin. Med. 2016, 18, 1364–1370. [Google Scholar]
- Colegio, O.R.; Itallie, C.V.; Rahner, C.; Anderson, J.M. Claudin extracellular domains determine paracellular charge selectivity and resistance but not tight junction fibril architecture. Am. J. Physiol. Cell Physiol. 2003, 284, C1346–C1354. [Google Scholar] [CrossRef] [PubMed]
- Waisberg, M.; Joseph, P.; Hale, B.; Beyersmann, D. Molecular and cellular mechanisms of cadmium carcinogenesis. Toxicology 2003, 192, 95–117. [Google Scholar] [CrossRef] [PubMed]
- Rana, K.; Verma, Y.; Rana, S.V.S. Possible Mechanisms of Liver Injury Induced by Cadmium Sulfide Nanoparticles in Rat. Biol. Trace Elem. Res. 2021, 199, 216–226. [Google Scholar] [CrossRef]
- Martel, J.; Marion, M.; Denizeau, F. Effect of cadmium on membrane potential in isolated rat hepatocytes. Toxicology 1990, 60, 161–172. [Google Scholar] [CrossRef]
- Li, H.Y.; Liang, L.Y.; Tao, W. Effects of astragalus injection on apoptosis and cellular mitochondrial membrane potential of cardiomyocytes in adriamycin-toxic mice. Guangxi Med. J. 2013, 35, 3. [Google Scholar]
- Wen, S.Q. Puerarin Alleviates Cadmium-Induced Neurotoxicity Mediated by Mitochondrial Damage in Rat Cerebral Cortical Neurons. Master’s Thesis, Yangzhou University, Yangzhou, China, 2023. [Google Scholar]
- Xu, D.H.; Li, J.H.; Li, S.S.; Liu, W.; Yang, T.Y.; Feng, Z. Antagonism of hepatic oxidative damage in subchronic cadmium-contaminated rats by lycopene sulphide and curcumin. J. Environ. Health 2014, 31, 4. [Google Scholar]
- He, X.; Chen, M.G.; Ma, Q. Activation of Nrf2 in defense against cadmium-induced oxidative stress. Chem. Res. Toxicol. 2008, 21, 1375–1383. [Google Scholar] [CrossRef]
- Gong, Z.G.; Wang, X.Y.; Wang, J.H.; Fan, R.F.; Wang, L. Trehalose prevents cadmium-induced hepatotoxicity by blocking Nrf2 pathway, restoring autophagy and inhibiting apoptosis. J. Inorg. Biochem. 2019, 192, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Zheyi, H.; Ye, J.; Yu, R. Long-term cadmium exposure impairs activation of Nrf2-mediated defense mechanism. Cancer Res. 2007, 67, 4999. [Google Scholar]
- Chen, J.Q.; Zhao, L.; Ding, X.H.; Wang, L.D.; Wen, Y.; Xie, X.; Peng, H. Tetramethylpyrazin inhibits high glucose-induced neutrophil extracellular capture network formation by modulating the Nrf2/HO-1 pathway. J. Army Med. Univ. 2022, 44, 2157–2164. [Google Scholar]
- Wang, X.; Mo, J.J.; Zhou, P.; Shi, H.; Huang, J.L. Effect of Traditional Chinese Medicine and Its Active Ingredients on Cardiovascular Disease Based on Nrf2/HO-1 Signaling Pathway. Chin. J. Exp. Tradit. Med. Formulae 2020, 26, 8. [Google Scholar]
- McCubrey, J.A.; Lahair, M.M.; Franklin, R.A. Reactive oxygen species-induced activation of the MAP kinase signaling pathways. Antioxid. Redox Signal. 2006, 8, 1775–1789. [Google Scholar] [CrossRef]
- Djukić-Ćosić, D.; Ćurčić Jovanović, M.; Plamenac Bulat, Z. Relation between lipid peroxidation and iron concentration in mouse liver after acute and subacute cadmium intoxication. J. Trace Elem. Med. Biol. 2008, 22, 66–72. [Google Scholar] [CrossRef]
