Mass-Spectrometry-Based Research of Cosmetic Ingredients
Abstract
:1. Introduction
2. Literature Research Methodology
3. MS Analysis of Cosmetic Ingredients
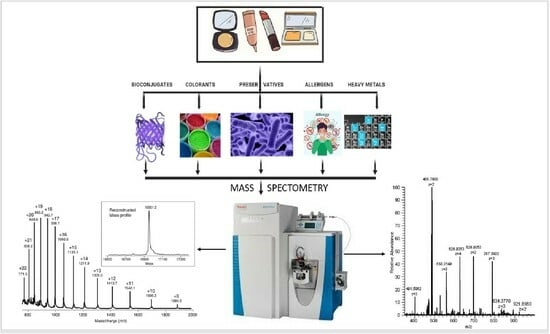
3.1. Analysis of Bioconjugates in Cosmetic Products
- -
- Lipids with manosyl erythritol (MEL)—these are glycolipids composed of a fatty acid ester, either 4-OD-manopyranosyl-erythritol or 1-OD-manopyranosyl-erythritol [56], produced by yeasts of the genus Pseudozyma, which have been shown to have a moisturizing action compared to natural ceramides on the skin. These glycolipids are used in antiwrinkle and skin-smoothing cosmetics [57].
- -
- Sophorolipids (SLP)—these are glycolipids composed of fatty acids of 16 or 18 carbon atoms bound to a sophorose as a hydrophilic part, produced by several species of Candida or other related yeast species. These glycolipids are used in detergents, lipsticks, lip creams, and eyeshadow [58].
- -
- Trehalose lipids—these are glycolipids composed of fatty acids linked to a disaccharide, trehalose, which is a nonreducing disaccharide in which two glucose molecules are linked in an α, α, 1,1-glycosidic bond.
3.2. Analysis of Preservatives in Cosmetic Products
3.2.1. HPLC-MS and UHPLC-MS
3.2.2. GC-MS
3.3. Analysis of Colorants in Cosmetic Products
3.4. Analysis of Allergens in Cosmetic Products
- (a)
- The ability to investigate compounds suitable for LC and GC in a single analysis;
- (b)
- A higher selectivity and specificity compared to HPLC or GC analysis only;
- (c)
- An analysis time at least six times faster than for HPLC and GC;
- (d)
- Solvent use is 95% lower than in existing HPLC methods.
3.5. Analysis of Heavy Metals in Cosmetic Products
| No. | Heavy Metal | Limits for Cosmetics (EU, Germany) | Limits for Cosmetics (USA) | Effects of Exposure on the Human Body |
|---|---|---|---|---|
| 1 | Mercury (Hg) | 0.1 ppm * | 1 ppm (colorants) | Renal, neurologic, and dermal toxicity [229], cutaneous changes reported include burning of the face, contact dermatitis, grey or blue–black facial discoloration, flushing, erythroderma, purpura, and gingivostomatitis. |
| 2 | Lead (Pb) | 2 ppm | 20 ppm (colorants) 10 ppm (lipsticks, lip glosses) | Affects the fetus and the central nervous system in Children [230,231], probably carcinogenic to humans [232,233], neurotoxic, nephrotoxic, and hepatotoxic and can also produce effects on the reproductive system, and can also affect fetal development through its passage via the placenta [234,235,236,237,238,239]. |
| 3 | Cadmium (Cd) | 0.1 ppm | - | Damage of the kidneys, fragility of the bones, carcinogenic in humans [240,241,242]. |
| 4 | Arsenic (As) | 0.5 ppm | 3 ppm (colorants) | Skin eruptions, alopecia, and striation of the nails, but also skin cancer [243], circulatory and peripheral nervous disorders, an increased risk of lung cancer, and a possible increase in the risk of gastrointestinal tract and the urinary system cancers [244]. |
| 5 | Nickel (Ni) | 10 ppm | - | Contact allergy, eyelid dermatitis, as well as irritation, eczema |
| 6 | Chromium (Cr) | - | - | contact allergy [245], carcinogenic in humans (Cr(VI)). |
| 7 | Antimony (Sb) | 0.5 ppm | - | Pneumoconiosis, alterations in pulmonary function, bronchitis, emphysema, gastrointestinal effects (abdominal pain, vomiting, diarrhea, and ulcers), dermatoses, and skin lesions [246,247,248], probably carcinogenic to humans (Sb trioxide). |
| 8 | Cobalt (Co) | - | - | Skin allergen causing allergic contact dermatitis (ACD) and eczema, possibly carcinogenic to humans. |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| No. | Samples | MS-Based Analysis | Observations/ Comments | Ref. |
|---|---|---|---|---|
| 1 | A total of 42 cosmetic products (12 rinse-off and 30 leave-on) purchased from nine different countries (USA and EU). | GC-MS | Simultaneous determination of 30 fragrances (including 24 listed allergens)—18 leave-on products contained at least one fragrance substance > 10 µg/g and 5 of the 12 rinse-off products contained at least one fragrance substance > 100 µg/g. | [166] |
| 2 | A total of 166 leave-on cosmetic products purchased within three years and stored at room temperature. | GC-MS and GC-MS/MS | The method was tested in order to identify the presence of 24 regulated allergens and 21 prohibited substances in the cosmetic products: 2–17 allergens were identified per sample, and only safrole (of the prohibited substances) was present in a concentration > LOQ in 12 out of 166 tested samples. | [144] |
| 3 | Six fragrance compositions. | GC HR-MS and GC-LR-MS | A total of 35 “difficult fragrance allergens” were quantified best by GC-orbitrap HR-MS. | [145] |
| 4 | A “Lily” matrix—a combination of about 50 raw materials, mainly constituted of essential oils, aromatic plant extracts, synthetic ingredients, water and ethanol. | GC-qMS | The validation of the GC-MS method for the quantification of the extended list of 57 fragrance allergens (which led to the updated NF EN 16274 standard in 2021 [276]). | [146] |
| 5 | One tested fragrance (CC02). | GC×GC qMS (SIM mode) | Validating a method to identify and quantify the suspected allergens (24) limited by EU regulations in fragrances by GC×GC-qMS. | [109] |
| 6 | Initial screening of 5 cosmetics (3 creams and 2 lotions) and subsequent screening of 123 cosmetics (71 creams and 52 lotions). | UHPLC-q-orbitrap HR-MS | Simultaneous screening of 100 restricted ingredients in cosmetics (39 antibodies, 40 glucocorticoids, 9 androgens, 8 progestogens, 4 antifungal agents. | [209] |
| 7 | Two commercial perfumes (“eau de toilette”). | GC–EI-MS | The 1460 and 1910 WatercolTM columns can reliably be used for the GC-MS analysis of EU-regulated volatile allergens in commercial perfumes (14 allergens identified in perfume 1 and 4 allergens in perfume 2) and showed complementary selectivity. | [150] |
| 8 | Three commercially available perfumes (Sakura Eau de Toilette, Moroccan Rose, White Musk). | GC-MPI-TOF-MS | A total of 26 allergens were identified (superior performance of MPI/MS over EI/MS for more reliable determination of the allergy compounds); the concentrations of methyl-2-octynoate (not written in the label of the bottle), citronellol, hexylcinnamaldehyde, and linalool in Sakura Eau de Toilette, and methyl-2-octynoate in White Musk (no label) is larger than the concentration specified by the Cosmetics Directive (0.001% for a leave-on sample). | [148] |
| 9 | A total of 20 commercial-scented plush toys (preserved in sealing packages before analysis). | Headspace (HS)-GC-MS | A total of 58 allergens were identified (natural extracts, which were unsuitable for a chromatography-based method, were not detected). | [149] |
| 10 | A total of 10 perfume products (7 eau de toilette, 2 aftershaves, and 1 eau de cologne) from the Swedish market. | 2D HPLC-ESI-MS/MS | Detection of hydroxiperoxides of limone and linalool (limonene-2-hydroperoxide (Lim-2-OOH), linalool-6-hydroperoxide (Lin-6-OOH), and linalool7-hydroperoxide (Lin-7-OOH, with strong sensitization potency), the highest concentrations of the measured hydroperoxides (445 ± 23 ppm of total linalool hydroperoxides) being observed in one after-shave product, which is likely able to elicit skin reactions in already sensitized individuals. | [208] |
| 11 | A total of 7 different matrices: 5 were homemade (DWL, fabric softener, liquid laundry detergent, milky hair shampoo, day cream), 1 (powder detergent) was provided by a detergent manufacturer, and 1 was a natural raw material (Peru balsam, Nelixia, Antigua, Guatemala). | GC-MS | The standard addition protocol allowed the analysis of suspected allergens in the investigated matrices and allowed the quantification of all compounds (15 allergens) except farnesol and Lyral, within a concentration range of 50–100 mg/L. | [147] |
| 12 | A total of 62 commercialized perfumes. | GC×GC-qMS | A total of 56 (69 analytes including isomers) suspected chemically defined fragrance allergens in perfumes were investigated (the majority of the analytes could be determined under or above the 10 mg/kg regulated limit (88–100%). | [170] |
| 13 | Citrus oils. | LC-MS | Quantification of 15 furocoumarins. | [209] |
| 14 | Various cosmetic and personal care products. | UPC2-MS/MS | The analysis of the 24 regulated and 6 additional compounds (4 nonregulated cosmetic allergens and 2 potential carcinogenic compounds, methyl eugenol and 4-allyl anisole) was achieved using the Xevo TQD in MRM mode with APCI ionization (+/−), coupled to an ACQUITY UPC2 System, in a 7 min run. The method is more than six times faster than existing HPLC and GC methods, with 95% less solvent usage than existing HPLC methods. | [201] |
| 15 | A total of 7 oils issued from plants. | GC-MS | The chromatographic procedure seemed to be slightly longer; however, the conditions showed good resolution for about 200 terpenoid compounds determined in general essential oil studies. From the 25 standard allergens studied, 19 showed a retained DL (detection limit) < 13 mg/L, and 5 were = 30–50 mg/L. These variations are well explained by the form of the peaks. GC-MS is considered a good technique for the determination of volatile substances. Results were obtained with good repeatability. | [151] |
| 16 | A total of 4 commercial perfumes purchased in a local store (Messina, Italy). | GC-MS | The GC-MS method described is a rapid (<5 min) and effective screening tool in the determination of 26 allergens contained in mediumly complex perfumes. The twin-filtered MS library search procedure was shown to be a powerful tool for reliable compound identification. As for all monodimensional methods, it may fail when the number of sample volatiles greatly exceeds the peak capacity of a single column: the reliable qualitative/quantitative determination of 10–20 skin sensitizers amongst 500–1000 other volatiles would be an arduous task. A good result may be attained for a 200-component fragrance and a bad one for a 150-compound perfume. The most appropriate approach to be used, if a multiple-choice exists, strictly depends on the analyst’s experience and judgment. | [28] |
| 17 | Randomly chosen 18 cosmetic products—5 shampoos, 7 creams and lotions, 2 eau de toilette, 1 deodorant spray, 1 lipstick, 1 face powder and 1 soap bar. | GC-MS | The GC-MS method has been developed for the routine analysis of 11 fragrance substances in cosmetics: cinnamic alcohol, cinnamic aldehyde, eugenol, hydroxy citronellal, a-amyl cinnamic aldehyde, geraniol, isoeugenol, coumarin, dihydrocoumarin, citronellal, and citral. DL of all of the target fragrance substances were ~1 ppm. | [168] |
| 18 | A total of 5 commercial perfumes (P1–P5). | All FM GC×GC–qMS | The FM GC×GC–qMS method is sufficiently sensitive for all the 54 allergens considered. Moreover, and if required, the HR untargeted analysis of perfume constituents can be performed. The FM model proposed is a low-cost and effective alternative to cryogenic modulation; both the hardware and operational costs are somewhat limited, with many of the well-known benefits of GC×GC maintained. | [107] |
| 19 | Fragrance concentrates provided in blind by IFRA-member companies. | GC-MS GC×GC-MS | To determine a more realistic LOQ (limit of quantitation) in the context of a fragrance concentrate, a fragrance concentrate (FT) was spiked with all allergens at various levels between 10 and 500 mg/L and analyzed by a single laboratory. The accuracy profile shows that the mean bias remains less than 20% at all spiking levels down to 10 mg/L. For 90% of determinations, the expected bias should be less than 35% down to a level of 20 mg/L and between −49 and 77% at 10 mg/L (i.e., between 5 and 18 mg/L). This range remains acceptable to set the LOQ at 10 mg/L, in view of the suspected allergens analysis complexity. Fragrance concentrates are very complex mixtures; the occurrence of coelutions is frequent—the two-columns × three-ion option best minimizes the consequences of coelution on the determination of suspected allergens. | [188] |
| 20 | A total of 70 commercial perfumes and colognes. | GC-MS | Contents of 52 cosmetic ingredients belonging to 4 different types of ingredients: 6 preservatives, 12 synthetic musks, 26 fragrance allergens, and 8 phthalates can be determined in a single run. All samples contained some of the target ingredients. Several samples do not comply with the regulations concerning the presence of phthalates. Musk’s data confirmed the trend of the replacement of nitromusks by polycyclic musks, as well as the noticeable introduction of macrocyclic musks in the perfume’s composition. The prohibited musk moskene has been detected in one sample in an appreciable concentration. The average number of fragrance allergens is 12 per sample; values > 1% have been found in some samples. Preservatives data show that parabens, although ubiquitous in other cosmetic products, are not widely used in perfumery. In contrast, the presence of BHT is indeed widespread. Only about 38% of the perfumes were adequately labeled for the allergens tested. | [153] |
| 21 | A model mixture of volatiles and essential oils of different complexity (mint, lavender, and vetiver essential oils). | GC×GC MS GC×GC FID | Profiling and fingerprinting of medium- to highly complex samples of interest in the flavor and fragrance field was investigated. Capillary flow technology reverse-inject differential flow modulator was implemented with different column configurations (lengths, diameters, and stationary phase coupling) and detector combinations (MS and FID) to evaluate its potential in the quantitative profiling and fingerprinting of medium- to highly complex essential oils, and a parallel dual-secondary column dual-detection configuration that has shown to improve the information potential also with thermally modulated GC×GC platforms (MS for identification FID for quantitation) was tested. Experimental results demonstrate that careful tuning of column dimensions and system configurations yields improved (a) selectivity, (b) operable carrier gas linear velocities at close-to-optimal values, (c) 2D separation power by extending the modulation period, and (d) handling of overloaded peaks without dramatic losses in resolution and quantitative accuracy. | [173] |
