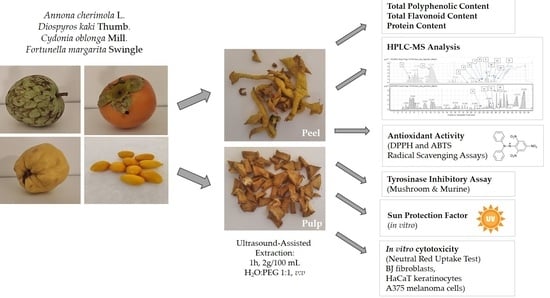
Pulp or Peel? Comparative Analysis of the Phytochemical Content and Selected Cosmetic-Related Properties of Annona cherimola L., Diospyros kaki Thumb., Cydonia oblonga Mill. and Fortunella margarita Swingle Pulp and Peel Extracts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Comparative Analysis of Total Phenolic, Flavonoid and Protein Contents between Peel and Pulp Hydroglycolic Extracts
2.2. HPLC-ESI-QTOF-MS/MS Fingerprinting of the Analyzed Extracts
2.3. Antioxidant Potential of Pulp and Peel Extracts
2.4. Skin Lightening Potential of Peel and Pulp Extracts—Inhibition of Tyrosinase
2.5. Sun Protecting Potential of Flesh and Peel Extracts—SPF In Vitro
2.6. The Influence of Peel and Pulp Extracts on the Viability of Skin Fibroblasts and Keratinocytes—In Vitro Cytotoxicity
2.7. In Vitro Cytotoxicity of Peel and Pulp Extracts on Human Melanoma Cells
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Plant Material and Extract Preparation
3.3. The Content of Total Polyphenols, Flavonoids and Proteins
3.4. HPLC-ESI-QTOF-MS/MS Fingerprinting of the Obtained Extracts
3.5. Antioxidant Activity—DPPH and ABTS Scavenging Assays
3.6. Tyrosinase Inhibitory Assay
3.7. Determination of the Sun Protection Factor (SPF)
3.8. In Vitro Cytotoxicity
3.9. Statistical Analysis
4. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Slavin, J.L.; Lloyd, B. Health Benefits of Fruits and Vegetables. Adv. Nutr. 2012, 3, 506–516. [Google Scholar] [CrossRef] [PubMed]
- FAO Food and Agricultural Organization of the United Nations. 2017. Available online: http://www.fao.org/faostat/en/#data (accessed on 8 February 2024).
- Fierascu, R.C.; Sieniawska, E.; Ortan, A.; Fierascu, I.; Xiao, J. Fruits By-Products—A Source of Valuable Active Principles. A Short Review. Front. Bioeng. Biotechnol. 2020, 8, 319. [Google Scholar] [CrossRef] [PubMed]
- Gorinstein, S.; Martín-Belloso, O.; Park, Y.-S.; Haruenkit, R.; Lojek, A.; Ĉíž, M.; Caspi, A.; Libman, I.; Trakhtenberg, S. Comparison of Some Biochemical Characteristics of Different Citrus Fruits. Food Chem. 2001, 74, 309–315. [Google Scholar] [CrossRef]
- Soong, Y.-Y.; Barlow, P.J. Antioxidant Activity and Phenolic Content of Selected Fruit Seeds. Food Chem. 2004, 88, 411–417. [Google Scholar] [CrossRef]
- Zuñiga-Martínez, B.S.; Domínguez-Avila, J.A.; Robles-Sánchez, R.M.; Ayala-Zavala, J.F.; Villegas-Ochoa, M.A.; González-Aguilar, G.A. Agro-Industrial Fruit Byproducts as Health-Promoting Ingredients Used to Supplement Baked Food Products. Foods 2022, 11, 3181. [Google Scholar] [CrossRef]
- Liu, J.-K. Natural Products in Cosmetics. Nat. Prod. Bioprospect. 2022, 12, 40. [Google Scholar] [CrossRef] [PubMed]
- Amberg, N.; Fogarassy, C. Green Consumer Behavior in the Cosmetics Market. Resources 2019, 8, 137. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Tundis, R.; Bonesi, M.; Menichini, F.; Mastellone, V.; Avallone, L.; Menichini, F. Radical Scavenging, Antioxidant and Metal Chelating Activities of Annona cherimola Mill. (Cherimoya) Peel and Pulp in Relation to Their Total Phenolic and Total Flavonoid Contents. J. Food Compos. Anal. 2012, 25, 179–184. [Google Scholar] [CrossRef]
- García-Salas, P.; Gómez-Caravaca, A.M.; Morales-Soto, A.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Identification and Quantification of Phenolic and Other Polar Compounds in the Edible Part of Annona cherimola and Its By-Products by HPLC-DAD-ESI-QTOF-MS. Food Res. Int. 2015, 78, 246–257. [Google Scholar] [CrossRef]
- Gorinstein, S.; Zachwieja, Z.; Folta, M.; Barton, H.; Piotrowicz, J.; Zemser, M.; Weisz, M.; Trakhtenberg, S.; Màrtín-Belloso, O. Comparative Contents of Dietary Fiber, Total Phenolics, and Minerals in Persimmons and Apples. J. Agric. Food Chem. 2001, 49, 952–957. [Google Scholar] [CrossRef]
- Jeong, D.-W.; Cho, C.H.; Lee, J.S.; Lee, S.H.; Kim, T.; Kim, D.-O. Deastringent Peel Extracts of Persimmon (Diospyros kaki Thunb. Cv. Cheongdo-Bansi) Protect Neuronal PC-12 and SH-SY5Y Cells against Oxidative Stress. J. Microbiol. Biotechnol. 2018, 28, 1094–1104. [Google Scholar] [CrossRef]
- Silva, B.M.; Andrade, P.B.; Valentão, P.; Ferreres, F.; Seabra, R.M.; Ferreira, M.A. Quince (Cydonia oblonga Miller) Fruit (Pulp, Peel, and Seed) and Jam: Antioxidant Activity. J. Agric. Food Chem. 2004, 52, 4705–4712. [Google Scholar] [CrossRef]
