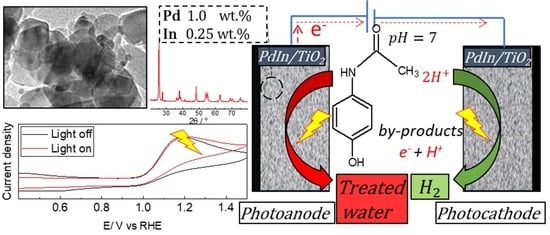
Pd:In-Doped TiO2 as a Bifunctional Catalyst for the Photoelectrochemical Oxidation of Paracetamol and Simultaneous Green Hydrogen Production
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization
2.1.1. UV-DRS Analysis
2.1.2. Physicochemical Properties
2.2. Hydrogen Evolution Reaction
2.3. Paracetamol Oxidation Reaction
3. Experimental
3.1. Catalyst Synthesis
3.2. Physicochemical Characterization
3.3. Photochemical Properties
3.4. Electrochemical Characterization
3.5. Photoelectrochemical Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feng, C.; Chen, Z.; Jing, J.; Sun, M.; Tian, J.; Lu, G.; Ma, L.; Li, X.; Hou, J. Significantly Enhanced Photocatalytic Hydrogen Production Performance of G-C3N4/CNTs/CdZnS with Carbon Nanotubes as the Electron Mediators. J. Mater. Sci. Technol. 2021, 80, 75–83. [Google Scholar] [CrossRef]
- Kim, D.; Yong, K. Boron Doping Induced Charge Transfer Switching of a C3N4/ZnO Photocatalyst from Z-Scheme to Type II to Enhance Photocatalytic Hydrogen Production. Appl. Catal. B 2021, 282, 119538. [Google Scholar] [CrossRef]
- Fernandez-Ibanez, P.; McMichael, S.; Rioja Cabanillas, A.; Alkharabsheh, S.; Tolosana Moranchel, A.; Byrne, J.A. New Trends on Photoelectrocatalysis (PEC): Nanomaterials, Wastewater Treatment and Hydrogen Generation. Curr. Opin. Chem. Eng. 2021, 34, 100725. [Google Scholar] [CrossRef]
- Truong, H.B.; Bae, S.; Cho, J.; Hur, J. Advances in Application of g-C3N4-Based Materials for Treatment of Polluted Water and Wastewater via Activation of Oxidants and Photoelectrocatalysis: A Comprehensive Review. Chemosphere 2022, 286, 131737. [Google Scholar] [CrossRef]
- Xie, L.; Wang, L.; Zhao, W.; Liu, S.; Huang, W.; Zhao, Q. WS2 Moiré Superlattices Derived from Mechanical Flexibility for Hydrogen Evolution Reaction. Nat. Commun. 2021, 12, 5070. [Google Scholar] [CrossRef]
- Yang, Z.Z.; Zhang, C.; Zeng, G.M.; Tan, X.F.; Huang, D.L.; Zhou, J.W.; Fang, Q.Z.; Yang, K.H.; Wang, H.; Wei, J.; et al. State-of-the-Art Progress in the Rational Design of Layered Double Hydroxide Based Photocatalysts for Photocatalytic and Photoelectrochemical H2/O2 Production. Coord. Chem. Rev. 2021, 446, 214103. [Google Scholar] [CrossRef]
- Wang, L.; Xie, L.; Zhao, W.; Liu, S.; Zhao, Q. Oxygen-Facilitated Dynamic Active-Site Generation on Strained MoS2 during Photo-Catalytic Hydrogen Evolution. Chem. Eng. J. 2021, 405, 127028. [Google Scholar] [CrossRef]
- Chen, Z.; Li, S.; Peng, Y.; Hu, C. Tailoring Aromatic Ring-Terminated Edges of g-C3N4 nanosheets for Efficient Photocatalytic Hydrogen Evolution with Simultaneous Antibiotic Removal. Catal. Sci. Technol. 2020, 10, 5470–5479. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, G.; Kumari, A.; Guo, C.; Naushad, M.; Vo, D.V.N.; Iqbal, J.; Stadler, F.J. Construction of Dual Z-Scheme g-C3N4/Bi4Ti3O12/Bi4O5I2 Heterojunction for Visible and Solar Powered Coupled Photocatalytic Antibiotic Degradation and Hydrogen Production: Boosting via I−/I3− and Bi3+/Bi5+ Redox Mediators. Appl. Catal. B 2021, 284, 119808. [Google Scholar] [CrossRef]
