Sensitive Electrochemical Detection of Carcinoembryonic Antigen Based on Biofunctionalized Nanochannel Modified Carbonaceous Electrode
Abstract
:1. Introduction
2. Results and Discussion
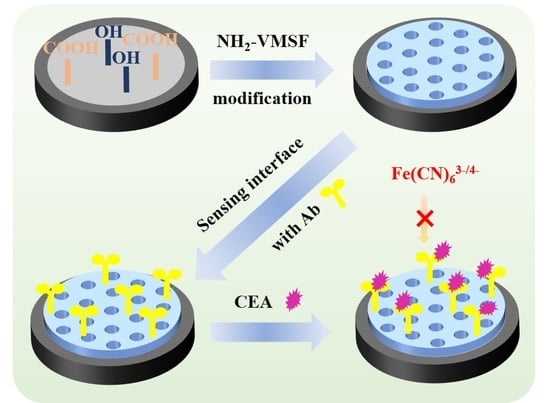
2.1. Strategy for Fabrication of Immunosensor by Growing VMSF on p-GCE
2.2. Characterization of p-GCE and NH2-VMSF/p-GCE
2.3. Morphology Characterization of NH2-VMSF
2.4. The Feasibility for the Construction of Immunosensor
2.5. Optimization of Immunosensor Construction Conditions
2.6. Electrochemical Detection of CEA
2.7. Selectivity, Reproducibility, and Stability of the Immunosensor
2.8. Real Sample Analysis
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Characterization and Instrumentation
3.3. Preparation of NH2-VMSF/p-GCE Electrode
3.4. Fabrication of the Immunosensor
3.5. Electrochemical Detection of CEA
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, C.; Holman, J.B.; Shi, Z.; Qiu, B.; Ding, W. On-chip modeling of tumor evolution: Advances, challenges and opportunities. Mater. Today Bio 2023, 21, 100724. [Google Scholar] [CrossRef]
- Mohammadpour, Z.; Majidzadeh-A, K. Applications of two-dimensional nanomaterials in breast cancer theranostics. ACS Biomater. Sci. Eng. 2020, 6, 1852–1873. [Google Scholar] [CrossRef] [PubMed]
- Jing, L.; Xie, C.; Li, Q.; Yang, M.; Li, S.; Li, H.; Xia, F. Electrochemical biosensors for the analysis of breast cancer biomarkers: From design to application. Anal. Chem. 2021, 94, 269–296. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Huang, G.; Wei, W.; Liu, J. Molecular imaging of renal cell carcinoma in precision medicine. Mol. Pharm. 2022, 19, 3457–3470. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chen, L.; Yang, K.; Wang, L.; Han, B.; Sun, S.; Wen, J. An electrochemiluminescence immunosensor based on functionalized metal organic layers as emitters for sensitive detection of carcinoembryonic antigen. Sens. Actuators B Chem. 2023, 393, 134317. [Google Scholar] [CrossRef]
- Song, Z.; Suo, Y.; Duan, S.; Zhang, S.; Liu, L.; Chen, B.; Cheng, Z. NIR-II fluorescent nanoprobe-labeled lateral flow biosensing platform: A high-performance point-of-care testing for carcinoembryonic antigen. Biosens. Bioelectron. 2023, 224, 115063. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.; Wu, L.; Wen, Y.; Tang, X.; Huang, Z.; Zhao, L. Photoelectrochemical immunosensor for carcinoembryonic antigen detection-an attempt for early cancer screening. Biosens. Bioelectron. 2023, 220, 114918. [Google Scholar] [CrossRef] [PubMed]
- Shao, K.; Xie, W.; Ling, Q.; Wang, T.; Zhang, H.; Teng, Y.; Ye, S.; Yuan, X.; Pan, Z. Dumbbell-like upconversion nanoparticles synthesized by controlled epitaxial growth for light-heat-color tri-modal sensing of carcinoembryonic antigen. Biosens. Bioelectron. 2023, 228, 115186. [Google Scholar] [CrossRef] [PubMed]
- Luong, J.H.T.; Vashist, S.K. Immunosensing procedures for carcinoembryonic antigen using graphene and nanocomposites. Biosens. Bioelectron. 2017, 89, 293–304. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, L.; Pei, J.; Tian, Y.; Liu, J. A reagentless electrochemical immunosensor for sensitive detection of carcinoembryonic antigen based on the interface with redox probe-modified electron transfer wires and effectively immobilized antibody. Front. Chem. 2022, 10, 939736. [Google Scholar] [CrossRef]
- Zhou, X.; Han, Q.; Zhou, J.; Liu, C.; Liu, J. Reagentless electrochemical detection of tumor biomarker based on stable confinement of electrochemical probe in bipolar silica nanochannel film. Nanomaterials 2023, 13, 1645. [Google Scholar] [CrossRef]
- Ghorbani, F.; Abbaszadeh, H.; Dolatabadi, J.E.N.; Aghebati-Maleki, L.; Yousefi, M. Application of various optical and electrochemical aptasensors for detection of human prostate specific antigen: A review. Biosens. Bioelectron. 2019, 142, 111484. [Google Scholar] [CrossRef]