- Fattouch, S.; Caboni, P.; Coroneo, V.; Tuberoso, C.I.G.; Angioni, A.; Dessi, S.; Marzouki, N.; Cabras, P. Antimicrobial Activity of Tunisian Quince (Cydonia oblonga Miller) Pulp and Peel Polyphenolic Extracts. J. Agric. Food Chem. 2007, 55, 963–969. [Google Scholar] [CrossRef]
- Sadek, E.S.; Makris, D.P.; Kefalas, P. Polyphenolic Composition and Antioxidant Characteristics of Kumquat (Fortunella margarita) Peel Fractions. Plant Foods Hum. Nutr. 2009, 64, 297–302. [Google Scholar] [CrossRef]
- Al Kazman, B.S.M.; Harnett, J.E.; Hanrahan, J.R. Traditional Uses, Phytochemistry and Pharmacological Activities of Annonacae. Molecules 2022, 27, 3462. [Google Scholar] [CrossRef] [PubMed]
- Quílez, A.M.; Fernández-Arche, M.A.; García-Giménez, M.D.; De La Puerta, R. Potential Therapeutic Applications of the Genus Annona: Local and Traditional Uses and Pharmacology. J. Ethnopharmacol. 2018, 225, 244–270. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, T.G.; Santos, F.; Sanches-Silva, A.; Beatriz Oliveira, M.; Bento, A.C.; Costa, H.S. Nutritional and Phytochemical Composition of Annona cherimola Mill. Fruits and by-Products: Potential Health Benefits. Food Chem. 2016, 193, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Jamkhande, P.G.; Ajgunde, B.R.; Jadge, D.R. Annona cherimola Mill. (Custard Apple): A Review on Its Plant Profile, Nutritional Values, Traditional Claims and Ethnomedicinal Properties. Orient. Pharm. Exp. Med. 2017, 17, 189–201. [Google Scholar] [CrossRef]
- Calzada, F.; Valdes, M.; Martínez-Solís, J.; Velázquez, C.; Barbosa, E. Annona cherimola Miller and Its Flavonoids, an Important Source of Products for the Treatment of Diabetes Mellitus: In Vivo and In Silico Evaluations. Pharmaceuticals 2023, 16, 724. [Google Scholar] [CrossRef] [PubMed]
- CosIng Cosmetic Ingredient Database of the European Commission. Available online: https://ec.europa.eu/growth/tools-databases/cosing/ (accessed on 4 February 2024).
- Rojas-García, A.; Rodríguez, L.; Cádiz-Gurrea, M.D.L.L.; García-Villegas, A.; Fuentes, E.; Villegas-Aguilar, M.D.C.; Palomo, I.; Arráez-Román, D.; Segura-Carretero, A. Determination of the Bioactive Effect of Custard Apple By-Products by In Vitro Assays. Int. J. Mol. Sci. 2022, 23, 9238. [Google Scholar] [CrossRef] [PubMed]
- Del Bubba, M.; Giordani, E.; Pippucci, L.; Cincinelli, A.; Checchini, L.; Galvan, P. Changes in Tannins, Ascorbic Acid and Sugar Content in Astringent Persimmons during on-Tree Growth and Ripening and in Response to Different Postharvest Treatments. J. Food Compos. Anal. 2009, 22, 668–677. [Google Scholar] [CrossRef]
- Murali, P.; Hamid; Shams, R.; Dar, A.H. Insights on Nutritional Profile, Nutraceutical Components, Pharmacological Potential, and Trending Utilization of Persimmon Cultivars: A Review. Food Chem. Adv. 2023, 3, 100431. [Google Scholar] [CrossRef]
- Direito, R.; Rocha, J.; Sepodes, B.; Eduardo-Figueira, M. From Diospyros kaki L. (Persimmon) Phytochemical Profile and Health Impact to New Product Perspectives and Waste Valorization. Nutrients 2021, 13, 3283. [Google Scholar] [CrossRef]
- Ashraf, M.U.; Muhammad, G.; Hussain, M.A.; Bukhari, S.N.A. Cydonia oblonga M., A Medicinal Plant Rich in Phytonutrients for Pharmaceuticals. Front. Pharmacol. 2016, 7, 163. [Google Scholar] [CrossRef] [PubMed]
- Khiljee, T.; Akhtar, N.; Khiljee, S.; Khiljee, B.; Rasheed, H.M.; Ansari, S.A.; Alkahtani, H.M.; Ansari, I.A. Gauging Quince Phytonutrients and Its 4% Emulgel Effect on Amplifying Facial Skin Moisturizing Potential. Gels 2023, 9, 934. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Meenu, M.; Xu, B. Recent Development in Bioactive Compounds and Health Benefits of Kumquat Fruits. Food Rev. Int. 2023, 39, 4312–4332. [Google Scholar] [CrossRef]
- Lou, S.-N.; Lai, Y.-C.; Hsu, Y.-S.; Ho, C.-T. Phenolic Content, Antioxidant Activity and Effective Compounds of Kumquat Extracted by Different Solvents. Food Chem. 2016, 197, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Fiume, M.M.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Safety Assessment of Propylene Glycol, Tripropylene Glycol, and PPGs as Used in Cosmetics. Int. J. Toxicol. 2012, 31, 245S–260S. [Google Scholar] [CrossRef] [PubMed]
