A New Glucosyl Flavone with Inhibitory Activity of Cancer Cell Viability and Other Bioactive Constituents from the Traditional Kurdish Plant Plantago loeflingii L.
Abstract
:1. Introduction
2. Results and Discussion
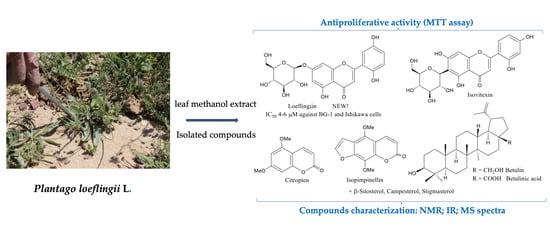
2.1. Phytochemical Investigation
2.2. Biological Activities of Isolated Compounds
3. Materials and Methods
3.1. General Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.3.1. Extraction of Plantago loeflingii Leaves
3.3.2. Chromatographic Separation of Plantago loeflingii Secondary Metabolites
3.3.3. Compounds Characterization
3.3.4. MTT Assay
Cell Cultures and Reagents
Cell Viability Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Solecki, R.S. Shanidar IV, a Neanderthal flower burial in northern Iraq. Science 1975, 190, 880–881. [Google Scholar] [CrossRef]
- Amin, H.I.M.; Ibrahim, M.F.; Hussain, F.H.S.; Sardar, A.S.; Vidari, G. Phytochemistry and ethnopharmacology of some medicinal plants used in the Kurdistan region of Iraq. Nat. Prod. Commun. 2016, 11, 291–296. [Google Scholar] [CrossRef]
- Abdullah, F.O.; Hussain, F.H.S.; Sardar, A.S.; Vita-Finzi, P.; Vidari, G. Phytochemistry and ethnopharmacology of medicinal plants used on Safeen Mountain in the Kurdistan region of Iraq. Nat. Prod. Commun. 2016, 11, 1923–1927. [Google Scholar] [CrossRef]
- Mati, E.; de Boer, H. Ethnobotany and trade of medicinal plants in the Qaysari Market, Kurdish autonomous region, Iraq. J. Ethnopharmacol. 2011, 133, 490–510. [Google Scholar] [CrossRef]
- Handa, S.S.; Rakesh, D.D.; Vasisht, K. Compendium of Medicinal and Aromatic Plants—Vol. 2-Asia; United Nations Industrial Development Organization and the International Centre for Science and High Technology (UNIDO-ICS): Trieste, Italy, 2006. [Google Scholar]
- Nahida, A. The most medicinal plants used in Iraq: Traditional knowledge. Adv. Environ. Biol. 2011, 5, 401–406. [Google Scholar]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef]
- Süntar, I. Importance of ethnopharmacological studies in drug discovery: Role of medicinal plants. Phytochem. Rev. 2020, 19, 1199–1209. [Google Scholar] [CrossRef]
- Fierascu, R.C.; Fierascu, I.; Ortan, A.; Georgiev, M.I.; Sieniawska, E. Innovative approaches for recovery of phytoconstituents from medicinal/aromatic plants and biotechnological production. Molecules 2020, 25, 309. [Google Scholar] [CrossRef] [PubMed]
- Amin, H.I.M.; Hussain, F.H.S.; Najmaldin, S.K.; Thu, Z.M.; Ibrahim, M.F.; Gilardoni, G.; Vidari, G. Phytochemistry and biological activities of Iris species growing in Iraqi Kurdistan and phenolic constituents of the traditional plant Iris postii. Molecules 2021, 26, 264. [Google Scholar] [CrossRef] [PubMed]
- Schwarzbach, A.E. Plantaginaceae. In Flowering Plants Dicotyledons. The Families and Genera of Vascular Plants; Kadereit, J.W., Ed.; Springer: Berlin/Heidelberg, Germany, 2004; Volume 7, pp. 327–329. [Google Scholar]
- Janković, T.; Gordana Zdunić, G.; Beara, I.; Balog, K.; Pljevljakušić, D.; Stešević, D.; Šavikin, K. Comparative study of some polyphenols in Plantago species. Biochem. Syst. Ecol. 2012, 42, 69–74. [Google Scholar] [CrossRef]
- Gonçalves, S.; Romano, A. The medicinal potential of plants from the genus Plantago (Plantaginaceae). Ind. Crops Prod. 2016, 83, 213–226. [Google Scholar] [CrossRef]
- Fierascu, R.C.F.; Fierascu, I.; Ortan, A.; Paunescu, A. Plantago media L.—Explored and potential applications of an underutilized plant. Plants 2021, 10, 265. [Google Scholar] [CrossRef] [PubMed]
- Abate, L.; Bachheti, R.K.; Tadesse, M.G.; Bachheti, A. Ethnobotanical uses, chemical constituents, and application of Plantago lanceolata L. J. Chem. 2022, 2022, 1532031. [Google Scholar] [CrossRef]
- Mayer, J.G. Plantain (Plantago lanceolata)—Medicinal plant of the year 2014. Z. Phytother. 2013, 34, 242–243. [Google Scholar] [CrossRef]