| 22 | Different cosmetics (leave-on and rinse-off products) from national and international brands were purchased from local sources. | GC-MS | Accuracy, precision, linearity, and LODs were evaluated to assess the performance of the proposed method. Quantitative recoveries (>75%) were obtained, and RSD values were <10% in all cases. The quantification limits were well below those set by the international cosmetic regulations, making this multicomponent analytical method suitable for routine control. A total of 25 fragrance allergens were identified All the samples contained several of the target cosmetic ingredients, with an average number of seven. The total fragrance allergen content was, in general, relatively high, even in baby care products, with values close to or up to 1%, for several samples, although the actual European Cosmetic Regulation was fulfilled. | [176] |
| 23 | A total of 60 household commodities, including perfumes, lotions, hair care products, and household cleaners, were purchased from retail stores in Albany, New York. | GC-MS | Concentrations of HHCB, AHTN, and HHCB-lactone in consumer products ranged from <5 ng/g to over 4000 μg/g, <5 ng/g to 451 μg/g, and <5 ng/g to 217 μg/g, respectively. The highest concentrations were found in perfumes, body creams, lotions, and deodorants. The results suggest that a wide variety of source materials exist for HHCB and AHTN and that these materials are used on a daily basis. | [177] |
| 24 | Different cosmetic products. | GC-MS | The analysis of suspected volatile allergens in products containing high-molecular-weight or nonvolatile compounds such as plant extracts, solid and liquid detergents, and shampoos was performed. Based on PTV injection with ALEX and GC-MS, nonvolatile matrices are retained in the liners filled with PDMS foam, while good analytical performance for the target solutes is preserved. This approach drastically shortens and simplifies the sample preparation step. The method also gives quasi-quantitative analyte recoveries for all solutes with the exception of methyl-2-octynoate and methyl-2-nonynoate. For various nonvolatile matrices, a single external calibration can be used, while for the two mentioned esters, internal standardization is presently carried out. Analyzing all target compounds in the different matrices with one single method is impossible; therefore, we proposed at various meetings to classify the different matrices into four classes. Class I consists of samples that contain volatile or semivolatile solutes, typically eluting on an apolar column between n-decane (retention index 1000) and n-docosane (retention index 2200). Class II also consists of samples containing only volatile and semivolatile solutes, but their complexity is very high (A100 solutes) and/or the concentration range is very broad (e.g., very low concentration of a target compound next to a very high concentration of matrix compound). Class III comprises nonvolatile samples (solutes eluting after n-hexacosane). Class IV matrices are finished products like soaps, liquid and solid detergents, etc. In these samples, the solutes are typically present at relatively low concentrations, while the matrix can be quite complex due to the presence of glycols and surfactants. | [156] |
| 25 | A total of 10 samples (several moisturizing and antiwrinkle creams and lotions, hand creams, and sunscreen and after-sun creams). | GC-MS | A new method based on solid-phase dispersion-pressurized liquid extraction (PLE) followed by GC-MS has been developed for the determination of 26 suspected fragrance allergens (all the regulated in the EU Cosmetics Directive amenable by GC, as well as pinene and methyl-eugenol) in cosmetic samples. The study revealed the presence of suspected allergens in all the analyzed samples, and half of the samples contained an elevated number of them. | [157] |
| 26 | A total of 26 cosmetic products (creams, emulsions, lotions, gels for the skin, bath and shower preparations, deodorants, hair-setting, hair-cleansing, and hair-conditioning products, shaving products, and sunbathing products). | GC-MS | MSPD and GC-MS were used for the rapid determination of 18 plasticizers (phthalates and adipates), 7 polycyclic musks, and 5 nitromusks, which makes a total of 30 targets in both rinse-off and leave-on cosmetic formulations. A total of 25 out of 30 targets were detected in the samples. The most frequently found compounds were galaxolide and tonalide, reaching concentrations above 0.1% (1000 g·g−1) and diethyl phthalate (between 0.7 and 357 g·g−1). The presence of banned substances such as dibutyl phthalate, diisobutyl phthalate, dimethoxyethyl phthalate, benzylbutyl phthalate, diethylhexyl phthalate, diisopentyl phthalate and dipentyl phthalate, musk ambrette, and musk tibetene was confirmed in 16 of the 26 personal care products (62%). | [154] |
| 27 | A broad range of cosmetics and personal care products (shampoos, body milk, moisturizing milk, toothpaste, hand creams, gloss lipstick, sunblock, deodorants, and liquid soaps, among others). | GC-MS | A practical, simple, and low-cost sample GC-MS and GC-MS/MS method has been developed for the rapid simultaneous determination of 38 cosmetic ingredients, 25 fragrance allergens, and 13 preservatives. The final miniaturized process required the use of only 0.1 g of sample and 1 mL of organic solvent for the final extract ready for analysis. The concentration levels ranged from the sub-parts per million to the parts per million. Several fragrances (linalool, farnesol, hexylcinnamal, and benzyl benzoate) have been detected at levels >0.1% (1000 g·g−1). With regard to preservatives, phenoxyethanol was the most frequently found additive, reaching a relatively high concentration (>1500 g·g−1) in 5 cosmetic products. BHT was detected in 8 samples, in 2 of them (a baby care product and a lipstick) at high concentrations (>1000 g·g−1). In 3 leave-on samples, methyl paraben was also found at high levels (>1700 g·g−1). Finally, triclosan was found at the maximum concentration limit (0.3%) laid down by the European regulation in 2 deodorant samples, and the total paraben concentration was close to the maximum concentration permitted (0.8%) in one leave-on sample (body milk). | [98] |
| 28 | Personal care products and sanitation products (n = 82) were obtained through the cooperation of several volunteers. The samples were divided into six categories: sanitation products (n = 14), perfumes (n = 19), deodorants (n = 4), hair care products (n = 12), shower and bath products (n = 18), and body lotions (n = 15). | GC-MS | An overview of the synthetic musk levels in 6 different personal care product categories was performed. Especially body lotions, perfumes, and deodorants contain high levels of synthetic musks. Maximum concentrations of HHCB, AHTN, MX, and MK were 22 mg·g−1, 8 mg·g−1, 26 μg·g−1, and 0.5 μg·g−1, respectively. By combining these results with the average usage of consumer products, low-, medium-, and high-exposure profiles through dermal application could be estimated. HHCB was the highest contributor to the total amount of synthetic musks in every exposure profile (18–23,700 lg·d1). Exposure to MK and MX did not increase substantially (10–20-fold) between low- and high-exposure profiles, indicating that these compounds cover a less broad range. In comparison, exposure to HHCB and AHTN increased up to 10,000 fold between low and high exposure. | [159] |
| 29 | A total of 73 household commodities were purchased in Kumamoto, Japan: perfumes (n¼13), fabric softeners (n¼11), shampoos (n¼11), body lotions (n¼9), body soap (n¼5), antiperspirants (n¼5), laundry detergents (n¼4) toilet deodorants (n¼4), body fragrances (n¼2), hair liquid (n¼2), sunscreen (n¼2), dish cleaner (n¼2), tooth powder (n¼2), and bath cleaner (n¼1). | GC-MS | Occurrence and concentrations of macrocyclic-, polycyclic-, and nitro musks in cosmetics and household commodities collected from Japan. The high concentrations and detection frequencies of Musk T, habanolide, and exaltolides were found in commercial products, suggesting their large production and usage in Japan. Polycyclic musks, HHCB and OTNE, also showed high concentrations in cosmetics and products. The estimated dairy intakes of Musk T and HHCB by the dermal exposure to commercial products were 7.8 and 7.9 μg/kg/day in humans, respectively, and perfume and body lotion are dominant exposure sources. The dairy intakes of HHCB by dust ingestions were 0.22 ng/kg/day in humans, which were approximately 5 orders of magnitude lower than those of dermal absorption from commercial household commodities. | [160] |
| 30 | Fragrance-free cosmetic samples (creams, body lotions, oils) were bought from commercial shops in Basel and stored at room temperature until preparation for recovery experiments (adding allergens in the range of 10 mg/kg). Additionally, for quality control, a hand cream (oil in water emulsion) of known fragrance content in the range of 4–15 mg/kg was used as a reference sample. | GC-MS | SEC combined with GC-MS was developed for the quantitation of 24 restricted allergenic fragrance compounds in cosmetic samples. Fragrance calibration has to be performed with propyl acetate as a solvent containing a constant proportion of matrix components. With the exception of hydroxycitronellal (66 ± 5%), all compounds showed good recovery rates in the range of 90–120%. The mean accuracy (relative error) was 1 ± 10% for all 24 compounds in five spiked creams (10 mg/kg per allergen) and 8 ± 34% in a reference sample (4–15 mg/kg). The most significant benefit compared to other methods is the flexible clean-up with SEC, which allows the determination of an extensive range of compounds in difficult matrices with GC-MS. | [180] |
| 31 | Personal care products were purchased from retail stores in Porto, Portugal: body and hair washes (n = 5), toilet soaps (n = 1), skin moisturizers (n = 4), roll-on deodorants (n = 1), and toothpaste (n = 1). | GC-MS | The developed and validated method using QuEChERS extraction followed by GC-MS was applied to the analysis of 12 samples, which revealed musk concentrations ranging from 2 ng/g (toothpaste) to 882,340 ng/g (perfumed body lotion). | [186] |
| 32 | Cosmetic samples from national and international brands were purchased from local markets in Beijing. | GC-MS | A total of 7 synthetic musks (musk ambrette, musk tibetene, musk moskene, musk ketone, musk xylene, phantolide, and tonalide) are extracted and prepurified by a mixture solution of water and isopropanol from cream and separated and purified by tandem columns containing SLE column and LC-Alumina-N SPE column. This pretreatment method combined with GC-MS/MS technology has been proved to be precise, accurate, and applicable to the routine analysis of 7 synthetic musks residues in cream samples. | [189] |
| 33 | A total of 12 commercially available essential oils were purchased from local chemical material stores in Taiwan. A total of 5 culinary herbs (holy basil, sweet basil, thyme, laurel, and rosemary) and 5 spices (cumin, cinnamon, nutmeg, cardamon, and clove) were purchased from local food retail stores in Kaohsiung, Taiwan. Samples of 4 commercially available aromatherapy massage oil products were obtained from randomly selected cosmeceutical stores in Taiwan. | GC-MS | A simple and quick sample preparation method was developed and used for preconcentration and extraction of six phenylpropenes, including anethole, estragole, eugenol, methyl eugenol, safrole, and myristicin, from oil samples by dual dispersive liquid–liquid microextraction. GC-MS was used for the determination and separation of compounds. Several experimental parameters affecting extraction efficiency were evaluated and optimized. For all analytes (10–1000 ng/mL), the limits of detection (S/N 3) ranged from 1.0 to 3.0 ng/mL; the limits of quantification (S/N 10) ranged from 2.5 to 10.0 ng/mL; and enrichment factors ranged from 3.2 to 37.1 times. Within-run and between-run relative standard deviations (n = 6) were less than 2.61% and 4.33%, respectively. Linearity was excellent, with determination coefficients (r2) above 0.9977. The experiments showed that the proposed method is simple, effective, and environmentally friendly for analyzing phenylpropenes in oil samples. | [163] |
| 34 | A total of 3 perfumes, 2 anti-hair loss products, 1 post-depilation mousse, 1 cream deodorant, and 3 different cream samples (body, sun, and hand creams). | GC-qMS | Qualitative and quantitative analysis of 24 volatile compounds listed as suspected allergens in cosmetics by the European Union was performed. The applicability of a headspace (HS) autosampler in combination with GC equipped with a programmable temperature vaporizer (PTV) and a qMS detector is explored. The method showed good precision and accuracy, and it is rapid, simple, and highly suitable for the determination of suspected allergens in different cosmetic products. | [181] |
| 35 | Commercial perfume samples and a cosmetic product (body cream) were obtained from a supermarket. | GC/GC×GC-MS | Suspected fragrance allergens were determined in cosmetic products using a combination of whole evaporation-dynamic headspace (FEDHS) with selectable GC/GC×GC-MS using capillary flow technology (CFT) and low thermal mass GC (LTM-GC). The FEDHS approach allows the nondiscriminating extraction and injection of both apolar and polar fragrance compounds without contamination of the analytical system by high-molecular-weight nonvolatile matrix compounds. The system is highly flexible and easy to use, and was applied to all classes of cosmetic samples, including water-containing matrices such as shower gels or body creams. | [182] |