- Gaweł-Bęben, K.; Bujak, T.; Nizioł-Łukaszewska, Z.; Antosiewicz, B.; Jakubczyk, A.; Karaś, M.; Rybczyńska, K. Stevia Rebaudiana Bert. Leaf Extracts as a Multifunctional Source of Natural Antioxidants. Molecules 2015, 20, 5468–5486. [Google Scholar] [CrossRef]
- Nizioł-Łukaszewska, Z.; Gaweł-Bęben, K.; Rybczyńska-Tkaczyk, K.; Jakubczyk, A.; Karaś, M.; Bujak, T. Biochemical Properties, UV-Protecting and Fibroblast Growth-Stimulating Activity of Plantago Lanceolata L. Extracts. Ind. Crops Prod. 2019, 138, 111453. [Google Scholar] [CrossRef]
- Lee, J.-H.; Lee, Y.-B.; Seo, W.-D.; Kang, S.-T.; Lim, J.-W.; Cho, K.-M. Comparative Studies of Antioxidant Activities and Nutritional Constituents of Persimmon Juice (Diospyros kaki L. Cv. Gapjubaekmok). Prev. Nutr. Food Sci. 2012, 17, 141–151. [Google Scholar] [CrossRef]
- Tardugno, R.; Gervasi, T.; Nava, V.; Cammilleri, G.; Ferrantelli, V.; Cicero, N. Nutritional and Mineral Composition of Persimmon Fruits (Diospyros kaki L.) from Central and Southern Italy. Nat. Prod. Res. 2022, 36, 5168–5173. [Google Scholar] [CrossRef] [PubMed]
- Bordiga, M.; Travaglia, F.; Giuffrida, D.; Mangraviti, D.; Rigano, F.; Mondello, L.; Arlorio, M.; Coïsson, J.D. Characterization of Peel and Pulp Proanthocyanidins and Carotenoids during Ripening in Persimmon “Kaki Tipo” Cv, Cultivated in Italy. Food Res. Int. 2019, 120, 800–809. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Rocchetti, G.; Zengin, G.; Ak, G.; Saber, F.R.; Montesano, D.; Lucini, L. The UHPLC-QTOF-MS Phenolic Profiling and Activity of Cydonia oblonga Mill. Reveals a Promising Nutraceutical Potential. Foods 2021, 10, 1230. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, A.S.; Silva, B.M.; Pereira, J.A.; Andrade, P.B.; Valentão, P.; Carvalho, M. Protective Effect of Quince (Cydonia oblonga Miller) Fruit against Oxidative Hemolysis of Human Erythrocytes. Food Chem. Toxicol. 2009, 47, 1372–1377. [Google Scholar] [CrossRef]
- Hanan, E.; Hasan, N.; Zahiruddin, S.; Ahmad, S.; Sharma, V.; Ahmad, F.J. Metabolite Profiling and Ameliorative Effect of Quince (Cydonia oblonga) Leaves against Doxorubicin Induced Cardiotoxicity in Wistar Rats. Food Biosci. 2023, 53, 102691. [Google Scholar] [CrossRef]
- Szychowski, P.J.; Munera-Picazo, S.; Szumny, A.; Carbonell-Barrachina, Á.A.; Hernández, F. Quality Parameters, Bio-Compounds, Antioxidant Activity and Sensory Attributes of Spanish Quinces (Cydonia oblonga Miller). Sci. Hortic. 2014, 165, 163–170. [Google Scholar] [CrossRef]
- Jayaprakasha, G.K.; Chidambara Murthy, K.N.; Etlinger, M.; Mantur, S.M.; Patil, B.S. Radical Scavenging Capacities and Inhibition of Human Prostate (LNCaP) Cell Proliferation by Fortunella margarita. Food Chem. 2012, 131, 184–191. [Google Scholar] [CrossRef]
- Mannino, G.; Gentile, C.; Porcu, A.; Agliassa, C.; Caradonna, F.; Bertea, C.M. Chemical Profile and Biological Activity of Cherimoya (Annona cherimola Mill.) and Atemoya (Annona atemoya) Leaves. Molecules 2020, 25, 2612. [Google Scholar] [CrossRef]
- Pu, F.; Ren, X.-L.; Zhang, X.-P. Phenolic Compounds and Antioxidant Activity in Fruits of Six Diospyros kaki Genotypes. Eur. Food Res. Technol. 2013, 237, 923–932. [Google Scholar] [CrossRef]
- Maulidiani, M.; Abdul-Hamid, N.A.; Abas, F.; Park, Y.S.; Park, Y.-K.; Kim, Y.M.; Gorinstein, S. Detection of Bioactive Compounds in Persimmon (Diospyros kaki) Using UPLC-ESI-Orbitrap-MS/MS and Fluorescence Analyses. Microchem. J. 2019, 149, 103978. [Google Scholar] [CrossRef]
- Jiménez-Sánchez, C.; Lozano-Sánchez, J.; Marti, N.; Saura, D.; Valero, M.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Characterization of Polyphenols, Sugars, and Other Polar Compounds in Persimmon Juices Produced under Different Technologies and Their Assessment in Terms of Compositional Variations. Food Chem. 2015, 182, 282–291. [Google Scholar] [CrossRef]
- Díaz-de-Cerio, E.; Aguilera-Saez, L.M.; Gómez-Caravaca, A.M.; Verardo, V.; Fernández-Gutiérrez, A.; Fernández, I.; Arráez-Román, D. Characterization of Bioactive Compounds of Annona cherimola L. Leaves Using a Combined Approach Based on HPLC-ESI-TOF-MS and NMR. Anal. Bioanal. Chem. 2018, 410, 3607–3619. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.A.O.; Vilela, C.; Camacho, J.F.; Cordeiro, N.; Gouveia, M.; Freire, C.S.R.; Silvestre, A.J.D. Profiling of Lipophilic and Phenolic Phytochemicals of Four Cultivars from Cherimoya (Annona cherimola Mill.). Food Chem. 2016, 211, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Kusunoki, K.; Fujita, M.; Matsuguma, M.; Baba, R.; Minari, Y.; Tadokoro, T.; Innami, S.; Maekawa, A. Studies on Microscopic Observations of Tissue Structures and Dietary Fiber Components in Persimmon (Diospyros kaki) Leaves. Food Preserv. Sci. 1997, 23, 65–75. [Google Scholar] [CrossRef]