- Adom, M.B.; Taher, M.; Mutalabisin, M.F.; Amri, M.S.; Abdul Kudos, M.B.; Wan Sulaiman, M.W.A.; Sengupta, P.; Susanti, D. Chemical constituents and medical benefits of Plantago major. Biomed. Pharmacother. 2017, 96, 348–360. [Google Scholar] [CrossRef] [PubMed]
- Samuelsen, A.B. The traditional uses, chemical constituents and biological activities of Plantago major L. A review. J. Ethnopharmacol. 2000, 72, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Franco, E.A.N.; Sanches-Silva, A.; Ribeiro-Santos, R.; de Melo, N.R. Psyllium (Plantago ovata Forsk): From evidence of health benefits to its food application. Trends Food Sci. Technol. 2020, 96, 166–175. [Google Scholar] [CrossRef]
- Townsend, C.C.; Guest, E.R. (Eds.) Flora of Iraq, Vol. 8: Monocotyledones Excluding Graminae; Ministry of Agriculture and Agrarian Reform: Baghdad, Iraq, 1985; p. 440. [Google Scholar]
- Ghazanfar, S.A.; Edmondson, J.R. (Eds.) Flora of Iraq, Vol. 5, Part 2: Lythraceae to Campanulaceae; Ministry of Agriculture and Agrarian Reform: Baghdad, Iraq, 2013; p. 349. [Google Scholar]
- Mabry, T.J.; Markham, K.R.; Thomas, K.R. The Systematic Identification of Flavonoids; Springer: New York, NY, USA, 1970. [Google Scholar]
- Agrawal, P.K.; Rastogi, R.P. 13C NMR spectroscopy of flavonoids. Heterocycles 1981, 16, 2181–2236. [Google Scholar]
- Lin, L.-C.; Pai, Y.-F.; Tsai, T.-H. Isolation of luteolin and luteolin-7-O-glucoside from Dendranthema morifolium Ramat Tzvel and their pharmacokinetics in rats. J. Agric. Food Chem. 2015, 63, 7700–7706. [Google Scholar] [CrossRef]
- Žemlička, L.; Fodran, P.; Lukeš, V.; Vagánek, A.; Slováková, M.; Staško, A.; Dubaj, T.; Liptaj, T.; Karabín, M.; Birošová, L.; et al. Physicochemical and biological properties of luteolin-7-O-β-D-glucoside (cynaroside) isolated from Anthriscus sylvestris (L.) Hoffm. Monatsh. Chem. 2014, 145, 1307–1318. [Google Scholar] [CrossRef]
- Jeong, S.H.; Ryu, Y.B.; Curtis-Long, M.J.; Ryu, H.W.; Baek, Y.S.; Kang, J.E.; Lee, W.S.; Park, K.H. Tyrosinase inhibitory polyphenols from roots of Morus lhou. J. Agric. Food Chem. 2009, 57, 1195–1203. [Google Scholar] [CrossRef]
- Puja, S.D.; Shahriar, K.R.; Hasan, C.M.; Ahsan, M. Flavonoids from the leaves of Bridelia stipularis with in vitro antioxidant and cytotoxicity activity. Pharmacol. Pharm. 2020, 11, 137–146. [Google Scholar] [CrossRef]
- Velázquez Fiza, M.P.; Díaz Lanza, A.M.; Fernández Matellano, L. Polyphenolic compounds from Plantago lagopus L. Z. Naturforsch. 2000, 55c, 877–880. [Google Scholar] [CrossRef]
- Zhang, Y.-Y.; Guo, Y.-Z.; Onda, M.; Hashimoto, K.; Ikeya, Y.; Okada, M.; Marumo, M.Y. Four flavonoids from Scutellaria baicalensis. Phytochemistry 1994, 35, 511–514. [Google Scholar] [CrossRef]
- Miyaichi, Y.; Hanamitsu, E.; Kizu, H.; Tomimori, T. Studies on the constituents of Scutellaria species (XXII). Constituents of the roots of Scutellaria amabilis HARA. Chem. Pharm. Bull. 2006, 54, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Magozwi, D.K.; Dinala, M.; Mokwana, N.; Siwe-Noundou, X.; Krause, R.W.M.; Sonopo, M.; McGaw, L.J.; Augustyn, W.A.; Tembu, V.J. Flavonoids from the genus Euphorbia: Isolation, structure, pharmacological activities and structure-activity relationships. Pharmaceuticals 2021, 14, 428. [Google Scholar] [CrossRef]
- He, M.; Min, J.-W.; Kong, W.-L.; He, X.-H.; Li, J.-X.; Peng, B.-W. A review on the pharmacological effects of vitexin and isovitexin. Fitoterapia 2016, 115, 74–85. [Google Scholar] [CrossRef]
- Kalinová, J.P.; Vrchotová, N.; Tříska, J. Vitexin and isovitexin levels in sprouts of selected plants. J. Food Compos. Anal. 2021, 100, 103895. [Google Scholar] [CrossRef]
- Natural Product Activity and Species Source (NPASS) Database. Available online: https://bidd.group/NPASS/ (accessed on 20 November 2023).