| 36 | The samples, analyzed for their content on illegal skin bleaching agents, were taken by inspectors affiliated with the Belgian federal public service “Animal, Plant and Food Directorate-General” (DG4) and the Belgium Federal Agency for Medicinal and Health Products (FAMHP) and were also used for this study. | GC-MS | A new headspace GC-MS method was capable of analyzing 24 volatile allergenic fragrances in complex cosmetic formulations, such as hydrophilic and lipophilic creams, lotions, and gels. This method was successfully validated using the total error approach. The trueness and precision deviations for all components were smaller than 8%, and the expectation tolerance limits did not exceed the acceptance limits of ±20% at the labeling limit—used to analyze 18 cosmetic samples that were already identified as being illegal on the EU market for containing forbidden skin-whitening substances. | [165] |
| 37 | Different personal care products: a hand cream water-in-oil (w/o), an eau de perfume, a shower gel, and an orange oil, and they were purchased from a local drugstore. | LC-MS | There is an advantage of the Direct EI LC-MS interface for the quantitation of principal components, as well as for the identification of unknown/undeclared ingredients Commercially available products were diluted with methanol and injected directly into a nano-LC column. Limonene, linalool, and citral were selected as target compounds because of their use as fragrances in toiletry and detergent products. Selected compounds are not detected with ESI because of their poor or very low response. No matrix effects were observed, and the repeatability was excellent even after several weeks of operation. The product’s composition was investigated in full scan mode to determine the presence of unknown or nonlisted ingredients. | [195] |
| 38 | A total of 10 randomly selected perfumes and similar products. | LC-MS | An LC-MS method for quantitative analysis of the potential oak moss allergens atranol and chloroatranol in perfumes and similar products was developed and validated. LOD for atranol and chloroatranol were 5.0 ng/mL and 2.4 ng/mL, respectively; the method based on LC-MS and LC-MS/MS with ESI in negative mode and SRM allowed the identification of these compounds at concentrations below those causing allergic skin reactions in oak-moss-sensitive patients. The recovery of chloratranol from spiked perfumes was 96 ± 4%. Low recoveries (49 ± 5%) were observed for atranol in spiked perfumes, indicating ion suppression caused by matrix components. | [197] |
References
- Frosch, P.J.; Menne, T.; Lepoittevin, J.P. Contact Dermatitis, 4th ed.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 493–495. [Google Scholar]
- González-Muñoz, P.; Conde-Salazar, L.; Vañó-Galván, S. Allergic Contact Dermatitis Caused by Cosmetic Products. Actas Dermosifiliogr. 2014, 105, 822–832. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.I.; Glaser, D.A. Cosmeceuticals: The new medicine of beauty. Mo Med. 2011, 108, 60–63. [Google Scholar] [PubMed]
- Iwata, H.; Shimada, K. Formulas, Ingredients and Production of Cosmetics: Technology of Skin-and Hair-Care Products in Japan; Springer: Tokyo, Japan, 2013; pp. 6–8. [Google Scholar]
- Feng, X.; Xu, X.; Liu, Z.; Xue, S.; Zhang, L. Novel functionalized magnetic ionic liquid green separation technology coupled with high performance liquid chromatography: A rapid approach for determination of estrogens in milk and cosmetics. Talanta 2020, 209, 120542. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Blanco, M.C.; Penafiel Barba, S.; Moliner-Martinez, Y.; Campins-Falco, P. Footprint of carbonyl compounds in hand scent by in-tube solid-phase mi- croextraction coupled to nano-liquid chromatography/diode array detection. J. Chromatogr. A 2019, 1596, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Cicourel, A.R.; van de Velde, B.; Verduin, J.; Janssen, H.G. Comprehensive off-line silver phase liquid chromatography x gas chromatography with flame ionization and vacuum ultraviolet detection for the detailed characterization of mineral oil aromatic hydrocarbons. J. Chromatogr. A 2019, 1607, 46039. [Google Scholar] [CrossRef] [PubMed]
- Chunin, N.; Phooplub, K.; Kaewpet, M.; Wattanasin, P.; Kanatharana, P.; Thavarungkul, P.; Thammakhet-Buranachai, C. A novel 3D-printed solid phase microextraction device equipped with silver-polyaniline coated pencil lead for the extraction of phthalate esters in cosmeceutical products. Anal. Chim. Acta 2019, 1091, 30–39. [Google Scholar] [CrossRef]
- Garcia-Cicourel, A.R.; van de Velde, B.; Roskam, G.; Janssen, H.G. Supercritical f luid chromatography as a rapid single-step method for the determination of mineral oil saturated and aromatic hydrocarbons in purified mineral oils for food and cosmetics applications. J. Chromatogr. A 2020, 1614, 460713. [Google Scholar] [CrossRef]
- Chindaphan, K.; Wongravee, K.; Nhujak, T.; Dissayabutra, T.; Srisa-Art, M. Online preconcentration and determination of chondroitin sulfate, dermatan sulfate and hyaluronic acid in biological and cosmetic samples using capillary electrophoresis. J. Sep. Sci. 2019, 42, 2867–2874. [Google Scholar] [CrossRef]
- Ko, H.Y.; Lin, Y.H.; Shih, C.J.; Chen, Y.L. Determination of phenylenediamines in hair colors derivatizated with 5-(4, 6-dichlorotriazinyl)aminofluorescein via micellar electrokinetic chromatography. J. Food Drug Anal. 2019, 27, 825–831. [Google Scholar] [CrossRef]
- Nkansah, M.A.; Owusu-Afriyie, E.; Opoku, F. Determination of lead and cadmium contents in lipstick and their potential health risks to con-sumers. J. Consum. Protect. Food Saf. 2018, 13, 367–373. [Google Scholar] [CrossRef]
- Rehan, I.; Gondal, M.A.; Rehan, K.; Sultana, S. Spectral diagnosis of health hazardous toxins in face foundation powders using laser induced breakdown spectroscopy and inductively coupled plasma-optical emission spectroscopy (ICP-OES). Talanta 2020, 217, 121007. [Google Scholar] [CrossRef] [PubMed]
- Al Alamein, A.M.A.; Elwy, H.M.; El-Din, S.H.S. Univariate and multivariate spectrophotometric methods for simultaneous determination of avobenzone and octinoxate in pure form and in cosmetic formulations: A comparative study. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 206, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Bonnier, F.; Miloudi, L.; Henry, S.; Bertrand, D.; Tauber, C.; Perse, X.; Yvergnaux, F.; Byrne, H.J.; Chourpa, I.; Munnier, E. Quantification of low-content encapsulated active cosmetic ingredients in complex semi-solid formulations by means of attenuated total reflectance-infrared spectroscopy. Anal. Bioanal. Chem. 2020, 412, 159–169. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, Z.; Yue, Z.; Gao, J.; Wu, S.; Zhang, Z.; Li, G. Rapid determination of trace nitrofurantoin in cosmetics by surface enhanced Raman spectroscopy using nanoarrayed hydroxyl polystyrene-based substrate. J. Raman Spectrosc. 2019, 50, 1094–1102. [Google Scholar] [CrossRef]
- Nicoletti, M.; Frezza, C.; Tomassini, L.; Serafini, M.; Bianco, A. Detection of picramic acid and picramate in henne products by NMR Spectroscopy. Nat. Prod. Res. 2019, 33, 2073–2078. [Google Scholar] [CrossRef]
- Alghamdi, A.F.; Messali, M. Green synthesis of new ionic liquid and its electrochemical determination at some detergents and cosmetics samples using differential pulse polarography. J. Mol. Liq. 2018, 266, 112–117. [Google Scholar] [CrossRef]
- Mildau, G. Chapter 4—General Review of Official Methods of Analysis of Cosmetics. In Analysis of Cosmetic Products, 2nd ed.; Salvador, A., Chisvert, A.B.T.-A., Eds.; Elsevier: Boston, MA, USA, 2018; pp. 67–83. [Google Scholar]
- Fernandes, G.M.; Silva, W.R.; Barreto, D.N.; Lamarca, R.S.; Lima Gomes, P.C.F.; da S Petruci, J.F.; Batista, A.D. Novel Approaches for Colorimetric Measurements in Analytical Chemistry—A Review. Anal. Chim. Acta 2020, 1135, 187–203. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, P.G.; Wittenberg, J.B.; Rua, D.; Krynitsky, A.J. Simultaneous Determination of Cosmetics Ingredients in Nail Products by Fast Gas Chromatography with Tandem Mass Spectrometry. J. Chromatogr. A 2016, 1446, 134–140. [Google Scholar] [CrossRef]
- Guć, M.; Cegłowski, M.; Pawlaczyk, M.; Kurczewska, J.; Reszke, E.; Schroeder, G. Application of FAPA Mass Spectrometry for Analysis of Fragrance Ingredients Used in Cosmetics. Measurement 2021, 168, 108326. [Google Scholar] [CrossRef]
- Guerra, E.; Llompart, M.; Garcia-Jares, C. Miniaturized Matrix Solid-Phase Dispersion Followed by Liquid Chromatography-Tandem Mass Spectrometry for the Quantification of Synthetic Dyes in Cosmetics and Foodstuffs Used or Consumed by Children. J. Chromatogr. A 2017, 1529, 29–38. [Google Scholar] [CrossRef]
- Shang, Y.; Meng, X.; Liu, J.; Song, N.; Zheng, H.; Han, C.; Ma, Q. Applications of mass spectrometry in cosmetic analysis: An overview. J. Chromatogr. A 2023, 1705, 464175. [Google Scholar] [CrossRef] [PubMed]
- Tuzimski, T.; Petruczynik, A. Review of New Trends in the Analysis of Allergenic Residues in Foods and Cosmetic Products. J. AOAC Int. 2020, 103, 997–1028. [Google Scholar] [CrossRef] [PubMed]
- Hamouda, A.F.; Felemban, S. A Bio-Indicator Pilot Study Screening Selected Heavy Metals in Female Hair, Nails, and Serum from Lifestyle Cosmetic, Canned Food, and Manufactured Drink Choices. Molecules 2023, 28, 5582. [Google Scholar] [CrossRef] [PubMed]
- Guerra, E.; Celeiro, M.; Lamas, J.P.; Llompart, M.; Garcia-Jares, C. Determination of Dyes in Cosmetic Products by Micro-Matrix Solid Phase Dispersion and Liquid Chromatography Coupled to Tandem Mass Spectrometry. J. Chromatogr. A 2015, 1415, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Mondello, L.; Sciarrone, D.; Casilli, A.; Tranchida, P.Q.; Dugo, P.; Dugo, G. Fast Gas Chromatography-Full Scan Quadrupole Mass Spectrometry for the Determination of Allergens in Fragrances. J. Sep. Sci. 2007, 30, 1905–1911. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Rivera, G.; Vila, M.; Lores, M.; Garcia-Jares, C.; Llompart, M. Development of a Multi-Preservative Method Based on Solid-Phase Microextraction–Gas Chromatography–Tandem Mass Spectrometry for Cosmetic Analysis. J. Chromatogr. A 2014, 1339, 13–25. [Google Scholar] [CrossRef]
- Muhammad, S.; H. P. S., A.K.; Abd Hamid, S.; Danish, M.; Marwan, M.; Yunardi, Y.; Abdullah, C.K.; Faisal, M.; Yahya, E.B. Characterization of Bioactive Compounds from Patchouli Extracted via Supercritical Carbon Dioxide (SC-CO2) Extraction. Molecules 2022, 27, 6025. [Google Scholar] [CrossRef]
- Li, Y. Analytical methods for the analysis of volatile natural products. Nat. Prod. Rep. 2023, 40, 922–956. [Google Scholar] [CrossRef]
- Ho, T.M.; Razzaghi, A.; Ramachandran, A.; Mikkonen, K.S. Emulsion Characterization via Microfluidic Devices: A Review on Interfacial Tension and Stability to Coalescence. Adv. Colloid Interface Sci. 2022, 299, 102541. [Google Scholar] [CrossRef]
- Eudier, F.; Savary, G.; Grisel, M.; Picard, C. Skin Surface Physico-Chemistry: Characteristics, Methods of Measurement, Influencing Factors and Future Developments. Adv. Colloid Interface Sci. 2019, 264, 11–27. [Google Scholar] [CrossRef]
- Chanamai, R.; McClements, D.J. Dependence of Creaming and Rheology of Monodisperse Oil-in-Water Emulsions on Droplet Size and Concentration. Colloids Surf. Physicochem. Eng. Asp. 2000, 172, 79–86. [Google Scholar] [CrossRef]
- Matos, M.; Gutiérrez, G.; Iglesias, O.; Coca, J.; Pazos, C. Characterization, Stability and Rheology of Highly Concentrated Monodisperse Emulsions Containing Lutein. Food Hydrocoll. 2015, 49, 156–163. [Google Scholar] [CrossRef]
- Hirschman, J.; Venkataramani, D.; Murphy, M.I.; Patel, S.M.; Du, J.; Amin, S. Application of Thin Gap Rheometry for High Shear Rate Viscosity Measurement in Monoclonal Antibody Formulations. Colloids Surf. Physicochem. Eng. Asp. 2021, 626, 127018. [Google Scholar] [CrossRef]
- Cano, M.; Borrego, V.; Roales, J.; Idígoras, J.; Lopes-Costa, T.; Mendoza, P.; Pedrosa, J.M. Rapid discrimination and counterfeit detection of perfumes by an electronic olfactory system. Sens. Actuators B Chem. 2011, 156, 319–324. [Google Scholar] [CrossRef]
- Penza, M.; Cassano, G.; Tortorella, F.; Zaccaria, G. Classification of Food, Beverages and Perfumes by WO3 Thin-Film Sensors Array and Pattern Recognition Techniques. Sens. Actuators B Chem. 2001, 73, 76–87. [Google Scholar] [CrossRef]
- Blank, I. Gas Chromatography-Olfactometry in Food Aroma Analisys. In Flavor, Fragrance and Odor Analysis; CRC Press: Boca Raton, FL, USA, 2002; pp. 297–331. [Google Scholar]
- Chemetsova, E.S.; Bromirski, M.; Scheibner, O.; Morlock, G.E. DART-Orbitrap MS: A novel mass spectrometric approach for the identification of phenolic compounds in propolis. Anal. Bioanal. Chem. 2012, 403, 2859–2867. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Zhang, J.; Hu, Z.; Hu, B.; Ding, L.; Jia, L.; Chen, H. Neutral desorption extractive electrospray ionization mass spectrometry for fast screening sunscreen agents in cream cosmetic products. Talanta 2011, 85, 1665–1671. [Google Scholar] [CrossRef]
- Salter, T.L.; Green, F.M.; Faruqui, N.; Gilmore, I.S. Analysis of personal care products on model skin surfaces using DESI and PADI ambient mass spectrometry. Analyst 2011, 136, 3274–3280. [Google Scholar] [CrossRef]
- Yang, S.; Han, J.; Huan, Y.; Cui, Y.; Zhang, X.; Chen, H.; Gu, H. Desorption electrospray ionization tandem mass spectrometry for detection of 24 carcinogenic aromatic amines in textiles. Anal. Chem. 2009, 81, 6070–6079. [Google Scholar]
- Campbell, I.S.; Tonand, A.T.; Mulligan, C.C. Direct detection of pharmaceuticals and personal care products from aqueous samples with thermally-assisted desorption electrospray ionization mass spectrometry. J. Am. Soc. Mass Spectrom. 2011, 22, 1285–1293. [Google Scholar] [CrossRef]
- Alberici, R.M.; Simas, R.C.; Sanvido, G.B.; Romão, W.; Lalli, P.M.; Benassi, M.; Cunha, I.B.; Eberlin, M.N. Ambient mass spectrometry: Bringing MS into the real world. Anal. Bioanal. Chem. 2010, 398, 265–294. [Google Scholar] [CrossRef]