- Wen, W.; Yan, X.; Zhu, C.; Du, D.; Lin, Y. Recent advances in electrochemical immunosensors. Anal. Chem. 2017, 89, 138–156. [Google Scholar] [CrossRef] [PubMed]
- Chikkaveeraiah, B.V.; Bhirde, A.A.; Morgan, N.Y.; Eden, H.S.; Chen, X. Electrochemical immunosensors for detection of cancer protein biomarkers. ACS Nano 2012, 6, 6546–6561. [Google Scholar] [CrossRef] [PubMed]
- Xue, R.; Liu, Y.S.; Huang, S.L.; Yang, G.Y. Recent progress of covalent organic frameworks applied in electrochemical sensors. ACS Sens. 2023, 8, 2124–2148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xue, W.; Xiao, K.; Visvanathan, C.; Tang, J.; Li, L. Selection and optimization of carbon-based electrode materials for flow-electrode capacitive deionization. Sep. Purif. Technol. 2023, 315, 123649. [Google Scholar] [CrossRef]
- Khumngern, S.; Choosang, J.; Kanatharana, P.; Thavarungkul, P.; Numnuam, A. Voltammetric sensor for an anti-cancer drug cisplatin based on bismuth nanoparticles/graphene modified glassy carbon electrode. Talanta 2024, 267, 125147. [Google Scholar] [CrossRef]
- Gong, J.; Tang, H.; Wang, M.; Lin, X.; Wang, K.; Liu, J. Novel three-dimensional graphene nanomesh prepared by facile electro-etching for improved electroanalytical performance for small biomolecules. Mater. Des. 2022, 215, 110506. [Google Scholar] [CrossRef]
- Zhu, D.; Tan, Y.; Zheng, L.; Lao, J.; Liu, J.; Yu, J.; Chen, P. Microneedle-coupled epidermal sensors for in-situ-multiplexed ion detection in interstitial fluids. ACS Appl. Mater. Interfaces 2023, 15, 14146–14154. [Google Scholar] [CrossRef]
- Phan, T.T.N.; Dinh, T.T.M.; Nguyen, M.D.; Li, D.; Phan, C.N.; Pham, T.K.; Nguyen, C.T.; Pham, T.H. Hierarchically structured LaFeO3 with hollow core and porous shell as efficient sensing material for ethanol detection. Sens. Actuators B Chem. 2022, 354, 131195. [Google Scholar] [CrossRef]
- Tang, Y.; Yang, K.; Hua, Z.; Yin, F.; Yuan, W. A new sensing material design based on chemically passivated phosphorene/porous two-dimensional polymer: Highly sensitive and selective detection of NO2. Sens. Actuators B Chem. 2021, 329, 129233. [Google Scholar] [CrossRef]
- Zhao, J.; Duan, W.; Liu, X.; Xi, F.; Wu, J. Microneedle patch integrated with porous silicon confined dual nanozymes for synergistic and hyperthermia-enhanced nanocatalytic ferroptosis treatment of melanoma. Adv. Funct. Mater. 2023, 33, 2308183. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, S.; Li, Y.; Liu, J. Facile synthesis of iron and nitrogen co-doped carbon dot nanozyme as highly efficient peroxidase mimics for visualized detection of metabolites. Molecules 2023, 28, 6064. [Google Scholar] [CrossRef]
- Vlassiouk, I.; Siwy, Z.S. Nanofluidic diode. Nano Lett. 2007, 7, 552–556. [Google Scholar] [CrossRef]
- Vlassiouk, I.; Kozel, T.R.; Siwy, Z.S. Biosensing with nanofluidic diodes. J. Am. Chem. Soc. 2009, 131, 8211–8220. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Yao, L.; Chen, K.; Su, B. Silica nanochannel membranes for electrochemical analysis and molecular sieving: A comprehensive review. Crit. Rev. Anal. Chem. 2019, 50, 424–444. [Google Scholar] [CrossRef]
- Gong, J.; Zhang, T.; Luo, T.; Luo, X.; Yan, F.; Tang, W.; Liu, J. Bipolar silica nanochannel array confined electrochemiluminescence for ultrasensitive detection of SARS-CoV-2 antibody. Biosens. Bioelectron. 2022, 215, 114563. [Google Scholar] [CrossRef]
- Huang, J.; Xu, S.; Yan, F.; Liu, J. Electrochemiluminescence enzyme biosensors for ultrasensitive determination of glucose using glucose dehydrogenase immobilized on vertical silica nanochannels. Sens. Actuators B Chem. 2024, 402, 135119. [Google Scholar] [CrossRef]