- Sharma, G.; Dionysiou, D.D.; Sharma, S.; Kumar, A.; Al-Muhtaseb, A.H.; Naushad, M.; Stadler, F.J. Highly Efficient Sr/Ce/Activated Carbon Bimetallic Nanocomposite for Photoinduced Degradation of Rhodamine B. Catal. Today 2019, 335, 437–451. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, L.; Liu, C.; Luo, J.; Crittenden, J.; Liu, X.; Cai, T.; Yuan, J.; Pei, Y.; Liu, Y. Photocatalytic Wastewater Purification with Simultaneous Hydrogen Production Using MoS2 QD-Decorated Hierarchical Assembly of ZnIn2S4 on Reduced Graphene Oxide Photocatalyst. Water Res. 2017, 121, 11–19. [Google Scholar] [CrossRef]
- Meng, S.; Chen, C.; Gu, X.; Wu, H.; Meng, Q.; Zhang, J.; Chen, S.; Fu, X.; Liu, D.; Lei, W. Efficient Photocatalytic H2 Evolution, CO2 Reduction and N2 Fixation Coupled with Organic Synthesis by Cocatalyst and Vacancies Engineering. Appl. Catal. B 2021, 285, 119789. [Google Scholar] [CrossRef]
- Wu, K.; Shang, Y.; Li, H.; Wu, P.; Li, S.; Ye, H.; Jian, F.; Zhu, J.; Yang, D.; Li, B.; et al. Synthesis and Hydrogen Production Performance of MoP/a-TiO2/Co-ZnIn2S4 Flower-like Composite Photocatalysts. Molecules 2023, 28, 4350. [Google Scholar] [CrossRef]
- Long, B.; He, H.; Yu, Y.; Cai, W.; Gu, Q.; Yang, J.; Meng, S. Bifunctional Hot Water Vapor Template-Mediated Synthesis of Nanostructured Polymeric Carbon Nitride for Efficient Hydrogen Evolution. Molecules 2023, 28, 4862. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, Z.; Meng, S.; Wang, Y.; Li, D. Regulating Charge Transfer over 3D Au/ZnO Hybrid Inverse Opal toward Efficiently Photocatalytic Degradation of Bisphenol A and Photoelectrochemical Water Splitting. Chem. Eng. J. 2020, 393, 124676. [Google Scholar] [CrossRef]
- Priyadarshini, M.; Sathe, S.M.; Ghangrekar, M.M. Hybrid Treatment Solutions for Removal of Micropollutant from Wastewaters. In Microconstituents in the Environment; Wiley: Hoboken, NJ, USA, 2023; pp. 491–512. [Google Scholar]
- Babuji, P.; Thirumalaisamy, S.; Duraisamy, K.; Periyasamy, G. Human Health Risks Due to Exposure to Water Pollution: A Review. Water 2023, 15, 2532. [Google Scholar] [CrossRef]
- Inostroza, P.A.; Carmona, E.; Arrhenius, Å.; Krauss, M.; Brack, W.; Backhaus, T. Target Screening of Chemicals of Emerging Concern (CECs) in Surface Waters of the Swedish West Coast. Data 2023, 8, 93. [Google Scholar] [CrossRef]