- Yaqub, S.; Farooq, U.; Shafi, A.; Akram, K.; Murtaza, M.A.; Kausar, T.; Siddique, F. Chemistry and Functionality of Bioactive Compounds Present in Persimmon. J. Chem. 2016, 2016, 1–13. [Google Scholar] [CrossRef]
- El Makhzangy, A.; Hamad, D.; El-Shawaf, A. Chemical and Bioactive Composition in Persimmon (Diospyros kaki) Fruits. Mathews J. Nutr. Diet. 2023, 6, 1–7. [Google Scholar] [CrossRef]
- Martínez-Las Heras, R.; Pinazo, A.; Heredia, A.; Andrés, A. Evaluation Studies of Persimmon Plant (Diospyros kaki) for Physiological Benefits and Bioaccessibility of Antioxidants by in Vitro Simulated Gastrointestinal Digestion. Food Chem. 2017, 214, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.M.; Andrade, P.B.; Gonçalves, A.C.; Seabra, R.M.; Oliveira, M.B.; Ferreira, M.A. Influence of Jam Processing upon the Contents of Phenolics, Organic Acids and Free Amino Acids in Quince Fruit (Cydonia oblonga Miller). Eur. Food Res. Technol. 2004, 218, 385–389. [Google Scholar] [CrossRef]
- Silva, B.M.; Andrade, P.B.; Ferreres, F.; Domingues, A.L.; Seabra, R.M.; Ferreira, M.A. Phenolic Profile of Quince Fruit (Cydonia oblonga Miller) (Pulp and Peel). J. Agric. Food Chem. 2002, 50, 4615–4618. [Google Scholar] [CrossRef]
- Kawaii, S.; Tomono, Y.; Katase, E.; Ogawa, K.; Yano, M. Quantitation of Flavonoid Constituents in Citrus Fruits. J. Agric. Food Chem. 1999, 47, 3565–3571. [Google Scholar] [CrossRef]
- Kawaii, S.; Tomono, Y.; Katase, E.; Ogawa, K.; Yano, M.; Koizumi, M.; Ito, C.; Furukawa, H. Quantitative Study of Flavonoids in Leaves of Citrus Plants. J. Agric. Food Chem. 2000, 48, 3865–3871. [Google Scholar] [CrossRef]
- Barreca, D.; Bellocco, E.; Caristi, C.; Leuzzi, U.; Gattuso, G. Kumquat (Fortunella Japonica Swingle) Juice: Flavonoid Distribution and Antioxidant Properties. Food Res. Int. 2011, 44, 2190–2197. [Google Scholar] [CrossRef]
- Quijano, C.E.; Pino, J.A. Volatile Compounds of Kumquat (Fortunella margarita (Lour.) Swingle) Leaf Oil. J. Essent. Oil Res. 2009, 21, 194–196. [Google Scholar] [CrossRef]
- Hamauzu, Y.; Yasui, H.; Inno, T.; Kume, C.; Omanyuda, M. Phenolic Profile, Antioxidant Property, and Anti-Influenza Viral Activity of Chinese Quince (Pseudocydonia Sinensis Schneid.), Quince (Cydonia oblonga Mill.), and Apple (Malus Domestica Mill.) Fruits. J. Agric. Food Chem. 2005, 53, 928–934. [Google Scholar] [CrossRef] [PubMed]
- Kołodziejczyk, K.; Sójka, M.; Abadias, M.; Viñas, I.; Guyot, S.; Baron, A. Polyphenol Composition, Antioxidant Capacity, and Antimicrobial Activity of the Extracts Obtained from Industrial Sour Cherry Pomace. Ind. Crops Prod. 2013, 51, 279–288. [Google Scholar] [CrossRef]
- Silva, B.M.; Andrade, P.B.; Martins, R.C.; Valentão, P.; Ferreres, F.; Seabra, R.M.; Ferreira, M.A. Quince (Cydonia oblonga Miller) Fruit Characterization Using Principal Component Analysis. J. Agric. Food Chem. 2005, 53, 111–122. [Google Scholar] [CrossRef]
- Wojdyło, A.; Oszmiański, J.; Bielicki, P. Polyphenolic Composition, Antioxidant Activity, and Polyphenol Oxidase (PPO) Activity of Quince (Cydonia oblonga Miller) Varieties. J. Agric. Food Chem. 2013, 61, 2762–2772. [Google Scholar] [CrossRef] [PubMed]
- Lou, S.-N.; Ho, C.-T. Phenolic Compounds and Biological Activities of Small-Size Citrus: Kumquat and Calamondin. J. Food Drug Anal. 2017, 25, 162–175. [Google Scholar] [CrossRef] [PubMed]
- Souza, C.S.E.; Anunciação, P.C.; Lucia, C.M.D.; Dôres, R.G.R.D.; Milagres, R.C.R.D.M.; Sant’Ana, H.M.P. Kumquat (Fortunella margarita): A Good Alternative for the Ingestion of Nutrients and Bioactive Compounds. Proceedings 2021, 70, 105. [Google Scholar] [CrossRef]
- Perez, S. Profile Physical and Phenolic-Chemical of Kumquat Influenced by the Environment Analyzed in Fresh. J. Ecol. Eng. 2022, 23, 196–203. [Google Scholar] [CrossRef]