- Zhou, H.; Ma, X.; Sailjoi, A.; Zou, Y.; Lin, X.; Yan, F.; Su, B.; Liu, J. Vertical silica nanochannels supported by nanocarbon composite for simultaneous detection of serotonin and melatonin in biological fluids. Sens. Actuators B Chem. 2022, 353, 131101. [Google Scholar] [CrossRef]
- Walcarius, A. Electroinduced surfactant self-assembly driven to vertical growth of oriented mesoporous films. Acc. Chem. Res. 2021, 54, 3563–3575. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, X.; Yan, F.; Lin, J. N-doped graphene quantum dots confined within silica nanochannels for enhanced electrochemical detection of doxorubicin. Molecules 2023, 28, 6443. [Google Scholar] [CrossRef]
- Zou, Y.; Zhou, X.; Xie, L.; Tang, H.; Yan, F. Vertically-ordered mesoporous silica films grown on boron nitride-graphene composite modified electrodes for rapid and sensitive detection of carbendazim in real samples. Front. Chem. 2022, 10, 939510. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, T.; Zhou, H.; Yan, F.; Liu, Y. Silica nanochannels boosting Ru(bpy)32+-mediated electrochemical sensor for the detection of guanine in beer and pharmaceutical samples. Front. Nutr. 2022, 9, 987442. [Google Scholar] [CrossRef]
- Wang, K.; Yang, L.; Huang, H.; Lv, N.; Liu, J.; Liu, Y. Nanochannel array on electrochemically polarized screen printed carbon electrode for rapid and sensitive electrochemical determination of clozapine in human whole blood. Molecules 2022, 27, 2739. [Google Scholar] [CrossRef]
- Serrano, M.B.; Despas, C.; Herzog, G.; Walcarius, A. Mesoporous silica thin films for molecular sieving and electrode surface protection against biofouling. Electrochem. Commun. 2015, 52, 34–36. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, T.; Dong, G.; Zhu, S.; Yan, F.; Liu, J. Direct and sensitive electrochemical detection of bisphenol A in complex environmental samples using a simple and convenient nanochannel-modified electrode. Front. Chem. 2022, 10, 900282. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Zhang, T.; Wang, M.; Yan, F.; Liu, J. Disposable electrochemical sensors for highly sensitive detection of chlorpromazine in human whole blood based on the silica nanochannel array modified screen-printed carbon electrode. Molecules 2022, 27, 8200. [Google Scholar] [CrossRef]
- Luo, X.; Zhang, T.; Tang, H.; Liu, J. Novel electrochemical and electrochemiluminescence dual-modality sensing platform for sensitive determination of antimicrobial peptides based on probe encapsulated liposome and nanochannel array electrode. Front. Nutr. 2022, 9, 962736. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Yang, L.; Yan, F.; Wang, K. Vertically-ordered mesoporous silica film based electrochemical aptasensor for highly sensitive detection of alpha-fetoprotein in human serum. Biosensors 2023, 13, 628. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, T.; Zheng, Y.; Liu, J. Dual-mode sensing platform for cancer antigen 15-3 determination based on a silica nanochannel array using electrochemiluminescence and electrochemistry. Biosensors 2023, 13, 317. [Google Scholar] [CrossRef]
- Gong, J.; Zhang, T.; Chen, P.; Yan, F.; Liu, J. Bipolar silica nanochannel array for dual-mode electrochemiluminescence and electrochemical immunosensing platform. Sens. Actuators B Chem. 2022, 368, 132086. [Google Scholar] [CrossRef]
- Zhang, T.; Xu, S.; Lin, X.; Liu, J.; Wang, K. Label-free electrochemical aptasensor based on the vertically-aligned mesoporous silica films for determination of aflatoxin B1. Biosensors 2023, 13, 661. [Google Scholar] [CrossRef]
- Chen, H.; Huang, J.; Zhang, R.; Yan, F. Dual-mode electrochemiluminescence and electrochemical sensor for alpha-fetoprotein detection in human serum based on vertically ordered mesoporous silica films. Front. Chem. 2022, 10, 1023998. [Google Scholar] [CrossRef]