- Lin, C.-M.; Chen, C.-T.; Lee, H.-H.; Lin, J.-K. Prevention of cellular ROS damage by isovitexin and related flavonoids. Planta Med. 2002, 68, 365–367. [Google Scholar] [CrossRef]
- Wolny, D.; Chodurek, E.; Dzierzewicz, Z. Antiproliferative effect of valproic acid and 5,7-dimethoxycoumarin against A2058 human melanoma cells. Acta Pol. Pharm. 2014, 71, 1056–1059. [Google Scholar] [PubMed]
- Cabral, C.E.; Klein, M.R. Phytosterols in the treatment of hypercholesterolemia and prevention of cardiovascular diseases. Arq. Bras. Cardiol. 2017, 109, 475–482. [Google Scholar] [CrossRef]
- Kim, T.H.; Lim, H.J.; Kim, M.S.; Lee, M.S. Dietary supplements for benign prostatic hyperplasia: An overview of systematic reviews. Maturitas 2012, 73, 180–185. [Google Scholar] [CrossRef]
- Kangsamaksin, T.; Chaithongyot, S.; Wootthichairangsan, C.; Hanchaina, R.; Tangshewinsirikul, C.; Svasti, J. Lupeol and stigmasterol suppress tumor angiogenesis and inhibit cholangiocarcinoma growth in mice via downregulation of tumor necrosis factor-α. PLoS ONE 2017, 12, e0189628. [Google Scholar] [CrossRef]
- Hordyjewska, A.; Ostapiuk, A.; Horecka, A.; Kurzepa, J. Betulin and betulinic acid: Triterpenoids derivatives with a powerful biological potential. Phytochem. Rev. 2019, 18, 929–951. [Google Scholar] [CrossRef]
- Lou, H.; Li, H.; Zhang, S.; Lu, H.; Chen, Q. A review on preparation of betulinic acid and its biological activities. Molecules 2021, 26, 5583. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Arora, S.; Singh, R. Isolation, characterization and biological activities of betulin from Acacia nilotica bark. Sci. Rep. 2022, 12, 9370. [Google Scholar] [CrossRef] [PubMed]
- Fotsis, T.; Pepper, M.S.; Aktas, E. Flavonoids, dietary-derived inhibitors of cell proliferation and in vitro angiogenesis. Cancer Res. 1997, 57, 2916–2921. [Google Scholar] [PubMed]
- Seelinger, G.; Merfort, I.; Wölfle, U.; Schempp, C.M. Anti-carcinogenic effects of the flavonoid luteolin. Molecules 2008, 13, 2628–2651. [Google Scholar] [CrossRef] [PubMed]
- Kawaii, S.; Tomono, Y.; Katase, E.; Ogawa, K.; Yano, M. Antiproliferative activity of flavonoids on several cancer cell lines. Biosci. Biotechnol. Biochem. 1999, 63, 896–899. [Google Scholar] [CrossRef] [PubMed]
- Amin, H.I.M.; Hussain, F.H.S.; Maggiolini, M.; Vidari, G. Bioactive constituents from the traditional Kurdish plant Iris persica. Nat. Prod. Commun. 2018, 13, 1127–1128. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Alley, M.C.; Scudiere, D.A.; Monks, A.; Hursey, M.L.; Czerwinski, M.J.; Fine, D.L.; Abbott, B.J.; Mayo, J.G.; Shoemaker, R.H.; Boyd, M.R. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res. 1988, 48, 589–601. [Google Scholar] [PubMed]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of Cell Viability by the MTT Assay. Cold Spring Harb. Protoc. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Riedhammer, C.; Halbritter, D.; Weissert, R. Peripheral blood mononuclear cells: Isolation, freezing, thawing, and culture. In Multiple Sclerosis. Methods in Molecular Biology; Weissert, R., Ed.; Humana Press: New York, NY, USA, 2014; Volume 1304. [Google Scholar] [CrossRef]