- Talaat, A.; Elgendy, Y.A.; Mohamed, H.F.; Saed, N.M.; Abd Elrouf, N.A.; Elgendy, H.A.; Elbalakousy, H.H.; Elmezaien, M.S.; Sayed, Y.M.; Hekal, Y.E.; et al. Ameliorative Effects of Frankincense Oil on Rats Treated with a Minimum Toxic Dose of Paracetamol. J. Med. Life Sci. 2023, 5, 155–175. [Google Scholar] [CrossRef]
- Zhang, C.; Li, T.; Zhang, J.; Yan, S.; Qin, C. Degradation of P-Nitrophenol Using a Ferrous-Tripolyphosphate Complex in the Presence of Oxygen: The Key Role of Superoxide Radicals. Appl. Catal. B 2019, 259, 118030. [Google Scholar] [CrossRef]
- Hashimoto, K.; Irie, H.; Fujishima, A. TiO2 Photocatalysis: A Historical Overview and Future Prospects. Jpn. J. Appl. Phys. 2005, 44, 8269–8285. [Google Scholar] [CrossRef]
- Daghrir, R.; Drogui, P.; Robert, D. Modified TiO2 for Environmental Photocatalytic Applications: A Review. Ind. Eng. Chem. Res. 2013, 52, 3581–3599. [Google Scholar] [CrossRef]
- Ni, M.; Leung, M.K.H.; Leung, D.Y.C.; Sumathy, K. A Review and Recent Developments in Photocatalytic Water-Splitting Using TiO2 for Hydrogen Production. Renew. Sustain. Energy Rev. 2007, 11, 401–425. [Google Scholar] [CrossRef]
- Yilmaz, P.; Lacerda, A.M.; Larrosa, I.; Dunn, S. Photoelectrocatalysis of Rhodamine B and Solar Hydrogen Production by TiO2 and Pd/TiO2 Catalyst Systems. Electrochim. Acta 2017, 231, 641–649. [Google Scholar] [CrossRef]
- Marchesini, F.A.; Mendow, G.; Picard, N.P.; Zoppas, F.M.; Aghemo, V.S.; Gutierrez, L.B.; Querini, C.A.; Miró, E.E. PdIn Catalysts in a Continuous Fixed Bed Reactor for the Nitrate Removal from Groundwater. Int. J. Chem. React. Eng. 2019, 17, 1–17. [Google Scholar] [CrossRef]
- Marchesini, F.A.; Gutierrez, L.B.; Querini, C.A.; Miró, E.E. Pt,In and Pd,In Catalysts for the Hydrogenation of Nitrates and Nitrites in Water. FTIR Characterization and Reaction Studies. Chem. Eng. J. 2010, 159, 203–211. [Google Scholar] [CrossRef]
- Sarkar, S.; Peter, S.C. An Overview on Pd-Based Electrocatalysts for the Hydrogen Evolution Reaction. Inorg. Chem. Front. 2018, 5, 2060–2080. [Google Scholar] [CrossRef]
- Merino-Garcia, I.; García, G.; Hernández, I.; Albo, J. An Optofluidic Planar Microreactor with Photoactive Cu2O/Mo2C/TiO2 heterostructures for Enhanced Visible Light-Driven CO2 conversion to Methanol. J. CO2 Util. 2023, 67, 102340. [Google Scholar] [CrossRef]
- Yang, Y.-K.; Jiao, C.-Q.; Meng, Y.-S.; Yao, N.-T.; Jiang, W.-J.; Liu, T. Substituent Effect on Metal-to-Metal Charge Transfer Behavior of Cyanide-Bridged {Fe2Co2} Square. Inorg. Chem. Commun. 2021, 130, 108712. [Google Scholar] [CrossRef]
- Chavez Zavaleta, R.; Fomichev, S.; Khaliullin, G.; Berciu, M. Effects of Reduced Dimensionality, Crystal Field, Electron-Lattice Coupling, and Strain on the Ground State of a Rare-Earth Nickelate Monolayer. Phys. Rev. B 2021, 104, 205111. [Google Scholar] [CrossRef]
- Dehury, T.; Kumar, S.; Rath, C. Structural Transformation and Bandgap Engineering by Doping Pr in HfO2 Nanoparticles. Mater. Lett. 2021, 302, 130413. [Google Scholar] [CrossRef]