- Sicari, V.; Poiana, M. Comparison of the Volatile Component of the Essential Oil of Kumquat (Fortunella margarita Swingle) Extracted by Supercritical Carbon Dioxide, Hydrodistillation and Conventional Solvent Extraction. J. Essent. Oil Bear. Plants 2017, 20, 87–94. [Google Scholar] [CrossRef]
- Agalar, H.G.; Temiz, B.; Demirci, B.; Baser, K.H.C. Drying Effects on The Volatile Compounds of Kumquat, Limequat and Mexican Lime Fruits. J. Essent. Oil Bear. Plants 2020, 23, 1395–1408. [Google Scholar] [CrossRef]
- Hoang, H.T.; Moon, J.-Y.; Lee, Y.-C. Natural Antioxidants from Plant Extracts in Skincare Cosmetics: Recent Applications, Challenges and Perspectives. Cosmetics 2021, 8, 106. [Google Scholar] [CrossRef]
- Platzer, M.; Kiese, S.; Herfellner, T.; Schweiggert-Weisz, U.; Miesbauer, O.; Eisner, P. Common Trends and Differences in Antioxidant Activity Analysis of Phenolic Substances Using Single Electron Transfer Based Assays. Molecules 2021, 26, 1244. [Google Scholar] [CrossRef]
- Fukai, S.; Tanimoto, S.; Maeda, A.; Fukuda, H.; Okada, Y.; Nomura, M. Pharmacological Activity of Compounds Extracted from Persimmon Peel (Diospyros kaki THUNB.). J. Oleo Sci. 2009, 58, 213–219. [Google Scholar] [CrossRef]
- Choe, J.-H.; Kim, H.-Y.; Kim, C.-J. Effect of Persimmon Peel (Diospyros kaki Thumb.) Extracts on Lipid and Protein Oxidation of Raw Ground Pork During Refrigerated Storage. Korean J. Food Sci. Anim. Resour. 2017, 37, 254–263. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin Whitening Agents: Medicinal Chemistry Perspective of Tyrosinase Inhibitors. J. Enzym. Inhib. Med. Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef] [PubMed]
- Strzępek-Gomółka, M.; Gaweł-Bęben, K.; Angelis, A.; Antosiewicz, B.; Sakipova, Z.; Kozhanova, K.; Głowniak, K.; Kukula-Koch, W. Identification of Mushroom and Murine Tyrosinase Inhibitors from Achillea Biebersteinii Afan. Extract. Molecules 2021, 26, 964. [Google Scholar] [CrossRef] [PubMed]
- Şöhretoğlu, D.; Sari, S.; Barut, B.; Özel, A. Tyrosinase Inhibition by Some Flavonoids: Inhibitory Activity, Mechanism by in Vitro and in Silico Studies. Bioorganic Chem. 2018, 81, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Panzella, L.; Napolitano, A. Natural and Bioinspired Phenolic Compounds as Tyrosinase Inhibitors for the Treatment of Skin Hyperpigmentation: Recent Advances. Cosmetics 2019, 6, 57. [Google Scholar] [CrossRef]
- Niu, C.; Aisa, H.A. Upregulation of Melanogenesis and Tyrosinase Activity: Potential Agents for Vitiligo. Molecules 2017, 22, 1303. [Google Scholar] [CrossRef]
- Ohguchi, K.; Nakajima, C.; Oyama, M.; Iinuma, M.; Itoh, T.; Akao, Y.; Nozawa, Y.; Ito, M. Inhibitory Effects of Flavonoid Glycosides Isolated from the Peel of Japanese Persimmon (Diospyros kaki ‘Fuyu’) on Melanin Biosynthesis. Biol. Pharm. Bull. 2010, 33, 122–124. [Google Scholar] [CrossRef]
- Li, W.; Tian, H.; Guo, F.; Wu, Y. Inhibition Characteristics and Mechanism of Tyrosinase Using Five Citrus Flavonoids: A Spectroscopic and Molecular Dynamics Simulation Study. J. Food Biochem. 2022, 46, e14484. [Google Scholar] [CrossRef]
- Fan, M.; Zhang, G.; Hu, X.; Xu, X.; Gong, D. Quercetin as a Tyrosinase Inhibitor: Inhibitory Activity, Conformational Change and Mechanism. Food Res. Int. 2017, 100, 226–233. [Google Scholar] [CrossRef]
- Bosch, R.; Philips, N.; Suárez-Pérez, J.; Juarranz, A.; Devmurari, A.; Chalensouk-Khaosaat, J.; González, S. Mechanisms of Photoaging and Cutaneous Photocarcinogenesis, and Photoprotective Strategies with Phytochemicals. Antioxidants 2015, 4, 248–268. [Google Scholar] [CrossRef] [PubMed]
- Sander, M.; Sander, M.; Burbidge, T.; Beecker, J. The Efficacy and Safety of Sunscreen Use for the Prevention of Skin Cancer. Cmaj 2020, 192, E1802–E1808. [Google Scholar] [CrossRef] [PubMed]
- Anbualakan, K.; Tajul Urus, N.Q.; Makpol, S.; Jamil, A.; Mohd Ramli, E.S.; Md Pauzi, S.H.; Muhammad, N. A Scoping Review on the Effects of Carotenoids and Flavonoids on Skin Damage Due to Ultraviolet Radiation. Nutrients 2022, 15, 92. [Google Scholar] [CrossRef] [PubMed]
- Boukamp, P.; Petrussevska, R.T.; Breitkreutz, D.; Hornung, J.; Markham, A.; Fusenig, N.E. Normal Keratinization in a Spontaneously Immortalized Aneuploid Human Keratinocyte Cell Line. J. Cell Biol. 1988, 106, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Riahi-Chebbi, I.; Haoues, M.; Essafi, M.; Zakraoui, O.; Fattouch, S.; Karoui, H.; Essafi-Benkhadir, K. Quince Peel Polyphenolic Extract Blocks Human Colon Adenocarcinoma LS174 Cell Growth and Potentiates 5-Fluorouracil Efficacy. Cancer Cell Int. 2015, 16, 1. [Google Scholar] [CrossRef] [PubMed]