- Yue, H.; He, F.; Zhao, Z.; Duan, Y. Plasma-based ambient mass spectrometry: Recent progress and applications. Mass Spectrom. Rev. 2023, 42, 95–130. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, D.N.; Sartor, S.d.B.; Ferreira, M.S.; Catharino, R.R. Cosmetic Analysis Using Matrix-Assisted Laser Desorption/Ionization Mass Spectrometry Imaging (MALDI-MSI). Materials 2013, 6, 1000–1010. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Lee, G.H.; Yoo, J.S. Analysis of Ceramides in Cosmetics by Reversed-Phase Liquid Chromatography/Electrospray Ionization Mass Spectrometry with Collision-Induced Dissociation. Rapid Commun. Mass Spectrom. 2003, 17, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Draelos, Z.D. New Treatments for Restoring Impaired Epidermal Barrier Permeability: Skin Barrier Repair Creams. Clin. Dermatol. 2012, 30, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.C.; Chang, T.M. Ceramide 1 and Ceramide 3 Act Synergistically on Skin Hydration and the Transepidermal Water Loss of Sodium Lauryl Sulfate-Irritated Skin. Int. J. Dermatol. 2008, 47, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Kircik, L.H.; Del Rosso, J.Q. Nonsteroidal Treatment of Atopic Dermatitis in Pediatric Patients with a Ceramide-Dominant Topical Emulsion Formulated with an Optimized Ratio of Physiological Lipids. J. Clin. Aesthet. Dermatol. 2011, 4, 25–31. [Google Scholar] [PubMed]
- Sparavigna, A.; Tenconi, B.; De Ponti, I. Preliminary Open-Label Clinical Evaluation of the Soothing and Reepithelialization Properties of a Novel Topical Formulation for Rosacea. Clin. Cosmet. Investig. Dermatol. 2014, 7, 275–283. [Google Scholar] [CrossRef]
- Puviani, M.; Agostinis, F.; Milani, M. Barrier Repair Therapy for Facial Atopic Eczema with a Non-Steroidal Emollient Cream Containing Rhamnosoft, Ceramides and Iso-Leucine. A Six-Case Report Series. Minerva Pediatr. 2014, 66, 307–311. [Google Scholar]
- Khan, N.R.; Rathod, V.K. Enzyme Catalyzed Synthesis of Cosmetic Esters and Its Intensification: A Review. Process Biochem. 2015, 50, 1793–1806. [Google Scholar] [CrossRef]
- Soberón-Chávez, G. Biosurfactants; Springer: Berlin/Heidelberg, Germany, 2011; Volume 20. [Google Scholar]
- Morita, T.; Kitagawa, M.; Suzuki, M.; Yamamoto, S.; Sogabe, A.; Yanagidani, S.; Imura, T.; Fukuoka, T.; Kitamoto, D. A Yeast Glycolipid Biosurfactant, Mannosylerythritol Lipid, Shows Potential Moisturizing Activity toward Cultured Human Skin Cells: The Recovery Effect of MEL-a on the SDS-Damaged Human Skin Cells. J. Oleo Sci. 2009, 58, 639–642. [Google Scholar] [CrossRef] [PubMed]
- Eiko, K.; Toshi, T. Dermatological Anti-Wrinkle Agent. World Patent 2008/001921, 3 January 2008. [Google Scholar]
- Hall, P.J.; Haverkamp, J.; Van Kralingen, C.G.; Schmidt, M. Laundry Detergent Composition Containing Synergistic Combination of Sophorose Lipid and Nonionic Surfactant. U.S. Patent Application No. 5520839, 28 May 1996. [Google Scholar]
- Alvani, K.; Qi, X.; Tester, R.F. Use of Carbohydrates, Including Dextrins, for Oral Delivery. Starch Staerke 2011, 63, 424–431. [Google Scholar] [CrossRef]
- Takatori, Y.; Akagi, S.; Sugiyama, H.; Inoue, J.; Kojo, S.; Morinaga, H.; Nakao, K.; Wada, J.; Makino, H. Icodextrin Increases Technique Survival Rate in Peritoneal Dialysis Patients with Diabetic Nephropathy by Improving Body Fluid Management: A Randomized Controlled Trial. Clin. J. Am. Soc. Nephrol. 2011, 6, 1337–1344. [Google Scholar] [CrossRef] [PubMed]
- Moreira, S.; Da Costa, R.M.G.; Guardáo, L.; Gärtner, F.; Vilanova, M.; Gama, M. In Vivo Biocompatibility and Biodegradability of Dextrin-Based Hydrogels. J. Bioact. Compat. Polym. 2010, 25, 141–153. [Google Scholar] [CrossRef]
- Harvey, D.J. Analysis of Carbohydrates and Glycoconjugates by Matrix-Assisted Laser Desorption/Ionization Mass Spectrometry: An Update for the Period 2005–2006. Mass Spectrom. Rev. 2011, 30, 1–100. [Google Scholar] [CrossRef] [PubMed]
- Bojin, L.A.; Georgescu, M.; Cojocariu, C.; Pascariu, M.C.; Purcarea, V.L.; Ivan, M.V.; Puiu, M.; Dehelean, C.; Serb, A.F.; Sisu, E.; et al. Structural investigation of raw and modified glycans by MALDI-TOF mass spectrometry. Farmacia 2020, 68, 891–897. [Google Scholar] [CrossRef]
- Kazmaier, T.; Roth, S.; Zapp, J.; Harding, M.; Kuhn, R. Quantitative analysis of malto-oligosaccharides by MALDI-TOF mass spectrometry, capillary electrophoresis and anion exchange chromatography. J. Anal. Chem. 1998, 361, 473–478. [Google Scholar] [CrossRef]
- Saavedra-Leos, Z.; Leyva-Porras, C.; Araujo-Díaz, S.B.; Toxqui-Terán, A.; Borrás-Enríquez, A.J. Technological Application of Maltodextrins According to the Degree of Polymerization. Molecules 2015, 20, 21067–21081. [Google Scholar] [CrossRef]
- Silva, D.M.; Nunes, C.; Pereira, I.; Moreira, A.S.P.; Domingues, M.R.M.; Coimbra, M.A.; Gama, F.M. Structural Analysis of Dextrins and Characterization of Dextrin-Based Biomedical Hydrogels. Carbohydr. Polym. 2014, 114, 458–466. [Google Scholar] [CrossRef]
- Darbre, P.D.; Harvey, P.W. Paraben Esters: Review of Recent Studies of Endocrine Toxicity, Absorption, Esterase and Human Exposure, and Discussion of Potential Human Health Risks. J. Appl. Toxicol. 2008, 28, 561–578. [Google Scholar] [CrossRef]
- Lee, M.R.; Lin, C.Y.; Li, Z.G.; Tsai, T.F. Simultaneous Analysis of Antioxidants and Preservatives in Cosmetics by Supercritical Fluid Extraction Combined with Liquid Chromatography-Mass Spectrometry. J. Chromatogr. A 2006, 1120, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Tahan, G.P.; Santos, N.d.K.S.; Albuquerque, A.C.; Martins, I. Determination of Parabens in Serum by Liquid Chromatography-Tandem Mass Spectrometry: Correlation with Lipstick Use. Regul. Toxicol. Pharmacol. 2016, 79, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Myers, E.A.; Pritchett, T.H.; Brettell, T.A. Determination of Preservatives in Cosmetics and Personal Care Products by LC–MS-MS. LCGC Suppl. 2015, 33, 16–22. [Google Scholar]
- Pedrouzo, M.; Borrull, F.; Marcé, R.M.; Pocurull, E. Ultra-High-Performance Liquid Chromatography-Tandem Mass Spectrometry for Determining the Presence of Eleven Personal Care Products in Surface and Wastewaters. J. Chromatogr. A 2009, 1216, 6994–7000. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.H.; Song, H.N. Development of a Liquid Chromatography/Tandem Mass Spectrometry Method for Monitoring of Long-Term Exposure to Parabens. Rapid Commun. Mass Spectrom. 2019, 33, 67–73. [Google Scholar] [CrossRef]
- Burnett, C.L.; Bergfeld, W.F.; Belsito, D.V.; Klaassen, C.D.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; Andersen, F.A. Final Report of the Safety Assessment of Methylisothiazolinone. Int. J. Toxicol. 2010, 29, 187S–213S. [Google Scholar] [CrossRef] [PubMed]
- Wittenberg, J.B.; Canas, B.J.; Zhou, W.; Wang, P.G.; Rua, D.; Krynitsky, A.J. Determination of Methylisothiazolinone and Methylchloroisothiazolinone in Cosmetic Products by Ultra High Performance Liquid Chromatography with Tandem Mass Spectrometry. J. Sep. Sci. 2015, 38, 2983–2988. [Google Scholar] [CrossRef]
- Lin, Q.B.; Wang, T.J.; Song, H.; Li, B. Analysis of Isothiazolinone Biocides in Paper for Food Packaging by Ultra-High-Performance Liquid Chromatography- Tandem Mass Spectrometry. Food Addit. Contam. 2010, 27, 1775–1781. [Google Scholar] [CrossRef]
- Saraji, M.; Mirmahdieh, S. Single-Drop Microextraction Followed by in-Syringe Derivatization and GC-MS Detection for the Determination of Parabens in Water and Cosmetic Products. J. Sep. Sci. 2009, 32, 988–995. [Google Scholar] [CrossRef]
- Ramírez, N.; Borrull, F.; Marcé, R.M. Simultaneous Determination of Parabens and Synthetic Musks in Water by Stir-Bar Sorptive Extraction and Thermal Desorption-Gas Chromatography-Mass Spectrometry. J. Sep. Sci. 2012, 35, 580–588. [Google Scholar] [CrossRef]
- Alcudia-León, M.C.; Lucena, R.; Cárdenas, S.; Valcárcel, M. Determination of Parabens in Waters by Magnetically Confined Hydrophobic Nanoparticle Microextraction Coupled to Gas Chromatography/Mass Spectrometry. Microchem. J. 2013, 110, 643–648. [Google Scholar] [CrossRef]
- González-Mariño, I.; Quintana, J.B.; Rodríguez, I.; Schrader, S.; Moeder, M. Fully Automated Determination of Parabens, Triclosan and Methyl Triclosan in Wastewater by Microextraction by Packed Sorbents and Gas Chromatography-Mass Spectrometry. Anal. Chim. Acta 2011, 684, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Prado, L.; Alvarez-Rivera, G.; Lamas, J.P.; Lores, M.; Garcia-Jares, C.; Llompart, M. Analysis of Multi-Class Preservatives in Leave-on and Rinse-off Cosmetics by Matrix Solid-Phase Dispersion. Anal. Bioanal. Chem. 2011, 401, 3293–3304. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.G.; Zhou, W. Rapid Determination of Parabens in Personal Care Products by Stable Isotope GC-MS/MS with Dynamic Selected Reaction Monitoring. J. Sep. Sci. 2013, 36, 1781–1787. [Google Scholar] [CrossRef]
- Yang, T.J.; Tsai, F.J.; Chen, C.Y.; Yang, T.C.C.; Lee, M.R. Determination of Additives in Cosmetics by Supercritical Fluid Extraction On-Line Headspace Solid-Phase Microextraction Combined with Gas Chromatography-Mass Spectrometry. Anal. Chim. Acta 2010, 668, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Haunschmidt, M.; Buchberger, W.; Klampfl, C.W.; Hertsens, R. Identification and Semi-Quantitative Analysis of Parabens and UV Filters in Cosmetic Products by Direct-Analysis-in-Real-Time Mass Spectrometry and Gas Chromatography with Mass Spectrometric Detection. Anal. Methods 2011, 3, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Ying, L.; Cao, Y.; Pan, G.; Zhou, L. Simultaneous Determination of Phthalates and Parabens in Cosmetic Products by Gas Chromatography/Mass Spectrometry Coupled with Solid Phase Extraction. Chin. J. Chromatogr. 2007, 25, 272–275. [Google Scholar]
- Guerra, E.; Alvarez-Rivera, G.; Llompart, M.; Garcia-Jares, C. Simultaneous Determination of Preservatives and Synthetic Dyes in Cosmetics by Single-Step Vortex Extraction and Clean-up Followed by Liquid Chromatography Coupled to Tandem Mass Spectrometry. Talanta 2018, 188, 251–258. [Google Scholar] [CrossRef]
- Guerra, E.; Llompart, M.; Garcia-Jares, C. Analysis of Dyes in Cosmetics: Challenges and Recent Developments. Cosmetics 2018, 5, 47. [Google Scholar] [CrossRef]
- Xian, Y.; Wu, Y.; Guo, X.; Lu, Y.; Luo, H.; Luo, D.; Chen, Y. Simultaneous Determination of 11 Restricted Dyes in Cosmetics by Ultra High-Performance Liquid Chromatography/Tandem Mass Spectrometry. Anal. Methods 2013, 5, 1965–1974. [Google Scholar] [CrossRef]
- Noguerol-Cal, R.; López-Vilariño, J.M.; Fernández-Martínez, G.; Barral-Losada, L.; González-Rodríguez, M.V. High-Performance Liquid Chromatography Analysis of Ten Dyes for Control of Safety of Commercial Articles. J. Chromatogr. A 2008, 1179, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Millbern, Z.; Trettin, A.; Wu, R.; Demmler, M.; Vinueza, N.R. Synthetic dyes: A mass spectrometry approach and applications. Mass Spectrom. Rev. 2022, 43, 327–344. [Google Scholar] [CrossRef] [PubMed]
- Han, K.M.; Kim, Y.K.; Seo, S.; Kim, J.H.; Lee, J.H.; Kim, H.l.; Cho, S. Analysis of 13 Banned Colorants in Cosmetics via Liquid Chromatographic and Mass Spectrometric Techniques. Appl. Sci. 2023, 13, 5967. [Google Scholar] [CrossRef]
- Qian, X.; Liu, H.; ZHU, X. Simultaneous Determination of 12 Synthetic Colorants in Cosmetics by SPE/UPLC-MS/MS. Chin. J. Chromatogr. 2014, 33, 527–532. [Google Scholar]
- Nizzia, J.L.; O’Leary, A.E.; Ton, A.T.; Mulligan, C.C. Screening of cosmetic ingredients from authentic formulations and environmental samples with desorption electrospray ionization mass spectrometry. Anal. Methods 2013, 5, 394–401. [Google Scholar] [CrossRef]
- Chen, M.; Bai, H.; Zhai, J.; Meng, X.; Guo, X.; Wang, C.; Wang, P.; Lei, H.; Niu, Z.; Ma, Q. Comprehensive Screening of 63 Coloring Agents in Cosmetics Using Matrix Solid-Phase Dispersion and Ultra-High-Performance Liquid Chromatography Coupled with Quadrupole-Orbitrap High-Resolution Mass Spectrometry. J. Chromatogr. A 2019, 1590, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Diepgen, T.L.; Ofenloch, R.F.; Bruze, M.; Bertuccio, P.; Cazzaniga, S.; Coenraads, P.J.; Elsner, P.; Goncalo, M.; Svensson, Å.; Naldi, L. Prevalence of Contact Allergy in the General Population in Different European Regions. Br. J. Dermatol. 2016, 174, 319–329. [Google Scholar] [CrossRef]
- Diepgen, T.L.; Ofenloch, R.; Bruze, M.; Cazzaniga, S.; Coenraads, P.J.; Elsner, P.; Goncalo, M.; Svensson, Å.; Naldi, L. Prevalence of Fragrance Contact Allergy in the General Population of Five European Countries: A Cross-Sectional Study. Br. J. Dermatol. 2015, 173, 1411–1419. [Google Scholar] [CrossRef]
- Thyssen, J.P.; Menné, T.; Linneberg, A.; Johansen, J.D. Contact Sensitization to Fragrances in the General Population: A Koch’s Approach May Reveal the Burden of Disease. Br. J. Dermatol. 2009, 160, 729–735. [Google Scholar] [CrossRef]
- Nielsen, N.H.; Menne, T. Allergic Contact Sensitization in an Unselected Danish Population. The Glostrup Allergy Study, Denmark. Acta Derm. Venereol. 1992, 72, 456–460. [Google Scholar] [CrossRef]
- Celeiro, M.; Guerra, E.; Lamas, J.P.; Lores, M.; Garcia-Jares, C.; Llompart, M. Development of a Multianalyte Method Based on Micro-Matrix-Solid-Phase Dispersion for the Analysis of Fragrance Allergens and Preservatives in Personal Care Products. J. Chromatogr. A 2014, 1344, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.; Silva, C.; Soares, P.; Garrido, E.M.; Borges, F.; Garrido, J. Isothiazolinone Biocides: Chemistry, Biological, and Toxicity Profiles. Molecules 2020, 25, 991. [Google Scholar] [CrossRef] [PubMed]
- Arribas, M.P.; Soro, P.; Silvestre, J.F. Allergic Contact Dermatitis to Fragrances. Part 1. Actas Dermo-Sifiliográficas 2012, 103, 874–879. [Google Scholar] [CrossRef] [PubMed]
- SCCS (Scientific Committee on Consumer Safety). Opinion on Fragrance Allergens in Cosmetic Products, Eur. Comm. SCCS/1459/(2012). Available online: https://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_102.pdf (accessed on 4 January 2024). [CrossRef]
- Salvador, A.; Chisvert, A. Analysis of Cosmetic Products, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2007; Volume 837, pp. 475–487. [Google Scholar]
- Bridges, B. Fragrance: Emerging Health and Environmental Concerns. Flavour Fragr. J. 2002, 17, 361–371. [Google Scholar] [CrossRef]
- Available online: https://ifrafragrance.org/docs/default-source/51st-amendment/ifra-standards---51st-amendment.pdf?sfvrsn=9bc6a23b_0 (accessed on 4 January 2024).