- Souza-Arroyo, V.; Fabián, J.J.; Bucio-Ortiz, L.; Miranda-Labra, R.U.; Gomez-Quiroz, L.E.; Gutiérrez-Ruiz, M.C. The mechanism of the cadmium-induced toxicity and cellular response in the liver. Toxicology 2022, 480, 153339. [Google Scholar] [CrossRef]
- Wang, L.; Gallagher, E.P. Role of Nrf2 antioxidant defense in mitigating cadmium-induced oxidative stress in the olfactory system of zebrafish. Toxicol. Appl. Pharmacol. 2013, 266, 177–186. [Google Scholar] [CrossRef]
- Liu, C.; Zhu, Y.; Lu, Z. Cadmium Induces Acute Liver Injury by Inhibiting Nrf2 and the Role of NF-κB, NLRP3, and MAPKs Signaling Pathway. Int. J. Environ. Res. Public Health 2019, 17, 138. [Google Scholar] [CrossRef]
- Famurewa, A.C.; Ugwu-Ejezie, C.S.; Iyare, E.E. Hepatoprotective effect of polyphenols isolated from virgin coconut oil against sub-chronic cadmium hepatotoxicity in rats is associated with improvement in antioxidant defense system. Drug Chem. Toxicol. 2019, 44, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, R.; Elangovan, P.; Pari, L. Protective role of tetrahydrocurcumin: An active polyphenolic curcuminoid on cadmium-inducedoxidative damage in rats. Appl. Biochem. Biotechnol. 2017, 183, 51–69. [Google Scholar] [CrossRef] [PubMed]
- Salama, S.A.; Arab, H.H.; Hassan, M.H.; Maghrabi, I.A. Cadmium-induced hepatocellular injury: Modulatory effects of γ-glutamyl cysteine on the biomarkers of inflammation, DNA damage, and apoptotic cell death. J. Trace Elem. Med. Biol. 2018, 52, 74–82. [Google Scholar] [CrossRef]
- Trachootham, D.; Zhang, W.; Huang, P. Oxidative Stress and Drug Resistance in Cancer; Springer US: New York, NY, USA, 2009. [Google Scholar]
- Almasmoum, H.; Refaat, B.; Ghaith, M.M.; Almaimani, R.A.; Idris, S.; Ahmad, J.; Abdelghany, A.H.; BaSalamah, M.A.; El-Boshy, M. Protective effect of Vitamin D3 against lead induced hepatotoxicity, oxidative stress, immunosuppressive and calcium homeostasis disorders in rat. Environ. Toxicol. Pharmacol. 2019, 72, 103246.1–103246.13. [Google Scholar] [CrossRef]
- Abd Allah ES, H.; Badary, D.M. Folic acid protects against lead acetate-induced hepatotoxicity by decreasing NF-κB, IL-1β production and lipid peroxidation mediataed cell injury. Pathophysiology 2017, 24, 39–44. [Google Scholar] [CrossRef]
- Ying, X.Q.; Pan, Y.X.; Lan, J.C.; Fang, Y.M.; Ding, Y.Y.; Gu, Z.Y. Preparation of pectin from fingered citron peel and its protective effect on cadmium-induced liver and kidney damage in mice. Food Biosci. 2023, 56, 103359. [Google Scholar] [CrossRef]
- Xiao, L.; Mei, G.C.; Wu, L.H.; Xie, Y.X. Study on the protective effect of Danshen TMP injection on acute liver injury in young rats with sepsis. Chin. J. Emerg. Med. 2019, 28, 1585–1589. [Google Scholar]
- Qian, C. Pharmacological effects of tetramethylpyrazine on cardiovascular. Inn. Mong. Tradit. Chin. Med. 2014, 33, 107–108. [Google Scholar]
- Li, W.M.; Liu, H.T.; Li, X.Y. The effect of tetramethylpyrazine on hydrogen peroxide-induced oxidative damage in human umbilical vein endothelial cells. Basic Clin. Pharmacol. Toxicol. 2010, 106, 45–52. [Google Scholar] [CrossRef]
- Fan, X.; Wang, E.; He, J. Ligustrazine protects homocysteine-induced apoptosis in human umbilical vein endothelial cells by modulating mitochondrial dysfunction. J. Cardiovasc. Transl. Res. 2019, 12, 591–599. [Google Scholar] [CrossRef]