- Iacopetta, D.; Fazio, A.; La Torre, C.; Barbarossa, A.; Ceramella, J.; Francomano, F.; Saturnino, C.; El-Kashef, H.; Alcaro, S.; Sinicropi, M.S. Annona cherimola Mill. Leaf Extracts Affect Melanoma Cells Growth and Progression. Foods 2022, 11, 2420. [Google Scholar] [CrossRef]
- Fukumoto, L.R.; Mazza, G. Assessing Antioxidant and Prooxidant Activities of Phenolic Compounds. J. Agric. Food Chem. 2000, 48, 3597–3604. [Google Scholar] [CrossRef] [PubMed]
- Matejić, J.; Džamić, A.; Mihajilov-Krstev, T.; Ranđelović, V.; Krivošej, Z.; Marin, P. Total Phenolic Content, Flavonoid Concentration, Antioxidant and Antimicrobial Activity of Methanol Extracts from Three Seseli L. Taxa. Open Life Sci. 2012, 7, 1116–1122. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Gaweł-Bęben, K.; Czech, K.; Strzępek-Gomółka, M.; Czop, M.; Szczepanik, M.; Lichtarska, A.; Kukula-Koch, W. Assessment of Cucurbita Spp. Peel Extracts as Potential Sources of Active Substances for Skin Care and Dermatology. Molecules 2022, 27, 7618. [Google Scholar] [CrossRef]
- Sayre, R.M.; Agin, P.P.; LeVee, G.J.; Marlowe, E. A comparison of in vivo and in vitro testing of sunscreening formulas. Photochem. Photobiol. 1979, 29, 559–566. [Google Scholar] [CrossRef]
- Mansur, J.d.S.; Breder, M.N.R.; d’Ascençäo Mansur, M.C.; Azulay, R.D. Determination of Sun Protection Factor by Spectrophotometry. An. Bras. Dermatol. 1986, 61, 121–124. [Google Scholar]
- Repetto, G.; Del Peso, A.; Zurita, J.L. Neutral Red Uptake Assay for the Estimation of Cell Viability/Cytotoxicity. Nat. Protoc. 2008, 3, 1125–1131. [Google Scholar] [CrossRef]
- Naman, M.; Masoodi, F.A.; Wani, S.M.; Ahad, T. Changes in Concentration of Pesticide Residues in Fruits and Vegetables during Household Processing. Toxicol. Rep. 2022, 9, 1419–1425. [Google Scholar] [CrossRef]
- Soliman, K.M. Changes in Concentration of Pesticide Residues in Potatoes during Washing and Home Preparation. Food Chem. Toxicol. 2001, 39, 887–891. [Google Scholar] [CrossRef] [PubMed]





| Annona cherimola | Diospyros kaki | Cydonia oblonga | Fortunella margarita | |||||
|---|---|---|---|---|---|---|---|---|
| Pulp | Peel | Pulp | Peel | Pulp | Peel | Pulp | Peel | |
| TPC [µg GAE/mL] | 357.06 *** ± 14.49 | 1596.06 ± 31.68 | 224.14 *** ± 5.36 | 778.31 ± 16.55 | 1188.28 *** ± 23.35 | 1814.94 ± 54.19 | 1299.39 ± 79.59 | 1341.06 ± 23.59 |
| TFC [µg QE/mL] | 3.38 *** ± 0.88 | 104.63 ± 3.31 | nd | nd | nd | 222.75 ± 6.50 | 81.50 *** ± 1.77 | 301.92 ± 48.16 |
| Protein [µg AE/mL] | nd | nd | nd | 4.45 ± 0.25 | 27.39 ± 3.57 | 28.65 ± 0.56 | nd | nd |
 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | RT [min] | Structure | Mexp. | Mcalc | Ions | Name | Error [ppm] | DBE | Compound Class | Ref. | Annona cherimola | |
| Pulp | Peel | |||||||||||
| 1 | 4.27 | C6H12O6 | 179.055 | 179.0561 | [M-H]− | Fructose | 7.17 | 1 | Organic compound | [10,45] | + | + |
| 2 | 4.69 | C7H12O6 | 191.0542 | 191.0561 | [M-H]− | Quinic acid | 9.95 | 2 | Organic compound | [10,45] | + | + |
| 3 | 5.47 | C5H9NO2 | 116.0711 | 116.0706 | [M-H]+ | Proline | 0.04 | 2 | Amino acids | [10,45] | + | + |
| 4 | 6.01 | C6H8O7 | 191.0189 | 191.0197 | [M-H]− | Citric acid | 4.30 | 3 | Organic compound | [10,45] | + | + |
| 5 | 8.69 | C6H6O6 | 173.0096 | 173.0092 | [M-H]− | Dehydroascorbic acid | 9.51 | 3 | Organic compound | [10,45] | + | + |
| 6 | 19.3 | C15H14O6 | 289.0695 | 289.0718 | [M-H]− | Catechin | 7.8 | 9 | Flavonoids | [10,45,46] | − | + |
| 7 | 20.42 | C16H22O10 | 373.1116 | 373.114 | [M-H]− | 3-beta-glucopyranosyloxy-2-hydroxy-1-(4-hydroxy-3-methophenyl)-propan-1-one | 6.47 | 6 | Phenolic compound | [10,45] | + | + |
| 8 | 20.11 | C14H18O9 | 329.0896 | 329.0878 | [M-H]− | Vanilic acid hexoside | −5.44 | 6 | Glucosides | [10,45] | − | + |
| 9 | 20.35 | C15H14O6 | 289.0691 | 289.0718 | [M-H]− | Epicatechin | 9.18 | 9 | Flavonoids | [10,45,46] | − | + |
| 10 | 20.52 | C14H20O8 | 315.1089 | 315.1085 | [M-H]− | Hydroxytyrosol 4-O-glucoside | −1.14 | 5 | Phenolic compound | [10,45] | + | + |
| 11 | 20.82 | C30H24O12 | 577.1392 | 577.1311 | [M-H]− | Procyanidin type B | −7.01 | 18 | Tannins | [10,45] | + | + |