- The European Parliament and the Council of the European Union. Regulations (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetic product. Off. J. Eur. Union L 2009, 342, 59–209. [Google Scholar]
- Commission Regulation (EU) 2023/1545 of 26 July 2023 Amending Regulation (EC) No 1223/2009 of the European Parliament and of the Council as Regards Labelling of Fragrance Allergens in Cosmetic Products. Available online: https://eur-lex.europa.eu/eli/reg/2023/1545/oj (accessed on 4 January 2024).
- Tranchida, P.Q.; Maimone, M.; Franchina, F.A.; Bjerk, T.R.; Zini, C.A.; Purcaro, G.; Mondello, L. Four-Stage (Low-)Flow Modulation Comprehensive Gas Chromatography-Quadrupole Mass Spectrometry for the Determination of Recently-Highlighted Cosmetic Allergens. J. Chromatogr. A 2016, 1439, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Villa, C.; Gambaro, R.; Mariani, E.; Dorato, S. High-Performance Liquid Chromatographic Method for the Simultaneous Determination of 24 Fragrance Allergens to Study Scented Products. J. Pharm. Biomed. Anal. 2007, 44, 755–76214. [Google Scholar] [CrossRef]
- Cordero, C.; Bicchi, C.; Joulain, D.; Rubiolo, P. Identification, Quantitation and Method Validation for the Analysis of Suspected Allergens in Fragrances by Comprehensive Two-Dimensional Gas Chromatography Coupled with Quadrupole Mass Spectrometry and with Flame Ionization Detection. J. Chromatogr. A 2007, 1150, 37–49. [Google Scholar] [CrossRef]
- Khan, M.R.; Khan, J.M.; Alqadami, A.A. A Simple Solvent Extraction and Ultra-Performance Liquid Chromatography-Tandem Mass Spectrometric Method for the Identification and Quantification of Rhodamine B in Commercial Lip Balm Samples. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2019, 2006, 72–77. [Google Scholar] [CrossRef]
- Arroyo Negrete, M.A.; Wrobel, K.; Acevedo Aguilar, F.J.; Yanez Barrientos, E.; Corrales Escobosa, A.R.; Wrobel, K. Determination of Fatty Acid Methyl Esters in Cosmetic Castor Oils by Flow Injection–Electrospray Ionization–High-Resolution Mass Spectrometry. Int. J. Cosmet. Sci. 2018, 40, 295–302. [Google Scholar] [CrossRef]
- Rubio, L.; Valverde-Som, L.; Sarabia, L.A.; Ortiz, M.C. Improvement in the Identification and Quantification of UV Filters and Additives in Sunscreen Cosmetic Creams by Gas Chromatography/Mass Spectrometry through Three-Way Calibration Techniques. Talanta 2019, 205, 120156. [Google Scholar] [CrossRef] [PubMed]
- Cerceau, C.I.; Barbosa, L.C.A.; Alvarenga, E.S.; Maltha, C.R.A.; Ismail, F.M.D. 1H-NMR and GC for Detection of Adulteration in Commercial Essential Oils of Cymbopogon ssp. Phytochem. Anal. 2020, 31, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Celeiro, M.; Vazquez, L.; Lamas, J.P.; Vila, M.; Garcia-Jares, C.; Llompart, M. Miniaturized Matrix Solid-Phase Dispersion for the Analysis of Ultraviolet Filters and Other Cosmetic Ingredients in Personal Care Products. Separations 2019, 6, 30. [Google Scholar] [CrossRef]
- Beltifa, A.; Belaid, A.; Lo Turco, V.; Machreki, M.; Ben Mansour, H.; Di Bella, G. Preliminary Evaluation of Plasticizer and BPA in Tunisian Cosmetics and Investigation of Hazards on Human Skin Cells. Int. J. Environ. Health Res. 2018, 28, 491–501. [Google Scholar] [PubMed]
- Abedi, G.; Talebpour, Z. Modified QuEChERS as a Novel Sample Preparation Method for Analysis of: N -Nitrosodiethanolamine in Shampoo by High Performance Liquid Chromatography. Anal. Methods 2017, 9, 5165–5173. [Google Scholar] [CrossRef]
- Huang, C.; Bian, C.; Wang, L.; Zhou, W.; Li, Y.; Li, B. Development and validation of a method for determining d-limonene and its oxidation products in vegetables and soil using GC–MS. Microchem. J. 2022, 179, 107470. [Google Scholar] [CrossRef]
- Belai, N.; White, S.R. Determination of Unsulfonated Aromatic Amines in FD and C Yellow No. 5 and FD and C Yellow No. 6 by Liquid Chromatography–Triple Quadrupole Mass Spectrometry. J. AOAC Int. 2019, 102, 580–589. [Google Scholar]
- Zhu, F.; Wu, X.; Li, F.; Wang, W.; Ji, W.; Huo, Z.; Xu, Y. Simultaneous Determination of 12 Antibacterial Drugs in Cream Disinfection Products with EMR-Lipid Cleanup Using Ultra-High-Performance Liquid Chromatography Tandem Mass Spectrometry. Anal. Methods 2019, 11, 4084–4092. [Google Scholar] [CrossRef]
- Schettino, L.; Benedé, J.L.; Chisvert, A. Determination of Nine Prohibited N-Nitrosamines in Cosmetic Products by Vortex-Assisted Dispersive Liquid-Liquid Microextraction Prior to Gas Chromatography-Mass Spectrometry. RSC Adv. 2023, 13, 2963–2971. [Google Scholar] [CrossRef]
- Guerra, E.; Lamas, J.P.; Llompart, M.; Garcia-Jares, C. Determination of Oxidative Hair Dyes Using Miniaturized Extraction Techniques and Gas Chromatography-Tandem Mass Spectrometry. Microchem. J. 2017, 132, 308–318. [Google Scholar] [CrossRef]
- Gładysz, M.; Król, M.; Własiuk, P.; Piwowar, M.; Zadora, G.; Kościelniak, P. Development and Evaluation of Semi-Destructive, Ultrasound Assisted Extraction Method Followed by Gas Chromatography Coupled to Mass Spectrometry Enabling Discrimination of Red Lipstick Samples. J. Chromatogr. A 2018, 1577, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Vila, M.; Facorro, R.; Lamas, J.P.; Garcia-Jares, C.; Dagnac, T.; Llompart, M. Determination of Fifteen Water and Fat-Soluble UV Filters in Cosmetics by Pressurized Liquid Extraction Followed by Liquid Chromatography Tandem Mass Spectrometry. Anal. Methods 2016, 8, 6787–6794. [Google Scholar] [CrossRef]
- Mostafa, A.; Shaaban, H. Development and Validation of a Dispersive Liquid-Liquid Microextraction Method for the Determination of Phthalate Esters in Perfumes Using Gas Chromatography-Mass Spectrometry. RSC Adv. 2018, 8, 26897–26905. [Google Scholar] [CrossRef] [PubMed]
- Farè, F.; Dei Cas, M.; Arnoldi, S.; Casagni, E.; Visconti, G.L.; Parnisari, G.; Bolchi, C.; Pallavicini, M.; Gambaro, V.; Roda, G. Determination of Methyldibromoglutaronitrile (MDBGN) in Skin Care Products by Gaschromatography-Mass Spectrometry Employing an Enhanced Matrix Removal (EMR) Lipid Clean-Up. Eur. J. Lipid Sci. Technol. 2018, 120, 1700525. [Google Scholar] [CrossRef]
- Farajzadeh, M.A.; Bakhshizadeh Aghdam, M.; Afshar Mogaddam, M.R.; Alizadeh Nabil, A.A. Simultaneous Derivatization and Lighter-than-Water Air-Assisted Liquid–Liquid Microextraction Using a Homemade Device for the Extraction and Preconcentration of Some Parabens in Different Samples. J. Sep. Sci. 2018, 41, 3105–3112. [Google Scholar] [CrossRef]
- Meng, X.; Ma, Q.; Bai, H.; Wang, Z.; Han, C.; Wang, C. Simultaneous Separation and Determination of 15 Organic UV Filters in Sunscreen Cosmetics by HPLC–ESI-MS/MS. Int. J. Cosmet. Sci. 2017, 39, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.H.; Fang, M.C.; Chen, Y.Z.; Huang, S.C.; Wang, D.Y. Quantitative Analysis of Fragrance Allergens in Various Matrixes of Cosmetics by Liquideliquid Extraction and GCeMS. J. Food Drug Anal. 2021, 29, 700. [Google Scholar] [CrossRef]
- Vazquez, L.; Celeiro, M.; Castiñeira-Landeira, A.; Dagnac, T.; Llompart, M. Development of a Solid Phase Microextraction Gas Chromatography Tandem Mass Spectrometry Methodology for the Analysis of Sixty Personal Care Products in Hydroalcoholic Gels-Hand Sanitizers-in the Context of COVID-19 Pandemic. Anal. Chim. Acta 2022, 1203, 339650. [Google Scholar] [CrossRef]
- Celeiro, M.; Garcia-Jares, C.; Llompart, M.; Lores, M. Recent Advances in Sample Preparation for Cosmetics and Personal Care Products Analysis. Molecules 2021, 26, 4900. [Google Scholar] [CrossRef]
- Peng, Y.; Wu, Z.; Huo, Y.; Chen, Y.; Lu, F.; Peng, Q.; Liang, Y. Simultaneous Determination of Ginsenosides Rg1, Re, and Rb1 and Notoginsenoside R1 by Solid Phase Extraction Followed by UHPLC-MS/MS and Investigation of Their Concentrations in Various Kinds of Cosmetics. Anal. Methods 2017, 9, 5441–5448. [Google Scholar] [CrossRef]
- Li, Y.; Chen, X.; Xia, L.; Xiao, X.; Li, G. Magnetic Metal-Organic Frameworks-101 Functionalized with Graphite-like Carbon Nitride for the Efficient Enrichment of Glucocorticoids in Cosmetics. J. Chromatogr. A 2019, 1606, 460382. [Google Scholar] [CrossRef] [PubMed]
- Miralles, P.; van Gemert, I.; Chisvert, A.; Salvador, A. Stir Bar Sorptive-Dispersive Microextraction Mediated by Magnetic Nanoparticles-Metal Organic Framework Composite: Determination of N-Nitrosamines in Cosmetic Products. J. Chromatogr. A 2019, 1604, 460465. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhou, L.; Xun, Z.; Wang, Q.; Lin, S.; Guo, X.; Cai, Y. Simultaneous Determination of Seven Nitrogen-Containing Phenyl Ethers in Cosmetics by Gas Chromatography with Mass Spectrometry and Dispersive Solid-Phase Extraction. J. Sep. Sci. 2017, 40, 1718–1723. [Google Scholar] [CrossRef] [PubMed]
- Xun, Z.; Liu, D.; Huang, R.; He, S.; Hu, D.; Guo, X.; Xian, Y. Simultaneous Determination of Eight Alkaloids and Oleandrin in Herbal Cosmetics by Dispersive Solid-Phase Extraction Coupled with Ultra High Performance Liquid Chromatography and Tandem Mass Spectrometry. J. Sep. Sci. 2017, 40, 1966–1973. [Google Scholar] [CrossRef]
- Vállez-Gomis, V.; Grau, J.; Benedé, J.L.; Chisvert, A.; Salvador, A. Reduced Graphene Oxide-Based Magnetic Composite for Trace Determination of Polycyclic Aromatic Hydrocarbons in Cosmetics by Stir Bar Sorptive Dispersive Microextraction. J. Chromatogr. A 2020, 1624, 461229. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, H.; Mostafa, A.; Alhajri, W.; Almubarak, L.; AlKhalifah, K. Development and Validation of an Eco-Friendly SPE-HPLC-MS Method for Simultaneous Determination of Selected Parabens and Bisphenol A in Personal Care Products: Evaluation of the Greenness Profile of the Developed Method. J. Liq. Chromatogr. Relat. Technol. 2018, 41, 621–628. [Google Scholar] [CrossRef]
- Alhooshani, K. Determination of Nitrosamines in Skin Care Cosmetics Using Ce-SBA-15 Based Stir Bar Supported Micro-Solid-Phase Extraction Coupled with Gas Chromatography Mass Spectrometry. Arab. J. Chem. 2020, 13, 2508–2516. [Google Scholar] [CrossRef]
- Duffy, E.; Albero, G.; Morrin, A. Headspace Solid-Phase Microextraction Gas Chromatography-Mass Spectrometry Analysis of Scent Profiles from Human Skin. Cosmetics 2018, 5, 62. [Google Scholar] [CrossRef]
- Masoum, S.; Gholami, A.; Ghaheri, S.; Bouveresse, D.J.R.; Cordella, C.B.Y.; Rutledge, D.N. Investigation of Fragrance Stability Used in the Formulation of Cosmetic and Hygienic Products Using Headspace Solid-Phase Microextraction by Nanostructured Materials Followed by Gas Chromatography with Mass Spectrometry. J. Sep. Sci. 2016, 39, 2760–2769. [Google Scholar] [CrossRef]
- Zhou, Y.; Abbas, F.; Wang, Z.; Yu, Y.; Yue, Y.; Li, X.; Yu, R.; Fan, Y. Hs–Spme–Gc–Ms and Electronic Nose Reveal Differences in the Volatile Profiles of Hedychium Flowers. Molecules 2021, 26, 5425. [Google Scholar] [CrossRef]
- Choi, N.R.; Kim, Y.P.; Ji, W.H.; Hwang, G.S.; Ahn, Y.G. Identification and Quantification of Seven Volatile N-Nitrosamines in Cosmetics Using Gas Chromatography/Chemical Ionization-Mass Spectrometry Coupled with Head Space-Solid Phase Microextraction. Talanta 2016, 148, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, B.R.; Horn, A.F.; Hyldig, G.; Taylor, R.; Blenkiron, P.; Jacobsen, C. Investigation of Lipid Oxidation in High- and Low-Lipid-Containing Topical Skin Formulations. J. Am. Oil Chem. Soc. 2017, 94, 1287–1300. [Google Scholar] [CrossRef]
- Ševčík, V.; Andraščíková, M.; Vavrouš, A.; Moulisová, A.; Vrbík, K.; Bendová, H.; Jírová, D.; Kejlová, K.; Hložek, T. Market Surveillance: Analysis of Perfuming Products for Presence of Allergens and Prohibited Substances. Chem. Pap. 2022, 76, 4989–5000. [Google Scholar] [CrossRef]
- Remy, P.A.; Pérès, C.; Dugay, J.; Corbi, E.; David, N.; Vial, J. How High-Resolution Mass Spectrometry Can Help for the Accurate Quantification of Difficult Fragrance Allergens. Flavour Fragr. J. 2021, 36, 243–255. [Google Scholar] [CrossRef]
- Pérès, C.; Corbi, E.; David, N.; Masson, J.; Cicchetti, E.; Kupfermann, N.; Kuropka, G.; Tacnet, N.; Roach, N.; Delacôte, A.M.; et al. Collaborative Validation of the Quantification Method for 57 Allergens in Ready to Inject Fragrance Samples. Flavour Fragr. J. 2023, 38, 464–475. [Google Scholar] [CrossRef]