- Bazan-Aguilar, A.; García, G.; Pastor, E.; Rodríguez, J.L.; Baena-Moncada, A.M. In-Situ Spectroelectrochemical Study of Highly Active Ni-Based Foam Electrocatalysts for Hydrogen Evolution Reaction. Appl. Catal. B 2023, 336, 122930. [Google Scholar] [CrossRef]
- López, M.; Exner, K.S.; Viñes, F.; Illas, F. Theoretical Study of the Mechanism of the Hydrogen Evolution Reaction on the V2C MXene: Thermodynamic and Kinetic Aspects. J. Catal. 2023, 421, 252–263. [Google Scholar] [CrossRef]
- Nematollahi, D.; Shayani-Jam, H.; Alimoradi, M.; Niroomand, S. Electrochemical Oxidation of Acetaminophen in Aqueous Solutions: Kinetic Evaluation of Hydrolysis, Hydroxylation and Dimerization Processes. Electrochim. Acta 2009, 54, 7407–7415. [Google Scholar] [CrossRef]
- Chenlo, F.; Moreira, R.; Pereira, G.; Vázquez, M.J. Viscosity of Binary and Ternary Aqueous Systems of NaH2PO4, Na2HPO4, Na3PO4, KH2PO4, K2HPO4, and K3PO4. J. Chem. Eng. Data 1996, 41, 906–909. [Google Scholar] [CrossRef]
- Pournaghi-Azar, M.H.; Kheradmandi, S.; Saadatirad, A. Simultaneous Voltammetry of Paracetamol, Ascorbic Acid, and Codeine on a Palladium-Plated Aluminum Electrode: Oxidation Pathway and Kinetics. J. Solid State Electrochem. 2010, 14, 1689–1695. [Google Scholar] [CrossRef]
- Zoubir, J.; Bakas, I.; Assabbane, A. A Simple Platform for the Electro-Catalytic Detection of the Dimetridazole Using an Electrochemical Sensor Fabricated by Electro-Deposition of Ag on Carbon Graphite: Application: Orange Juice, Tomato Juice and Tap Water. Heliyon 2021, 7, e07542. [Google Scholar] [CrossRef]









| Photocatalyst | EDLCA (mF/cm2) | EDLCC (mF/cm2) | Electroactive Surface Area (cm2) |
|---|---|---|---|
| GC | 0.00003 | −0.00003 | 0.6 |
| TiO2 | 0.00011 | −0.0001 | 2.5 |
| PdIn/TiO2 | 0.0004 | −0.0004 | 8.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sacco, N.; Iguini, A.; Gamba, I.; Marchesini, F.A.; García, G. Pd:In-Doped TiO2 as a Bifunctional Catalyst for the Photoelectrochemical Oxidation of Paracetamol and Simultaneous Green Hydrogen Production. Molecules 2024, 29, 1073. https://doi.org/10.3390/molecules29051073
Sacco N, Iguini A, Gamba I, Marchesini FA, García G. Pd:In-Doped TiO2 as a Bifunctional Catalyst for the Photoelectrochemical Oxidation of Paracetamol and Simultaneous Green Hydrogen Production. Molecules. 2024; 29(5):1073. https://doi.org/10.3390/molecules29051073
Chicago/Turabian StyleSacco, Nicolás, Alexander Iguini, Ilaria Gamba, Fernanda Albana Marchesini, and Gonzalo García. 2024. "Pd:In-Doped TiO2 as a Bifunctional Catalyst for the Photoelectrochemical Oxidation of Paracetamol and Simultaneous Green Hydrogen Production" Molecules 29, no. 5: 1073. https://doi.org/10.3390/molecules29051073