| 12 | 22.93 | C32H38O20 | 741.1862 | 741.1884 | [M-H]− | Calabricoside | 2.92 | 14 | Phenolic compound | [10,45] | + | + |
| 14 | 23.84 | C27H30O16 | 609.1454 | 609.1461 | [M-H]− | Rutoside | 1.16 | 13 | Phenolic compound | [45] | − | + |
| 15 | 25.09 | C30H24O12 | 575.1206 | 575.1195 | [M-H]− | Procyanidin type A | −1.91 | 19 | Tannins | [10,45] | + | − |
| 16 | 25.19 | C21H19O11 | 447.0922 | 447.0933 | [M-H]− | Kaempferol hexoside I | 2.42 | 12 | Phenolic compound | [10,45] | + | − |
 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | RT [min] | Structure | Mexp. | Mcalc | Ions | Name | Error [ppm] | DBE | Compound Class | Ref. | Diospyros kaki | |
| Pulp | Peel | |||||||||||
| 1 | 4.51 | C6H12O7 | 195.0491 | 195.0510 | [M-H]− | Glucuronic acid | 9.83 | 1 | Organic acids | [43] | + | + |
| 2 | 4.53 | C6H10O7 | 193.0360 | 193.0354 | [M-H]− | Hexuronic acid | −3.21 | 2 | Organic acids | [47] | + | + |
| 3 | 4.56 | C5H10O6 | 165.0420 | 165.0405 | [M-H]− | Xylonic acid | −9.26 | 1 | organic acids | [43] | + | + |
| 4 | 5.77 | C4H6O5 | 133.0157 | 133.0142 | [M-H]− | Malic acid | −5.62 | 2 | Organic acids | [43] | + | + |
| 5 | 8.85 | C6H8O7 | 191.0198 | 191.0197 | [M-H]− | Citric acid | −0.38 | 3 | Organic acids | [43] | + | + |
| 6 | 14.07 | C4H6O4 | 117.0183 | 117.0193 | [M-H]− | p-coumaric acid | 8.75 | 2 | Organic acid | [48] | + | - |
| 7 | 22.8 | C15H14O6 | 289.0684 | 289.0718 | [M-H]− | Epicatechin | 11.59 | 9 | Flavonoids | [43] | + | + |
| 8 | 16.33 | C13H16O10 | 331.0702 | 331.0671 | [M-H]− | Glucogallin isomer 1 | −9.42 | 6 | Phenolic compound | [43] | + | + |
| 9 | 18.05 | C7H6O5 | 169.0155 | 169.0142 | [M-H]− | Gallic acid | −7.37 | 5 | Phenolic acid | [48] | + | + |
| 10 | 20.22 | C13H16O10 | 331.0702 | 331.0671 | [M-H]− | Glucogallin isomer 2 | −9.42 | 6 | Phenolic compound | [43] | + | + |
| 11 | 20.36 | C8H8O4 | 167.0368 | 167.035 | [M-H]− | Vanillic acid | −10.82 | 5 | Phenolic acids | [48,49] | − | + |
| 12 | 22.66 | C10H10O4 | 195.0632 | 195.0652 | [M-H]+ | Ferulic acid | 10.23 | 6 | Phenolic acids | [48,49] | + | + |
| 13 | 24.84 | C21H24O10 | 435.1319 | 435.1297 | [M-H]− | Phloridzin | −5.11 | 10 | Phenolic compound | [42] | + | − |
| 14 | 24.93 | C15H10O4 | 287.0549 | 287.055 | [M-H]+ | Kaempferol | 0.40 | 11 | Flavonoids | [50] | − | + |
| 15 | 25.0 | C8H14O4 | 173.0828 | 173.0819 | [M-H]− | Propylglutaric acid | −4.98 | 2 | Organic acids | [43] | + | + |
| 16 | 33.14 | C8H6O4 | 165.022 | 165.0193 | [M-H]+ | O-phtalic acid | −16.07 | 6 | Organic compound | [49] | + | − |
 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | RT [min] | Structure | Mexp. | Mcalc | Ions | Name | Error [ppm] | DBE | Compound Class | Ref. | Cydonia oblonga | |
| Pulp | Peel | |||||||||||
| 1 | 4.77 | C7H12O6 | 191.0565 | 191.0561 | [M-H]− | Quinic acid | −2.02 | 2 | Organic acids | [39] | + | + |
| 2 | 6.36 | C4H6O5 | 133.0149 | 133.0142 | [M-H]− | Malic acid | −4.87 | 2 | Organic acids | [39,57] | + | + |
| 3 | 6.64 | C12H18O11 | 337.0778 | 337.0776 | [M-H]− | Ascorbyl glucoside | −0.49 | 4 | Glucosides | - | + | + |
| 4 | 9.49 | C6H8O7 | 191.0208 | 191.0197 | [M-H]− | Citric acid | −5.59 | 3 | Organic acids | [39] | + | + |
| 5 | 21.31 | C16H18O9 | 353.0869 | 353.0878 | [M-H]− | Chlorogenic bxsacid | 2.56 | 8 | Phenolic acids | [52,57] | + | + |
| 6 | 21.45 | C18H16O9 | 375.0706 | 375.0722 | [M-H]− | Limocitrol | 4.14 | 11 | Flavonoids | [58] | + | + |
| 7 | 22.43 | C16H18O9 | 353.0879 | 353.0878 | [M-H]− | Neochlorogenic acid | −0.27 | 8 | Phenolic acids | [52,57] | + | + |
| 8 | 23.13 | C16H18O9 | 353.0882 | 353.0878 | [M-H]− | (Z)-chlorogenic acid | −1.11 | 8 | Phenolic acids | [52,57] | − | + |
| 9 | 23.68 | C27H30O16 | 609.1462 | 609.1461 | [M-H]− | Rutoside | −0.15 | 13 | Flavonoids | [52] | + | − |
| 10 | 23.8 | C19H30O18 | 449.1707 | 449.1664 | [M-H]− | Luteolin glucoside | −9.44 | 5 | Flavonoids | [36] | + | + |
| 11 | 24.29 | C27H30O15 | 593.1512 | 593.1509 | [M-H]− | Vicenin-2 | 0.65 | 13 | Flavonoids | [59] | − | + |
| 12 | 26.29 | C30H26O13 | 593.1301 | 593.1301 | [M-H]− | Kaempferol 3-rutinoside | −0.06 | 18 | Flavonoids | [60] | − | + |
| 13 | 24.34 | C21H20O12 | 463.0919 | 463.0882 | [M-H]− | Quercitin-3-galactoside | −7.97 | 12 | Flavonoids | [60] | + | + |
| 14 | 24.94 | C21H20O12 | 447.0933 | 447.0933 | [M-H]− | Kaempferol-3-O-galactoside | −0.48 | 12 | Flavonoids | [60] | − | + |