- Shen, H.; Chen, Y.J.; Chen, J.Y.; Sun, Y.; Huang, S. Grub polypeptide extracts protect against oxidative stress through the NRF2-ARE signaling pathway. Anim. Cells Syst. 2021, 25, 405–415. [Google Scholar]
- Tang, H.P.; Cha, N.; Liu, X.Y.; Ma, X.F.; Li, J.F.; Li, L.C. Forsythiaside A suppresses LPS-induced inflammation and oxidative stress by inhibiting PI3K/Akt pathway and activating Nrf2/HO-1 pathway. Cell. Mol. Immunol. 2021, 37, 7. [Google Scholar]
- Zhang, C.Y.; Xuan, B.Y. A total of 143 cases of gestational hypertension were treated with the combination of traditional Chinese and Western medicine. Zhejiang J. Tradit. Chin. Med. 2012, 47, 188. [Google Scholar]
- Chen, S.Y.; Hao, G.; Wang, H.R.; Cheng, P.Y.; Li, Y.M. Tetramethylpyrazine induces heme oxygenase-1 expression and attenuates myocardial ischemia/reperfusion injury in rats. J. Biomed. Sci. 2006, 13, 731–740. [Google Scholar] [CrossRef]
- Lu, C.F.; Xu, W.; Shao, J.; Zhang, F.; Chen, A.P.; Zheng, S.Z. Nrf2 Activation Is Required for Ligustrazine to Inhibit Hepatic Steatosis in Alcohol-Preferring Mice and Hepatocytes. Toxicol. Sci. 2017, 155, 432–443. [Google Scholar] [CrossRef]
- Zhu, Y.C.; Chen, Y.Y.; Tian, H.C.; Wu, L.F.; Huang, Y.L.; Sun, B.L. Effects of Different Proportions of Broussonetia Papyrifera Leaves Powder on Growth Performance, Organ Indexes and Serum Biochemical Indexes in Mice. J. Domest. Anim. Ecol. 2022, 43, 24–28. [Google Scholar]








| Items | CON Group | Cd Group | Cd + 100 mg/kg TMP Group | Cd + 150 mg/kg TMP Group | Cd + 200 mg/kg TMP Group | P Trend | ||
|---|---|---|---|---|---|---|---|---|
| Treatment Effect | Linear | Quadratic | ||||||
| Starting Weight (g) | 27.92 ± 0.54 | 27.84 ± 0.50 | 27.92 ± 0.48 | 27.92 ± 0.35 | 27.88 ± 0.42 | 0.991 | 0.881 | 0.928 |
| Final Weight (g) | 32.02 ± 0.49 a | 29.15 ± 0.27 d | 29.87 ± 0.51 c | 30.20 ± 0.29 c | 30.98 ± 0.35 b | <0.05 | 0.543 | 0.010 |
| Items | CON Group | Cd Group | Cd + 100 mg/kg TMP Group | Cd + 150 mg/kg TMP Group | Cd + 200 mg/kg TMP Group | P Trend | ||
|---|---|---|---|---|---|---|---|---|
| Treatment Effect | Linear | Quadratic | ||||||
| Heart Index (%) | 0.60 ± 0.08 | 0.61 ± 0.15 | 0.65 ± 0.11 | 0.62 ± 0.18 | 0.99 ± 1.00 | 0.246 | 0.080 | 0.101 |
| Liver Index (%) | 3.98 ± 0.53 | 4.00 ± 0.35 | 3.78 ± 0.31 | 3.78 ± 0.31 | 3.85 ± 0.25 | 0.487 | 0.183 | 0.327 |
| Spleen Index (%) | 0.36 ± 0.10 | 0.34 ± 0.07 | 0.34 ± 0.12 | 0.37 ± 0.17 | 0.33 ± 0.12 | 0.930 | 0.760 | 0.930 |
| Lung Index (%) | 0.68 ± 0.10 | 0.64 ± 0.07 | 0.68 ± 0.20 | 0.70 ± 0.16 | 0.64 ± 0.09 | 0.702 | 0.887 | 0.885 |
| Kidney Index (%) | 1.36 ± 0.18 | 1.41 ± 0.18 | 1.41 ± 0.14 | 1.34 ± 0.14 | 1.33 ± 0.10 | 0.656 | 0.387 | 0.404 |
| Items | CON Group | Cd Group | Cd + 100 mg/kg TMP Group | Cd + 150 mg/kg TMP Group | Cd + 200 mg/kg TMP Group | P Trend | ||
|---|---|---|---|---|---|---|---|---|
| Treatment Effect | Linear | Quadratic | ||||||
| Cd (mg/kg) | 0.04 ± 0.00 e | 7.57 ± 0.41 a | 5.52 ± 0.18 b | 4.77 ± 0.31 c | 4.27 ± 0.13 d | <0.05 | 0.241 | 0.003 |