- Debonneville, C.; Chaintreau, A. Online Clean-up of Volatile Compounds in Complex Matrices for GC-MS Quantification: Testing with Fragranced Consumer Products. Flavour Fragr. J. 2014, 29, 267–276. [Google Scholar] [CrossRef]
- Shibuta, S.; Imasaka, T.; Imasaka, T. Determination of Fragrance Allergens by Ultraviolet Femtosecond Laser Ionization Mass Spectrometry. Anal. Chem. 2016, 88, 10693–10700. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Q.; Li, H.; Lv, Q.; Wang, W.; Bai, H. Rapid and Green Determination of 58 Fragrance Allergens in Plush Toys. J. Sep. Sci. 2018, 41, 657–668. [Google Scholar] [CrossRef]
- Mazzucotelli, M.; Minteguiaga, M.A.; Sgorbini, B.; Sidisky, L.; Marengo, A.; Rubiolo, P.; Bicchi, C.; Cagliero, C. Ionic Liquids as Water-Compatible GC Stationary Phases for the Analysis of Fragrances and Essential Oils: Quantitative GC–MS Analysis of Officially-Regulated Allergens in Perfumes. J. Chromatogr. A 2020, 1610, 460567. [Google Scholar] [CrossRef]
- Chen, J.; Yi, Z.; Sun, R.; Ning, W.; Zhou, C.; Tian, Z.; Sun, C.; Li, Y. Analysis of Fragrance Allergens in Personal Care Products, Toys, and Water Samples: A Review. J. AOAC Int. 2022, 105, 396–412. [Google Scholar] [CrossRef]
- Rico, F.; Mazabel, A.; Egurrola, G.; Pulido, J.; Barrios, N.; Marquez, R.; García, J. Meta-Analysis and Analytical Methods in Cosmetics Formulation: A Review. Cosmetics 2024, 11, 1. [Google Scholar] [CrossRef]
- Sanchez-Prado, L.; Llompart, M.; Lamas, J.P.; Garcia-Jares, C.; Lores, M. Multicomponent Analytical Methodology to Control Phthalates, Synthetic Musks, Fragrance Allergens and Preservatives in Perfumes. Talanta 2011, 85, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, R.; Wang, Z.; Zhang, Q.; Bai, H.; Lv, Q. Optimization of headspace for GC-MS analysis of fragrance allergens in wooden children’s products using response surface methodology. Sep. Sci. Plus 2019, 2, 26–37. [Google Scholar] [CrossRef]
- Abedi, G.; Talebpour, Z.; Jamechenarboo, F. The survey of analytical methods for sample preparation and analysis of fragrances in cosmetics and personal care products. TrAC-Trends Anal. Chem. 2018, 102, 41–59. [Google Scholar] [CrossRef]
- David, F.; Devos, C.; Joulain, D.; Chaintreau, A.; Sandra, P. Determination of Suspected Allergens in Non-Volatile Matrices Using PTV Injection with Automated Liner Exchange and GC-MS. J. Sep. Sci. 2006, 29, 1587–1594. [Google Scholar] [CrossRef] [PubMed]
- Lamas, J.P.; Sanchez-Prado, L.; Garcia-Jares, C.; Lores, M.; Llompart, M. Development of a Solid Phase Dispersion-Pressurized Liquid Extraction Method for the Analysis of Suspected Fragrance Allergens in Leave-on Cosmetics. J. Chromatogr. A 2010, 1217, 8087–8094. [Google Scholar] [CrossRef] [PubMed]
- Llompart, M.; Celeiro, M.; Pablo Lamas, J.; Sanchez-Prado, L.; Lores, M.; Garcia-Jares, C. Analysis of Plasticizers and Synthetic Musks in Cosmetic and Personal Care Products by Matrix Solid-Phase Dispersion Gas Chromatography-Mass Spectrometry. J. Chromatogr. A 2013, 1293, 10–19. [Google Scholar] [CrossRef]
- Roosens, L.; Covaci, A.; Neels, H. Concentrations of Synthetic Musk Compounds in Personal Care and Sanitation Products and Human Exposure Profiles through Dermal Application. Chemosphere 2007, 69, 1540–1547. [Google Scholar] [CrossRef]
- Nakata, H.; Hinosaka, M.; Yanagimoto, H. Macrocyclic-, Polycyclic-, and Nitro Musks in Cosmetics, Household Commodities and Indoor Dusts Collected from Japan: Implications for Their Human Exposure. Ecotoxicol. Environ. Saf. 2015, 111, 248–255. [Google Scholar] [CrossRef]
- Chisvert, A.; López-Nogueroles, M.; Salvador, A. Essential Oils: Analytical Methods to Control the Quality of Perfumes. In Natural Products; Ramawat, K., Mérillon, J.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 3295–3299. [Google Scholar]
- Martín-Pozo, L.; del Carmen Gómez-Regalado, M.; Moscoso-Ruiz, I.; Zafra-Gómez, A. Analytical methods for the determination of endocrine disrupting chemicals in cosmetics and personal care products: A review. Talanta 2021, 234, 122642. [Google Scholar] [CrossRef]
- Tsai, C.J.; Li, J.H.; Feng, C.H. Dual Dispersive Liquid-Liquid Microextraction for Determination of Phenylpropenes in Oils by Gas Chromatography-Mass Spectrometry. J. Chromatogr. A 2015, 1410, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Pratiwi, R.; Auliya As, N.N.; Yusar, R.F.; Shofwan, A.A.A. Analysis of Prohibited and Restricted Ingredients in Cosmetics. Cosmetics 2022, 9, 87. [Google Scholar] [CrossRef]
- Desmedt, B.; Canfyn, M.; Pype, M.; Baudewyns, S.; Hanot, V.; Courselle, P.; De Beer, J.O.; Rogiers, V.; De Paepe, K.; Deconinck, E. HS-GC-MS Method for the Analysis of Fragrance Allergens in Complex Cosmetic Matrices. Talanta 2015, 131, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Fardin-Kia, A.R.; Zhou, W. Development and Validation of a Gas Chromatography–Mass Spectrometry Method for Determination of 30 Fragrance Substances in Cosmetic Products. Sep. Sci. Plus 2020, 3, 496–510. [Google Scholar] [CrossRef]
- Belhassen, E.; Bressanello, D.; Merle, P.; Raynaud, E.; Bicchi, C.; Chaintreau, A.; Cordero, C. Routine quantification of 54 allergens in fragrances using comprehensive two-dimensional gas chromatography-quadrupole mass spectrometry with dual parallel secondary columns. Part I: Method development. Flavour Fragr. J. 2017, 33, 63–74. [Google Scholar] [CrossRef]
- Rastogi, S.C. Analysis of Fragrances in Cosmetics by Gas Chromatography–Mass Spectrometry. J. High Resolut. Chromatogr. 1995, 18, 653–658. [Google Scholar] [CrossRef]
- Giménez Arnau, E.; Andersen, K.E.; Bruze, M.; Frosch, P.J.; Johansen, J.D.; Menné, T.; Rastogi, S.C.; White, I.R.; Lepoittevin, J.P. Identification of Lilial® as a Fragrance Sensitizer in a Perfume by Bioassay-Guided Chemical Fractionation and Structure-Activity Relationships. Contact Dermat. 2000, 43, 351–358. [Google Scholar] [CrossRef]
- Rey, A.; Corbi, E.; Pérès, C.; David, N. Determination of Suspected Fragrance Allergens Extended List by Two-Dimensional Gas Chromatography-Mass Spectrometry in Ready-to-Inject Samples. J. Chromatogr. A 2015, 1404, 95–103. [Google Scholar] [CrossRef]
- Leijs, H.; Broekhans, J.; Van Pelt, L.; Mussinan, C. Quantitative Analysis of the 26 Allergens for Cosmetic Labeling in Fragrance Raw Materials and Perfume Oils. J. Agric. Food Chem. 2005, 53, 5487–5491. [Google Scholar] [CrossRef]
- Debonneville, C.; Chaintreau, A. Quantitation of Suspected Allergens in Fragrances-Part II. Evaluation of Comprehensive Gas Chromatography-Conventional Mass Spectrometry. J. Chromatogr. A 2004, 1027, 109–115. [Google Scholar] [CrossRef]
- Cordero, C.; Rubiolo, P.; Cobelli, L.; Stani, G.; Miliazza, A.; Giardina, M.; Firor, R.; Bicchi, C. Potential of the Reversed-Inject Differential Flow Modulator for Comprehensive Two-Dimensional Gas Chromatography in the Quantitative Profiling and Fingerprinting of Essential Oils of Different Complexity. J. Chromatogr. A 2015, 1417, 79–95. [Google Scholar] [CrossRef] [PubMed]
- Cordero, C.; Rubiolo, P.; Reichenbach, S.E.; Carretta, A.; Cobelli, L.; Giardina, M.; Bicchi, C. Method Translation and Full Metadata Transfer from Thermal to Differential Flow Modulated Comprehensive Two Dimensional Gas Chromatography: Profiling of Suspected Fragrance Allergens. J. Chromatogr. A 2017, 1480, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Cagliero, C.; Bicchi, C.; Cordero, C.; Liberto, E.; Rubiolo, P.; Sgorbini, B. Analysis of Essential Oils and Fragrances with a New Generation of Highly Inert Gas Chromatographic Columns Coated with Ionic Liquids. J. Chromatogr. A 2017, 1495, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Prado, L.; Lamas, J.P.; Alvarez-Rivera, G.; Lores, M.; Garcia-Jares, C.; Llompart, M. Determination of Suspected Fragrance Allergens in Cosmetics by Matrix Solid-Phase Dispersion Gas Chromatography-Mass Spectrometry Analysis. J. Chromatogr. A 2011, 1218, 5055–5062. [Google Scholar] [CrossRef] [PubMed]
- Reiner, J.L.; Kannan, K. A Survey of Polycyclic Musks in Selected Household Commodities from the United States. Chemosphere 2006, 62, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Debonneville, C.; Thomé, M.A.; Chaintreau, A. Hyphenation of quadrupole MS to GC and comprehensive two-dimensional GC for the analysis of suspected allergens: Review and improvement. J. Chromatogr. Sci. 2004, 2, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Yusa, V.; Ye, X.; Calafat, A.M. Methods for the determination of biomarkers of exposure to emerging pollutants in human specimens. TrAC Trends Anal. Chem. 2012, 38, 129–142. [Google Scholar] [CrossRef]
- Niederer, M.; Bollhalder, R.; Hohl, C. Determination of Fragrance Allergens in Cosmetics by Size-Exclusion Chromatography Followed by Gas Chromatography-Mass Spectrometry. J. Chromatogr. A 2006, 1132, 109–116. [Google Scholar] [CrossRef]
- Del Nogal Sánchez, M.; Pérez-Pavón, J.L.; Moreno Cordero, B. Determination of Suspected Allergens in Cosmetic Products by Headspace-Programmed Temperature Vaporization-Fast Gas Chromatography-Quadrupole Mass Spectrometry. Anal. Bioanal. Chem. 2010, 397, 2579–2591. [Google Scholar] [CrossRef]
- Devos, C.; Ochiai, N.; Sasamoto, K.; Sandra, P.; David, F. Full Evaporation Dynamic Headspace in Combination with Selectable One-Dimensional/Two-Dimensional Gas Chromatography-Mass Spectrometry for the Determination of Suspected Fragrance Allergens in Cosmetic Products. J. Chromatogr. A 2012, 1255, 207–215. [Google Scholar] [CrossRef]
- Bothe, F.; Dettmer, K.; Engewald, W. Determination of Perfume Oil in Household Products by Headspace Solid-Phase Microextraction and Fast Capillary Gas Chromatography. Chromatographia 2003, 57, S199–S206. [Google Scholar] [CrossRef]
- Liu, S.; Zhu, Y. Determination of 27 fragrances in cosmetics and perfume raw materials by gas chromatography-mass spectrometry. Se Pu Chin. J. Chromatogr. 2019, 37, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Kaloustian, J.; Mikail, C.; El-Moselhy, T.; Abou, L.; Portugal, H. GC-MS Analysis of Allergens in Plant Oils Meant to Cosmetics. Oléagineux Corp. Gras Lipides 2007, 14, 110–115. [Google Scholar] [CrossRef]
- Homem, V.; Avelino Silva, J.; Cunha, C.; Alves, A.; Santos, L. New Analytical Method for the Determination of Musks in Personal Care Products by Quick, Easy, Cheap, Effective, Rugged, and Safe Extraction Followed by GC-MS. J. Sep. Sci. 2013, 36, 2176–2184. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wene, M.J. Measurement of Gas-Liquid Partition Coefficient and Headspace Concentration Profiles of Perfume Materials by Solid-Phase Microextraction and Capillary Gas Chromatography-Mass Spectrometry. J. Chromatogr. Sci. 2000, 38, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Bassereau, M.; Chaintreau, A.; Duperrex, S.; Joulain, D.; Leijs, H.; Loesing, G.; Owen, N.; Sherlock, A.; Schippa, C.; Thorel, P.J.; et al. GC-MS Quantification of Suspected Volatile Allergens in Fragrances. 2. Data Treatment Strategies and Method Performances. 2. Data Treatment Strategies and Method Performances. J. Agric. Food Chem. 2007, 55, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Tang, H.; Chen, D.; Xu, T.; Li, L. Analysis of 7 Synthetic Musks in Cream by Supported Liquid Extraction and Solid Phase Extraction Followed by GC-MS/MS. Talanta 2014, 120, 248–254. [Google Scholar] [CrossRef]
- de Hoffmann, E.; Stroobant, V. Mass Spectrometry, 3rd ed.; John Willey and Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Stilo, F.; Gabetti, E.; Bicchi, C.; Carretta, A.; Peroni, D.; Reichenbach, S.E.; Cordero, C.; McCurry, J. A Step Forward in the Equivalence between Thermal and Differential-Flow Modulated Comprehensive Two-Dimensional Gas Chromatography Methods. J. Chromatogr. A 2020, 1627, 461396. [Google Scholar] [CrossRef]
- IFRA. Guidelines on the Environmental Assessment of Natural Complex Substances (NCS). 2016. Available online: https://ifrafragrance.org/docs/default-source/guidelines/23702_gd_2016_05_27_efeo_ifra_guidelines_on_the_environmental_assessment_of_natural_complex_substances_ (accessed on 3 February 2024).