- Vakili Zahir, N.; Nakhjavani, M.; Hajian, P.; Shirazi, F.H.; Mirzaei, H. Evaluation of silibinin effects on the viability of HepG2 (Human hepatocellular liver carcinoma) and HUVEC (Human Umbilical Vein Endothelial) cell lines. Iran. J. Pharm. Res. 2018, 17, 261–267. [Google Scholar]
- Ghasemi, M.; Turnbull, T.; Sebastian, S.; Kempson, I. The MTT assay: Utility, limitations, pitfalls, and interpretation in bulk and single-cell analysis. Int. J. Mol. Sci. 2021, 22, 12827. [Google Scholar] [CrossRef]
- Larsson, P.; Engqvist, H.; Biermann, J.; Rönnerman, E.W.; Forssell-Aronsson, E.; Kovács, A.; Karlsson, P.; Helou, K.; Parris, T.Z. Optimization of cell viability assays to improve replicability and reproducibility of cancer drug sensitivity screens. Sci. Rep. 2020, 10, 5798. [Google Scholar] [CrossRef]
- Ahmadian, S.; Barar, J.; Saei, A.A.; Fakhree, M.A.; Omidi, Y. Cellular toxicity of nanogenomedicine in MCF-7 cell line: MTT assay. J. Vis. Exp. 2009, 26, 1191. [Google Scholar] [CrossRef]
- Kang, N.-H.; Hwang, K.-A.; Lee, H.-R.; Choi, D.-W.; Choi, K.-C. Resveratrol regulates the cell viability promoted by 17β-estradiol or bisphenol A via down-regulation of the cross-talk between estrogen receptor α and insulin growth factor-1 receptor in BG-1 ovarian cancer cells. Food Chem. Toxicol. 2013, 59, 373–379. [Google Scholar] [CrossRef]
- Tsai, C.-C.; Chang, Y.-H.; Chang, C.-C.; Cheng, Y.-M.; Ou, Y.-C.; Chien, C.-C.C.; Hsu, Y.-C. Induction of apoptosis in endometrial cancer (Ishikawa) cells by Pogostemon cablin aqueous extract (PCAE). Int. J. Mol. Sci. 2015, 16, 12424–12435. [Google Scholar] [CrossRef]
- Orengo, A.M.; Spoletini, L.; Procopio, A.; Favoni, R.E.; De Cupis, A.; Ardizzoni, A.; Castagneto, B.; Ribotta, M.; Betta, P.G.; Ferrini, S.; et al. Establishment of four new mesothelioma cell lines: Characterization by ultrastructural and immunophenotypic analysis. Eur. Respir. J. 1999, 13, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Ko, W.G.; Kang, T.H.; Lee, S.J.; Kim, Y.C.; Lee, B.H. Effects of luteolin on the inhibition of proliferation and induction ofapoptosis in human myeloid leukaemia cells. Phytother. Res. 2002, 16, 295–298. [Google Scholar] [CrossRef]
- Singh Tuli, H.; Rath, P.; Chauhan, A.; Sak, K.; Aggarwal, D.; Choudhary, R.; Sharma, U.; Vashishth, K.; Sharma, S.; Kumar, M.; et al. Luteolin, a potent anticancer compound: From chemistry to cellular interactions and synergetic perspectives. Cancers 2022, 14, 5373. [Google Scholar] [CrossRef]
- Lin, Y.; Shi, R.; Wang, X.; Shen, H.M. Luteolin, a flavonoid with potential for cancer prevention and therapy. Curr. Cancer Drug Targets 2008, 8, 634–646. [Google Scholar] [CrossRef]
- Horinaka, M.; Yoshida, T.; Shiraishi, T.; Nakata, S.; Wakada, M.; Nakanishi, R.; Nishino, H.; Matsui, H.; Sakai, T. Luteolin induces apoptosis via death receptor 5 upregulation in human malignant tumor cells. Oncogene 2005, 24, 7180–7189. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, H.E.; Kotlyar, V.; Nudelman, A. NMR Chemical shifts of common laboratory solvents as trace impurities. J. Org. Chem. 1997, 62, 7512–7515. [Google Scholar] [CrossRef] [PubMed]