 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | RT [min] | Structure | Mexp. | Mcalc | Ions | Name | Error [ppm] | DBE | Compound Class | Ref. | Fortunella margarita | |
| Pulp | Peel | |||||||||||
| 1 | 24.72 | C28H34O15 | 609.1831 | 609.1884 | [M-H]− | Hesperidin | −0.99 | 12 | Flavonoids | [29,61] | + | − |
| 2 | 6.58 | C15H10O7 | 301.0353 | 301.0354 | [M-H]− | Quercetin | 0.25 | 11 | Flavonoids | [61] | − | + |
| 3 | 6.73 | C15H11O5+ | 272.0684 | 272.0684 | [M-H]− | Luteolinidin | 2.28 | 9.5 | Flavonoids | [62] | − | + |
| 4 | 10.05 | C6H8O7 | 191.0172 | 191.0197 | [M-H]− | Citric acid | 13.16 | 3 | Organic acids | [63] | + | − |
| 5 | 21.27 | C15H12O5 | 271.0626 | 271.0616 | [M-H]− | Naringenin | −5.16 | 10 | Flavonoids | [61] | + | − |
| 6 | 21.57 | C10H16O | 153.1276 | 153.1274 | [M-H]+ | Carveol | −1.37 | 3 | Monoterpenoid alcohols | [64] | + | + |
| 7 | 22.07 | C27H32O15 | 595.1625 | 595.1668 | [M-H]− | Eriocitrin | 7.29 | 12 | Flavonoids | [29] | + | + |
| 8 | 22.32 | C27H30O15 | 593.1478 | 593.1512 | [M-H]− | Vicenin | 5.71 | 13 | Flavonoids | [29] | + | + |
| 9 | 23.48 | C27H34O15 | 597.1869 | 597.1825 | [M-H]− | 3,5-di-C-beta-glucopyranosylphoretin | −7.37 | 11 | Flavonoids | [29] | + | + |
| 10 | 23.58 | C27H30O14 | 577.1535 | 577.1563 | [M-H]− | Rhoifolin | 7.35 | 11 | Flavonoids | [29] | + | + |
| 11 | 23.68 | C28H32O15 | 607.1721 | 607.1668 | [M-H]− | 3,6-di-C-glucosylacacetin | −8.64 | 3 | Flavonoids | [61] | + | + |
| 12 | 24.69 | C28H34O14 | 593.1827 | 593.1876 | [M-H]− | Poncirin | 8.21 | 12 | Flavonoids | [29] | + | − |
| 13 | 24.78 | C28H32O14 | 591.1703 | 591.1719 | [M-H]− | Acacetin-6-C-neohesperidoside (isomargaritene) | 2.75 | 13 | Flavonoids | [29] | + | + |
| 14 | 25.04 | C15H24O | 221.1905 | 221.19 | [M-H]+ | Spathulenol | −2.31 | 4 | Terpenes | [65] | + | − |
| 15 | 25.29 | C10H16O | 153.1288 | 153.1274 | [M-H]+ | p-mentha-1,5-8-ol | −9.26 | 3 | Menthane monoterpenoids | [65] | + | − |
| 16 | 25.39 | C28H32O14 | 591.1771 | 591.1719 | [M-H]− | Acetin-7-C-neohesperidoside (fortunellin) | −8.73 | 13 | Flavonoids | [29] | + | + |
| 17 | 25.94 | C28H32O15 | 607.1659 | 607.1668 | [M-H]− | 3,6-di-C-glucosylacacetin | 1.55 | 13 | Flavonoids | [61] | + | − |
| 18 | 26.24 | C28H34O14 | 593.1836 | 593.1876 | [M-H]− | Neoporcirin | 6.7 | 12 | Flavonoids | [29] | + | + |
| 19 | 26.85 | C29H50O2 | 431.3867 | 431.3843 | [M-H]+ | Vitamin E | 6.37 | 4 | Organic compounds | [62] | + | + |
| Annona cherimola | Diospyros kaki | Cydonia oblonga | Fortunella margarita | |||||
|---|---|---|---|---|---|---|---|---|
| Peel | Pulp | Peel | Pulp | Peel | Pulp | Peel | Pulp | |
| 5% | 1.29 d ± 0.06 | 0.18 a ± 0.03 | 1.39 d ± 0.02 | 0.79 b ± 0.11 | 2.87 d ± 0.22 | 1.50 c ± 0.07 | 7.48 c ± 0.40 | 6.41 c ± 0.77 |
| 2.5% | 0.80 c ± 0.05 | 0.05 a ± 0.00 | 0.76 ab ± 0.16 | 0.52 ab ± 0.03 | 1.39 c ± 0.07 | 0.75 ab ± 0.03 | 2.85 a ± 0.03 | 2.46 a ± 0.06 |
| 1% | 0.58 b ± 0.05 | 0.04 a ± 0.05 | 0.51 ac ± 0.01 | 0.25 c ± 0.01 | 0.97 b ± 0.16 | 0.41 a ± 0.02 | 1.81 ab ± 0.16 | 1.01 b ± 0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lasota, M.; Lechwar, P.; Kukula-Koch, W.; Czop, M.; Czech, K.; Gaweł-Bęben, K. Pulp or Peel? Comparative Analysis of the Phytochemical Content and Selected Cosmetic-Related Properties of Annona cherimola L., Diospyros kaki Thumb., Cydonia oblonga Mill. and Fortunella margarita Swingle Pulp and Peel Extracts. Molecules 2024, 29, 1133. https://doi.org/10.3390/molecules29051133
Lasota M, Lechwar P, Kukula-Koch W, Czop M, Czech K, Gaweł-Bęben K. Pulp or Peel? Comparative Analysis of the Phytochemical Content and Selected Cosmetic-Related Properties of Annona cherimola L., Diospyros kaki Thumb., Cydonia oblonga Mill. and Fortunella margarita Swingle Pulp and Peel Extracts. Molecules. 2024; 29(5):1133. https://doi.org/10.3390/molecules29051133
Chicago/Turabian StyleLasota, Magdalena, Paulina Lechwar, Wirginia Kukula-Koch, Marcin Czop, Karolina Czech, and Katarzyna Gaweł-Bęben. 2024. "Pulp or Peel? Comparative Analysis of the Phytochemical Content and Selected Cosmetic-Related Properties of Annona cherimola L., Diospyros kaki Thumb., Cydonia oblonga Mill. and Fortunella margarita Swingle Pulp and Peel Extracts" Molecules 29, no. 5: 1133. https://doi.org/10.3390/molecules29051133