- Famiglini, G.; Termopoli, V.; Palma, P.; Capriotti, F.; Cappiello, A. Rapid LC-MS Method for the Detection of Common Fragrances in Personal Care Products without Sample Preparation. Electrophoresis 2014, 35, 1339–1345. [Google Scholar] [CrossRef]
- Hiserodt, R.; Chen, L. An LC/MS/MS Method for the Analysis of Furocoumarins in Citrus Oil. In Recent Advances in the Analysis of Food and Flavors; American Chemical Society: Washington, DC, USA, 2012; Volume 1098, pp. 71–88. [Google Scholar]
- Bossi, R.; Rastogi, S.C.; Bernard, G.; Gimenez-Arnau, E.; Johansen, J.D.; Lepoittevin, J.P.; Menné, T. A Liquid Chromatography-Mass Spectrometric Method for the Determination of Oak Moss Allergens Atranol and Chloroatranol in Perfumes. J. Sep. Sci. 2004, 27, 537–540. [Google Scholar] [CrossRef]
- Palfi Salavat, M.C.; Racea, R.C.; Drăghici, G.; Seclaman, E.P.; Munteanu, M.; Mușat, O.; Ungureanu, E.; Milcu, A.I.; Boruga, M.V.; Laura Rusu, L.; et al. Polyphenols content and in vitro antitumour activity of hydroalcoholic extract of Viscum album in two pigmented and unpigmented skin cancer cell lines. Farmacia 2022, 70, 807–815. [Google Scholar] [CrossRef]
- Bettero, A.; Benassi, C.A. Determination of Coumarin and 6-Methylcoumarin in Cosmetics by High-Performance Liquid Chromatography. J. Pharm. Biomed. Anal. 1983, 1, 229–233. [Google Scholar] [CrossRef]
- Wilm, M. Principles of Electrospray Ionization. Mol. Cell. Proteom. 2011, 10, M111.009407. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, C.; Hanley, L. Ion Sources for Mass Spectrometric Identification and Imaging of Molecular Species. Nat. Prod. Rep. 2014, 31, 756–767. [Google Scholar] [CrossRef] [PubMed]
- Kauppila, T.J.; Kuuranne, T.; Meurer, E.C.; Eberlin, M.N.; Kotiaho, T.; Kostiainen, R. Atmospheric Pressure Photoionization Mass Spectrometry. Ionization Mechanism and the Effect of Solvent on the Ionization of Naphthalenes. Anal. Chem. 2002, 74, 5470–5479. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.; Jones, M.; Dubant, S. Fast Analysis of Cosmetic Allergens Using UltraPerformance Convergence Chromatography (UPC2) with MS Detection. Waters, Application Notes 2017. Available online: https://www.waters.com/webassets/cms/library/docs/720005553en.pdf (accessed on 2 February 2024).
- Liu, Q.; Zenobi, R. Rapid Analysis of Fragrance Allergens by Dielectric Barrier Discharge Ionization Mass Spectrometry. Rapid Commun. Mass Spectrom. 2021, 35, e9021. [Google Scholar] [CrossRef] [PubMed]
- Rankin-Turner, S.; Reynolds, J.C.; Turner, M.A.; Heaney, L.M. Applications of Ambient Ionization Mass Spectrometry in 2021: An Annual Review. Anal. Sci. Adv. 2022, 3, 67–89. [Google Scholar] [CrossRef]
- Marques, L.D.A.; Catharino, R.R.; Bruns, R.E.; Eberlin, M.N. Electrospray Ionization Mass Spectrometry Fingerprinting of Perfumes: Rapid Classification and Counterfeit Detection. Rapid Commun. Mass Spectrom. 2006, 20, 3654–3658. [Google Scholar] [CrossRef]
- Haddad, R.; Catharino, R.R.; Marques, L.A.; Eberlin, M.N. Perfume Fingerprinting by Easy Ambient Sonic-Spray Ionization Mass Spectrometry: Nearly Instantaneous Typification and Counterfeit Detection. Rapid Commun. Mass Spectrom. 2008, 22, 3662–3666. [Google Scholar] [CrossRef]
- Chingin, K.; Gamez, G.; Chen, H.; Zhu, L.; Zenobi, R. Rapid Classification of Perfumes by Extractive Electrospray Ionization Mass Spectrometry (EESI-MS). Rapid Commun. Mass Spectrom. 2008, 22, 2009–2014. [Google Scholar] [CrossRef]
- Meng, X.; Bai, H.; Guo, T.; Niu, Z.; Ma, Q. Broad screening of illicit ingredients in cosmetics using ultra-high-performance liquid chromatography-hybrid quadrupole-Orbitrap mass spectrometry with customized accurate-mass database and mass spectral library. J. Chromatogr. A 2017, 1528, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Ramzi, A.; Ahmadi, H.; Sadiktsis, I.; Nilsson, U. A two-dimensional non-comprehensive reversed/normal phase high-performance liquid chromatography/tandem mass spectrometry system for determination of limonene and linalool hydroperoxides. J. Chromatogr. A 2018, 1566, 102–110. [Google Scholar] [CrossRef]
- Frerot, E.; Decorzant, E. Quantification of total furocoumarins in citrus oils by HPLC coupled with UV, fluorescence, and mass detection. J. Agric. Food Chem. 2004, 52, 6879–6886. [Google Scholar] [CrossRef] [PubMed]
- European Commission. CosIng—Cosmetics Ingredients. Available online: https://ec.europa.eu/growth/tools-databases/cosing/reference/annexes/list/II (accessed on 12 January 2024).
- Bundesamt fur Verbraucherschutz und Lebensmittelsicherheit (BVL). Technically avoidable heavy metal contents in cosmetic products. J. Verbr. Lebensm. 2016, 12, 51–53. [Google Scholar]
- Food and Drug Administration. FDA’s Testing of Cosmetics for Arsenic, Cadmium, Chromium, Cobalt, Lead, Mercury, and Nickel Content. Available online: https://www.fda.gov/cosmetics/potential-contaminants-cosmetics/fdas-testing-cosmetics-arsenic-cadmium-chromium-cobalt-lead-mercury-and-nickel-content (accessed on 10 January 2024).
- The Association of Southeast Asian Nations. Available online: https://www.asean.org/wp-content/uploads/images/archive/MRA-Cosmetic/Doc-3.pdf (accessed on 10 January 2024).
- Health Sciences Authority. Available online: https://www.hsa.gov.sg/docs/default-source/hprg-cosmetics/asean-guidelines-on-limits-of-contaminants-for-cosmetics-ver-3.pdf (accessed on 10 January 2024).
- CIRS C&K Testing. Available online: https://www.cirs-ck.com/en/new-chinese-standard-for-cosmetics-published (accessed on 1 January 2024).
- Available online: https://laws-lois.justice.gc.ca/eng/regulations/C.R.C.,_c._869/index.html (accessed on 1 January 2024).
- Government of Canada. Available online: https://www.canada.ca/en/health-canada/services/consumer-product-safety/reports-publications/industry-professionals/guidance-heavy-metal-impurities-cosmetics.html (accessed on 1 January 2024).
- Omolaoye, J.A.; Uzairu, A.; Gimba, C.E. Heavy metal assessment of some eye shadow products imported into Nigeria from China. Arch. Appl. Sci. Res. 2010, 2, 76–84. [Google Scholar]
- Lavilla, I.; Cabaleiro, N.; Costas, M.; de la Calle, I.; Bendicho, C. Ultrasound-assisted emulsification of cosmetic samples prior to elemental analysis by different atomic spectrometric techniques. Talanta 2009, 80, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Bocca, B.; Forte, G.; Petrucci, F.; Cristaudo, A. Levels of nickel and other potentially allergenic metals in Ni-tested commercial body creams. J. Pharm. Biomed Anal. 2007, 44, 1197–1202. [Google Scholar] [CrossRef] [PubMed]
- Environmental Defence Canada. Available online: https://environmentaldefence.ca/wp-content/uploads/2016/01/HeavyMetalHazard-FINAL.pdf (accessed on 1 January 2024).
- Volpe, M.G.; Nazzaro, M.; Coppola, R.; Rapuano, F.; Aquino, R.P. Determination and assessments of selected heavy metals in eye shadow cosmetics from China, Italy, and USA. Microchem. J. 2012, 101, 65–69. [Google Scholar] [CrossRef]
- Piccinini, P.; Piecha, M.; Torrent, S.F. European survey on the content of lead in lip products. J. Pharm. Biomed. Anal. 2013, 76, 225–233. [Google Scholar] [CrossRef]
- Hepp, N.M. Determination of total lead in 400 lipsticks on the U.S. market using a validated microwave-assisted digestion, inductively coupled plasma-mass spectrometric method. J. Cosmet. Sci. 2012, 63, 159–176. [Google Scholar]
- Hepp, N.M.; Mindak, W.R.; Cheng, J. Determination of total lead in lipstick: Development and validation of a microwave-assisted digestion, inductively coupled plasma-mass spectrometric method. J. Cosmet. Sci. 2009, 60, 405–414. [Google Scholar] [CrossRef]
- Uram, E.; Bischofer, B.P.; Hagermann, S. Market Analysis of Some Mercury Containing Products and Their Mercury-Free Alternatives in Selected Regions; Gesellschaft für Anlagenund Reaktorsicherheit: Brunswick, Germany, 2010; pp. 1–140. [Google Scholar]
- Georgescu, M.; Drăghici, G.A.; Oancea, E.-F.; Dehelean, C.A.; Şoica, C.; Vlăduţ, N.-V.; Nica, D.V. Effects of Cadmium Sulfate on the Brown Garden Snail Cornu aspersum: Implications for DNA Methylation. Toxics 2021, 9, 306. [Google Scholar] [CrossRef] [PubMed]
- Grosser, Z.; Davidowski, L.; Thompson, L. The Determination of Metals in Cosmetics; Application Note ICP-Mass Spectrometry PerkinElmer, Inc.: Shelton, CT, USA, 2011; pp. 1–6. [Google Scholar]
- Graeme, K.A.; Pollack, C.V., Jr. Heavy metal toxicity, Part I: Arsenic and mercury. J. Emerg. Med. 1998, 16, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Bellinger, D.C. Very low lead exposures and children’s neurodevelopment. Curr. Opin. Pediatr. 2008, 20, 172–177. [Google Scholar] [CrossRef]
- Sanders, T.; Liu, Y.; Buchner, V.; Tchounwou, P.B. Neurotoxic effects and biomarkers of lead exposure: A review. Rev. Environ. Health 2009, 24, 15–45. [Google Scholar] [CrossRef]
- EPA’s IRIS Program. Available online: https://iris.epa.gov/static/pdfs/0277_summary.pdf (accessed on 1 January 2024).
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans Volume 87: Inorganic and Organic Lead Compounds; International Agency for Research in Cancer: Lyon, France, 2006. [Google Scholar]
- Patrick, L. Lead toxicity, a review of the literature. Part 1: Exposure, evaluation, and treatment. Altern. Med. Rev. 2006, 11, 2–22. [Google Scholar] [PubMed]
- Karri, V.; Schuhmacher, M.; Kumar, V. Heavy metals (Pb, Cd, As and MeHg) as risk factors for cognitive dysfunction: A general review of metal mixture mechanism in brain. Environ. Toxicol. Pharmacol. 2016, 48, 203–213. [Google Scholar] [CrossRef]
- Chauhan, A.S.; Bhadauris, R.; Singh, A.K.; Lodhi, S.S.; Chaturvedi, D.K.; Tomar, V.S. Determination of lead and cadmium in cosmetic products. J. Chem. Pharm. Res. 2010, 2, 92–97. [Google Scholar]
- Ayenimo, J.G.; Yusuf, A.M.; Adekunle, A.S.; Makinde, O.W. Heavy metal exposure from personal care products. Bull. Environ. Contam. Toxicol. 2010, 84, 8–14. [Google Scholar] [CrossRef]
- Horowitz, Y.; Greenberg, D.; Ling, G.; Lifshitz, M. Acrodynia: A case report of two siblings. Arh. Dis. Child. 2002, 86, 453. [Google Scholar] [CrossRef]
- Sprinkle, R.V. Leaded eye cosmetics: A cultural cause of elevated lead levels in children. J. Fam. Pract. 1995, 40, 358–362. [Google Scholar] [PubMed]
- Rafati Rahimzadeh, M.; Rafati Rahimzadeh, M.; Kazemi, S.; Moghadamnia, A.A. Cadmium toxicity and treatment: An update. Casp. J. Intern. Med. 2017, 8, 135–145. [Google Scholar]
- Draghici, G.A.; Dehelean, C.A.; Moaca, A.E.; Moise, M.L.; Pinzaru, I.; Vladut, V.N.; Banatean-Dunea, I.; Nica, D. Cadmium nitrate and DNA methylation in gastropods: Comparison between ovotestis and hepatopancreas. PeerJ 2023, 11, e15032. [Google Scholar] [CrossRef] [PubMed]
- Waalkes, M.P. Cadmium carcinogenesis. Mutat. Res. Mol. Mech. Mutagen. 2003, 533, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Guy, R.; Hostynek, J.J.; Hinz, R.S.; Lorence, C.R. Metals and the Skin: Topical Effects and Systemic Absorption, 3rd ed.; CRC Press: New York, NY, USA, 1999. [Google Scholar]
- Chou, S.; Harper, C.; Ingerman, L.; Llados, F.; Colman, J.; Chappell, L.; Osier, M.; Odin, M.; Sage, G. Toxicological Profile for Arsenic; Agency for Toxic Substances and Disease Registry: Atlanta, Georgia, 2007. [Google Scholar]
- Thyssen, J.P.; Johansen, J.D.; Menné, T. Contact allergy epidemics and their controls. Contact Dermat. 2007, 56, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Iavicoli, I.; Caroli, S.; Alimonti, A.; Petrucci, F.; Carelli, G. Biomonitoring of a worker population exposed to low antimony trioxide levels. J. Trace Elem. Med. Biol. 2002, 16, 33–39. [Google Scholar] [CrossRef]
- White, G.P., Jr.; Mathias, C.G.; Davin, J.S. Dermatitis in workers exposed to antimony in a melting process. J. Occup. Med. 1993, 35, 392–395. [Google Scholar]
- Agency for Toxic Substances and Disease Registry. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp23.pdf (accessed on 1 January 2024).