| Items | CON Group | Cd Group | Cd + 100 mg/kg TMP Group | Cd + 150 mg/kg TMP Group | Cd + 200 mg/kg TMP Group | P Trend | ||
|---|---|---|---|---|---|---|---|---|
| Treatment Effect | Linear | Quadratic | ||||||
| GSH (μmol/gport) | 548.90 ± 33.09 a | 404.47 ± 29.73 d | 448.08 ± 21.95 c | 487.06 ± 22.28 bc | 515.86 ± 21.61 ab | <0.05 | 0.873 | 0.002 |
| MDA (nmol/mgport) | 2.92 ± 0.11 d | 4.34 ± 0.13 a | 3.25 ± 0.17 b | 3.17 ± 0.12 bc | 2.97 ± 0.10 cd | <0.05 | 0.057 | 0.174 |
| T-SOD (U/mgport) | 13.51 ± 0.73 d | 11.40 ± 0.71 e | 14.70 ± 0.13 c | 15.63 ± 0.16 b | 16.51 ± 0.35 a | <0.05 | 1.688 × 10−8 | 1.818 × 10−7 |
| T-AOC (mmol/gport) | 1.74 ± 0.11 b | 1.26 ± 0.05 c | 1.90 ± 0.10 ab | 2.05 ± 0.12 ab | 2.24 ± 0.42 a | <0.05 | 2.189 × 10−4 | 1.389 × 10−3 |
| Items | CON Group | Cd Group | Cd + 100 mg/kg TMP Group | Cd + 150 mg/kg TMP Group | Cd + 200 mg/kg TMP Group | P Trend | ||
|---|---|---|---|---|---|---|---|---|
| Treatment Effect | Linear | Quadratic | ||||||
| T-SOD (U/mL) | 220.63 ± 22.66 c | 181.55 ± 14.97 d | 223.19 ± 30.48 bc | 249.16 ± 22.25 b | 282.13 ± 20.09 a | <0.05 | 8.558 × 10−6 | 1.934 × 10−6 |
| T-AOC (U/mL) | 0.84 ± 0.03 a | 0.73 ± 0.02 b | 0.77 ± 0.03 b | 0.84 ± 0.03 a | 0.86 ± 0.01 a | <0.05 | 0.066 | 0.063 |
| GSH (nmol/mL) | 259.03 ± 20.02 d | 204.19 ± 36.55 e | 311.59 ± 20.73 c | 360.47 ± 22.10 b | 407.14 ± 26.17 a | <0.05 | 2.874 × 10−7 | 2.806 × 10−7 |
| MDA (nmol/mL) | 16.67 ± 0.84 c | 20.88 ± 1.13 a | 18.58 ± 1.23 b | 17.19 ± 1.15 bc | 13.17 ± 1.17 d | <0.05 | 6.167 × 10−3 | 1.165 × 10−3 |
| Items | CON Group | Cd Group | Cd + 100 mg/kg TMP Group | Cd + 150 mg/kg TMP Group | Cd + 200 mg/kg TMP Group | P Trend | ||
|---|---|---|---|---|---|---|---|---|
| Treatment Effect | Linear | Quadratic | ||||||
| HO-1 | 1.09 ± 0.03 e | 1.19 ± 0.03 d | 1.26 ± 0.04 c | 1.36 ± 0.05 b | 1.54 ± 0.09 a | <0.05 | 1.027 × 10−11 | 2.5 × 10−11 |
| Nrf2 | 1.08 ± 0.05 e | 1.17 ± 0.04 d | 1.28 ± 0.03 c | 1.34 ± 0.04 b | 1.42 ± 0.03 a | <0.05 | 4.629 × 10−11 | 7.063 × 10−10 |
| Gene | Accession Number | Primer Sequence (5′-3′) | Product Length (bp) |
|---|---|---|---|
| Nrf2 | NM-O10902.4 | F:AGCGGTAGTATCAGCCA R: GCCCAGTCCCTCAATAGC | 150 |
| HO-1 | NM-O10442.2 | F: TGTTGCGCTCTATCTCC R: GTACACATCCAAGCCGAG | 136 |
| β-actin | NM-007393 | F: CGCTCGTTGCCAATAGTG R: GCTGTGCTATGTTGCTCTAG | 117 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, X.; Zhao, S.; Guo, Z.; Zhu, Y.; Zhang, S.; Li, D.; Shu, G. Tetramethylpyrazine Antagonizes the Subchronic Cadmium Exposure-Induced Oxidative Damage in Mouse Livers via the Nrf2/HO-1 Pathway. Molecules 2024, 29, 1434. https://doi.org/10.3390/molecules29071434
Hu X, Zhao S, Guo Z, Zhu Y, Zhang S, Li D, Shu G. Tetramethylpyrazine Antagonizes the Subchronic Cadmium Exposure-Induced Oxidative Damage in Mouse Livers via the Nrf2/HO-1 Pathway. Molecules. 2024; 29(7):1434. https://doi.org/10.3390/molecules29071434
Chicago/Turabian StyleHu, Xue, Siqi Zhao, Ziming Guo, Yiling Zhu, Shuai Zhang, Danqin Li, and Gang Shu. 2024. "Tetramethylpyrazine Antagonizes the Subchronic Cadmium Exposure-Induced Oxidative Damage in Mouse Livers via the Nrf2/HO-1 Pathway" Molecules 29, no. 7: 1434. https://doi.org/10.3390/molecules29071434