- Ersöz, T.; Harput, Ü.Ş.; Saragoğlu, İ.; Çaliş, I.; Ogihara, Y. Phenolic compounds from Scutellaria pontica. Turk. J. Chem. 2002, 26, 581–588. [Google Scholar]
- Zhou, G.; Yan, R.; Wang, X.; Li, S.; Lin, J.; Liu, J.; Zhao, Z. The overlooked rotational isomerism of C-glycosyl flavonoids. Phytochem. Rev. 2019, 18, 443–461. [Google Scholar] [CrossRef]
- Davoust, D.; Massias, M.; Molho, D. 13C-NMR investigation of flavonoid C-β-D-glucosides. Detection of a conformational equilibrium. Org. Magn. Reson. 1980, 13, 218–219. [Google Scholar] [CrossRef]
- Osborne, A.G. 13C NMR Spectral studies of some methoxycoumarin derivatives. A re-assignment for citropten (limettin) and an examination of peri-proximity effects for the methyl-methoxy and methoxy-methyl couples. Magn. Reson. Chem. 1989, 27, 348–354. [Google Scholar] [CrossRef]
- Lee, H.-S.; Eun-Nam Kim, E.-N.; Jeong, G.-S. Ameliorative effect of citropten isolated from Citrus aurantifolia peel extract as a modulator of T cell and intestinal epithelial cell activity in DSS-induced colitis. Molecules 2022, 27, 4633. [Google Scholar] [CrossRef]
- Qiu, H.; Xiao, X.; Li, G. Separation and purification of furanocoumarins from Toddalia asiatica (L.) Lam. using microwave-assisted extraction coupled with high-speed counter-current chromatography. J. Sep. Sci. 2012, 35, 901–906. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, T.; Johnson, J.A.; Webster, D.; Gray, C.A. The Canadian medicinal plant Heracleum maximum contains antimycobacterial diynes and furanocoumarins. J. Ethnopharmacol. 2013, 147, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Yili, A.; Mutalipu; Aisa, H.A.; Isaev, M.I. Betulinic acid and sterols from Astragalus altaicus. Chem. Nat. Compd. 2009, 45, 592–594. [Google Scholar] [CrossRef]





| IC50 (µM) ± SD | ||||||
|---|---|---|---|---|---|---|
| Compound/Cell Line | MCF7 | IST-MES1 | BG-1 Cells | Ishikawa Cells | HUVE Cells | PBM Cells |
| loeflingiin (1) | 17 ± 2 | 13 ± 3 | 4 ± 0.3 | 6 ± 0.5 | >40 | >40 |
| isovitexin (2) | >50 | >50 | >50 | >50 | >50 | >50 |
| cisplatin | 17 ± 4 | 10 ± 3 | 12 ± 3 | 12 ± 2 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amin, H.I.M.; Amin, K.Y.M.; Armijos, C.; Hussain, F.H.S.; Jawhar, Z.H.; Caprioglio, D.; Mella, M.; Vidari, G. A New Glucosyl Flavone with Inhibitory Activity of Cancer Cell Viability and Other Bioactive Constituents from the Traditional Kurdish Plant Plantago loeflingii L. Molecules 2024, 29, 1079. https://doi.org/10.3390/molecules29051079
Amin HIM, Amin KYM, Armijos C, Hussain FHS, Jawhar ZH, Caprioglio D, Mella M, Vidari G. A New Glucosyl Flavone with Inhibitory Activity of Cancer Cell Viability and Other Bioactive Constituents from the Traditional Kurdish Plant Plantago loeflingii L. Molecules. 2024; 29(5):1079. https://doi.org/10.3390/molecules29051079
Chicago/Turabian StyleAmin, Hawraz Ibrahim M., Kamaran Younis M. Amin, Chabaco Armijos, Faiq H. S. Hussain, Zanko Hassan Jawhar, Diego Caprioglio, Mariella Mella, and Giovanni Vidari. 2024. "A New Glucosyl Flavone with Inhibitory Activity of Cancer Cell Viability and Other Bioactive Constituents from the Traditional Kurdish Plant Plantago loeflingii L." Molecules 29, no. 5: 1079. https://doi.org/10.3390/molecules29051079