- Ammann, A.A. Inductively coupled plasma mass spectrometry (ICP MS): A versatile tool. J. Mass Spectrom. 2007, 42, 419–427. [Google Scholar] [CrossRef]
- Wilschefski, S.C.; Baxter, M.R. Inductively Coupled Plasma Mass Spectrometry: Introduction to Analytical Aspects. Chem. Biochem. Rev. 2019, 40, 115–133. [Google Scholar] [CrossRef]
- Salama, A.K. Assessment of Metals in Cosmetics Commonly Used in Saudi Arabia. Environ. Monit. Assess. 2015, 188, 553–564. [Google Scholar] [CrossRef]
- Aldayel, O.; Hefne, J.; Alharbi, K.N.; Al-Ajyan, T. Heavy Metals Concentration in Facial Cosmetics. Nat. Prod. Chem. Res. 2018, 6, 303–311. [Google Scholar]
- Bobaker, A.M.; Alakili, I.; Sarmani, S.B.; Al-Ansari, N.; Yaseen, Z.M. Determination and Assessment of the Toxic Heavy Metal Elements Abstracted from the Traditional Plant Cosmetics and Medical Remedies: Case Study of Libya. Int. J. Environ. Res. Public Health 2019, 16, 1957. [Google Scholar] [CrossRef] [PubMed]
- Rubio, L.; Barrulas, P.; Costa, M.; Garcia-Jares, C.; Lores, M.; Barrocas Dias, C. The chemistry behind the body art: Unveiling the elemental profile and heavy metal content of natural tattoos and dyes by ICP-MS. RSC Adv. 2022, 12, 34414–34424. [Google Scholar] [CrossRef] [PubMed]
- Kilic, S.; Kilic, M.; Soylak, M. The Determination of Toxic Metals in some Traditional Cosmetic Products and Health Risk Assessment. Biol. Trace Elem. Res. 2021, 199, 2272–2277. [Google Scholar] [CrossRef] [PubMed]
- Perez, A.L.; Nembhard, M.; Monnot, A.; Bator, D.; Madonick, E.; Gaffney, S.H. Child and adult exposure and health risk evaluation following the use of metal- and metalloid-containing costume cosmetics sold in the United States. Regul. Toxicol. Pharmacol. 2016, 84, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Salles, F.J.; Paniz, F.P.; Batista, B.L.; Nardocci, A.C.; Olympio, K.P.K. Potentially Toxic Elements in Costume Cosmetics Used by Children and Adults Are Associated with Cancer Risk. Int. J. Environ. Res. Public Health 2023, 20, 531. [Google Scholar] [CrossRef] [PubMed]
- Domeradzka-Gajda, K.; Nocuń, M.; Roszak, J.; Janasik, B.; Quarles, C.D., Jr.; Wąsowicz, W.; Grobelny, J.; Tomaszewska, E.; Celichowski, G.; Ranoszek-Soliwoda, K.; et al. A study on the in vitro percutaneous absorption of silver nanoparticles in combination with aluminum chloride, methyl paraben or di-n-butyl phthalate. Toxicol. Lett. 2017, 272, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Rujido-Santos, I.; Herbello-Hermelo, P.; Barciela-Alonso, M.C.; Bermejo-Barrera, P.; Moreda-Piñeiro, A. Study Design on the Presence of Metals in Moisturisers, and Compliance with Regulation (EC) No. 1223/2009 of the European Parliament and of the Council of the European Union, on Cosmetic Products. Cosmetics 2022, 9, 82. [Google Scholar] [CrossRef]
- Papadopoulos, A.; Assimomytis, N.; Varvaresou, A. Sample Preparation of Cosmetic Products for the Determination of Heavy Metals. Cosmetics 2022, 9, 21. [Google Scholar] [CrossRef]
- Kopru, S.; Soylak, M. Inductively coupled plasma-mass spectrometry (ICP-MS) detection of trace metal contents of children cosmetics. Opt. Quant. Electron. 2024, 56, 399. [Google Scholar] [CrossRef]
- Nica, D.V.; Draghici, G.A.; Andrica, F.M.; Popescu, S.; Coricovac, D.E.; Dehelea, C.A.; Gergen, I.I.; Kovatsi, L.; Coleman, M.D.; Tsatsakis, A. Short-term effects of very low dose cadmium feeding on copper, manganese and iron homeostasis: A gastropod perspective. Environ. Toxicol. Pharmacol. 2019, 65, 9–13. [Google Scholar] [CrossRef] [PubMed]
- U.S. EPA. Exposure Factors Handbook 2011 Edition (Final Report); U.S. Environmental Protection Agency: Washington, DC, USA, 2011. [Google Scholar]
- The California Environmental Protection Agency’s Office of Environmental Health Hazard Assessment (OEHHA). Available online: https://oehha.ca.gov/media/downloads/crnr/2011handtomouthpb.pdf (accessed on 1 January 2024).
- The European Consumer Organisation (BEUC). Available online: http://www.beuc.eu/safety/nanotechnology (accessed on 1 January 2024).
- Durán, N.; Durán, M.; de Jesus, M.B.; Seabra, A.B.; Favaro, W.J.; Nakazato, G. Silver nanoparticles: A new view on mechanistic aspects on antimicrobial activity. Nanomedicine 2016, 12, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Vlachou, E.; Chipp, E.; Shale, E.; Wilson, Y.T.; Papini, R.; Moiemen, N.S. The safety of nanocrystalline silver dressings on burns: A study of systemic silver absorption. Burns 2007, 33, 979–985. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Q.; Kempf, M.; Mott, J.; Chang, H.E.; Francis, R.; Liu, P.Y.; Cuttle, L.; Olszowy, H.; Kravchuk, O.; Mill, J.; et al. Silver absorption on burns after the application of ActicoatTM: Data from pediatric patients and a porcine burn model. J. Burn Care Res. 2009, 30, 341–348. [Google Scholar] [CrossRef]
- Moiemen, N.S.; Shale, E.; Drysdale, K.J.; Smith, G.; Wilson, Y.T.; Papini, R. Acticoat dressings and major burns: Systemic silver absorption. Burns 2011, 37, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Pfurtscheller, K.; Petnehazy, T.; Goessler, W.; Bubalo, V.; Kamolz, L.P.; Trop, M. Transdermal uptake and organ distribution of silver from two different wound dressings in rats after a burn trauma. Wound Repair Regen. 2014, 22, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Bianco, C.; Adami, G.; Crosera, M.; Larese, F.; Casarin, S.; Castagnoli, C.; Stella, M.; Maina, G. Silver percutaneous absorption after exposure to silver nanoparticles: A comparison study of three human skin graft samples used for clinical applications. Burns 2014, 40, 1390–1396. [Google Scholar] [CrossRef]
- Bianco, C.; Kezic, S.; Crosera, M.; Svetlicic, V.; Šegota, S.; Maina, G.; Romano, C.; Larese, F.; Adami, G. In vitro percutaneous penetration and characterization of silver from silver-containing textiles. Int. J. Nanomed. 2015, 10, 1899–1908. [Google Scholar] [CrossRef]
- Larese, F.F.; D’Agostin, F.; Crosera, M.; Adami, G.; Renzi, N.; Bovenzi, M.; Maina, G. Human skin penetration of silver nanoparticles through intact and damaged skin. Toxicology 2009, 255, 33–37. [Google Scholar] [CrossRef]
- European Commission—Public Health. Available online: https://health.ec.europa.eu/document/download/47f167ec-b5db-4ec9-9d12-3d807bf3e526_en (accessed on 1 January 2024).
- Commission Decision of 9 February 2006 amending Decision 96/335/EC establishing an inventory and a common nomenclature of ingredients employed in cosmetic products (2006/257/EC). Off. J. Eur. Union 2006, L97, 1–528.
- NF EN 16274; Method for Analysis of Allergens—Quantification of an Extended List of 57 Suspected Allergens in Ready to Inject Fragrance Materials by Gas Chromatography Mass Spectrometry. 2021. Available online: https://www.boutique.afnor.org/en-gb/standard/nf-en-16274/method-for-analysis-of-allergens-quantification-of-an-extended-list-of-57-s/fa191311/264795# (accessed on 1 January 2024).


| Paraben | Monoisotopic Mass (Da) | Concentration (ng/g, Mean ± SD) | Range (ng/g, Mean ± SD) |
|---|---|---|---|
| MP | 152.047348 | 123.6 ± 61.6 | 48.3–224.2 |
| EP | 166.062988 | 64.5 ± 43.5 | 11.5–158.3 |
| PP | 180.078644 | 136.9 ± 48.5 | 70.2–214.5 |
| BP | 194.094299 | 74.2 ± 27.5 | 25.4–111.1 |
| BeP | 228.078644 | 55.6 ± 24.3 | 15.3–100.2 |
| Sample Nail Polish | Color | Aspect |
|---|---|---|
| AHC | Dark blue | Creamy |
| CCR | Light blue | Creamy–shiny |
| HTT | Brown | Simple |
| ICM | Red | Metallic |
| ICR | Orange-Golden | Creamy |
| IPC | Orange | Simple |
| RCR | Red | Creamy |
| RMT | Pink | Metallic |
| SLB | Golden | Simple |
| No. | Dye Agent | Molecular Ion (m/z) | Molecular Formula | Structural Formula |
|---|---|---|---|---|
| 1 | Basic Violet 1 | 358.22696 | C24H28N3 |  |
| 2 | Basic Violet 10 | 443.23227 | C28H31N2O3 |  |
| 3 | Basic Violet 3 | 372.24277 | C25H30N3 |  |
| 4 | Pigment Red 3 | 308.10236 | C17H13N3O3 |  |
| 5 | Pigment Red 48:4 | 421.02478 | C18H13ClN2O6S |  |
| 6 | Solvent Blue 35 | 351.20642 | C22H26N2O2 |  |
| 7 | Solvent Red 23 | 353.13892 | C22H16N4O |  |
| 8 | Acid Orange 20 | 327.0441 [M − Na+]− | C16H11N2O4S |  |
| 9 | Pigment Red 48:2 | 419.01062 | C18H11CClN2O6S |  |
| 10 | Pigment Red 49 | 377.05936 | C20H14N2O4S |  |
| 11 | Pigment Red 53:1 | 375.02057 |  |
| No. | Fragrance Allergen | Molecular Formula | MW |
|---|---|---|---|
| 1 | Alpha isomethylionone | C14H22O | 206.32 |
| 2 | Amyl cinnamal (Jasmonal A) | C14H18O | 202.29 |
| 3 | Amyl cinnamyl alcohol | C14H20O | 204.31 |
| 4 | Anisyl alcohol | C8H10O2 | 138.16 |
| 5 | Benzyl alcohol | C7H8O | 108.14 |
| 6 | Benzyl benzoate | C14H12O2 | 212.24 |
| 7 | Benzyl cinnamate | C16H14O2 | 238.28 |
| 8 | Benzyl salicylate | C14H12O3 | 228.24 |
| 9 | Butylphenyl methylpropional (Lilial) | C14H20O | 204.31 |
| 10 | Cinnamal | C9H8O | 132.16 |
| 11 | Cinnamyl alcohol | C9H10O | 134.17 |
| 12 | Citral | C10H16O | 152.24 |
| 13 | Citronellol | C10H20O | 156.26 |
| 14 | Coumarin | C9H6O2 | 146.14 |
| 15 | Eugenol | C10H12O2 | 164.20 |
| 16 | Farnesol | C15H26O | 222.37 |
| 17 | Geraniol | C10H18O | 154.25 |
| 18 | Hexyl cinnamal (Jasmonal h) | C15H20O | 216.32 |
| 19 | Hydroxycitronellal | C10H20O2 | 172.26 |
| 20 | Hydroxyisohexyl 3-cyclohexene carboxaldehyde (Lyral) | C13H22O2 | 210.31 |
| 21 | Isoeugenol | C10H12O2 | 164.20 |
| 22 | Limonene | C10H16 | 136.23 |
| 23 | Linalool | C10H18O | 154.25 |
| 24 | Methyl 2-octynoate | C9H14O2 | 154.21 |
| No. | Heavy Metal | Cosmetic Products |
|---|---|---|
| 1 | Mercury (Hg) | Creams (antiseptic, skin-lightening) and some mascaras |
| 2 | Lead (Pb) | Lipsticks, eyeliners, lip glosses, and hair dyes |
| 3 | Cadmium (Cd) | Blush, eyeshadow, and face powders |
| 4 | Arsenic (As) | Blush, eyeshadow, and face powders |
| 5 | Nickel (Ni) | Foundations, eyeshadow, and mascaras |
| 6 | Chromium (Cr) | Eyeshadow, lipsticks, and face powders |
| 7 | Aluminum (Al) | Foundations, eyeshadow, and mascaras |
| 8 | Copper (Cu) | Blush, eyeshadow, and some lipsticks |
| 9 | Antimony (Sb) | Blush, eyeshadow, and mascara |
| 10 | Zinc (Zn) | Foundations, sunscreens, and face powders |
| 11 | Manganese (Mn) | Blush, eyeshadow, and some lipsticks |
| 12 | Cobalt (Co) | Hair dyes and some eyeshadows |
| 13 | Selenium (Se) | Hair dyes and some face powders |
| 14 | Barium (Ba) | Blush, eyeshadow, and face powders |
| 15 | Beryllium (Be) | Eyeshadow and face powders |
| 16 | Thallium (Tl) | Hair dyes and some face powders |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serb, A.F.; Georgescu, M.; Onulov, R.; Novaconi, C.R.; Sisu, E.; Bolocan, A.; Sandu, R.E. Mass-Spectrometry-Based Research of Cosmetic Ingredients. Molecules 2024, 29, 1336. https://doi.org/10.3390/molecules29061336
Serb AF, Georgescu M, Onulov R, Novaconi CR, Sisu E, Bolocan A, Sandu RE. Mass-Spectrometry-Based Research of Cosmetic Ingredients. Molecules. 2024; 29(6):1336. https://doi.org/10.3390/molecules29061336
Chicago/Turabian StyleSerb, Alina Florina, Marius Georgescu, Robert Onulov, Cristina Ramona Novaconi, Eugen Sisu, Alexandru Bolocan, and Raluca Elena Sandu. 2024. "Mass-Spectrometry-Based Research of Cosmetic Ingredients" Molecules 29, no. 6: 1336. https://doi.org/10.3390/molecules29061336