- Walcarius, A.; Sibottier, E.; Etienne, M.; Ghanbaja, J. Electrochemically assisted self-assembly of mesoporous silica thin films. Nat. Mater. 2007, 6, 602–608. [Google Scholar] [CrossRef]
- Xia, N.; Huang, Y.; Cui, Z.; Liu, S.; Deng, D.; Liu, L.; Wang, J. Impedimetric biosensor for assay of caspase-3 activity and evaluation of cell apoptosis using self-assembled biotin-phenylalanine network as signal enhancer. Sens. Actuators B Chem. 2020, 320, 128436. [Google Scholar] [CrossRef]
- Xuan, L.; Liao, W.; Wang, M.; Zhou, H.; Ding, Y.; Yan, F.; Liu, J.; Tang, H.; Xi, F. Integration of vertically-ordered mesoporous silica-nanochannel film with electro-activated glassy carbon electrode for improved electroanalysis in complex samples. Talanta 2021, 225, 122066. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Deng, D.; Wu, D.; Hou, W.; Wang, L.; Li, N.; Sun, Z. Duplex-specific nuclease-based electrochemical biosensor for the detection of microRNAs by conversion of homogeneous assay into surface-tethered electrochemical analysis. Anal. Chim. Acta 2021, 1149, 338199. [Google Scholar] [CrossRef] [PubMed]
- Engstrom, R.C.; Strasser, V.A. Characterization of electrochemically pretreated glassy carbon electrodes. Anal. Chem. 1984, 56, 136–141. [Google Scholar] [CrossRef]
- Santhiago, M.; Maroneze, C.M.; Silva, C.C.C.; Camargo, M.N.L.; Kubota, L.T. Electrochemical oxidation of glassy carbon provides similar electrochemicalresponse as graphene oxide prepared by tour or hummers routes. ChemElectroChem 2015, 2, 761. [Google Scholar] [CrossRef]
- Alam, A.U.; Deen, M.J. Bisphenol A electrochemical sensor using graphene oxide and beta-cyclodextrin-functionalized multi-walled carbon nanotubes. Anal. Chem. 2020, 92, 5532–5539. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Bao, J.; Huo, D.; Zeng, Y.; Wang, X.; Samalo, M.; Zhao, J.; Zhang, S.; Shen, C.; Hou, C. Au doped poly-thionine and poly-m-Cresol purple: Synthesis and their application in simultaneously electrochemical detection of two lung cancer markers CEA and CYFRA21-1. Talanta 2021, 224, 121816. [Google Scholar] [CrossRef]
- Zhao, L.; Li, C.; Qi, H.; Gao, Q.; Zhang, C. Electrochemical lectin-based biosensor array for detection and discrimination of carcinoembryonic antigen using dual amplification of gold nanoparticles and horseradish peroxidase. Sens. Actuators B Chem. 2016, 235, 575–582. [Google Scholar] [CrossRef]
- Akbari Nakhjavani, S.; Khalilzadeh, B.; Afsharan, H.; Hosseini, N.; Ghahremani, M.H.; Carrara, S.; Tasoglu, S.; Omidi, Y. Electrochemiluminescent immunosensor for detection of carcinoembryonic antigen using luminol-coated silver nanoparticles. Microchim. Acta 2023, 190, 77. [Google Scholar] [CrossRef]
- Sun, Y.; Hou, Y.; Cao, T.; Luo, C.; Wei, Q. A chemiluminescence sensor for the detection of α-fetoprotein and carcinoembryonic antigen based on dual-aptamer functionalized magnetic silicon composite. Anal. Chem. 2023, 95, 7387–7395. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Y.; Deng, D.; Luo, L.; He, H.; Wang, Z. Water-dispersible graphene/amphiphilic pyrene derivative nanocomposite: High AuNPs loading capacity for CEA electrochemical immunosensing. Sens. Actuators B Chem. 2017, 248, 966–972. [Google Scholar] [CrossRef]
- Direksilp, C.; Parinyanitikul, N.; Ariyasajjamongkol, N.; Sirivat, A. A label-free electrochemical immunosensor based on 11-mercaptoundecanoic acid grafted chitosan and poly(N-methylaniline) for the detection of carcinoembryonic antigen. Bioelectrochemistry 2023, 152, 108446. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, L.; Ai, Y.; Liu, Y.; Sun, H.; Liang, Q. Self-polymerized dopamine-decorated Au NPs and coordinated with Fe-MOF as a dual binding sites and dual signal-amplifying electrochemical aptasensor for the detection of CEA. ACS Appl. Mater. Interfaces 2020, 12, 5500–5510. [Google Scholar] [CrossRef]
- Ma, K.; Yang, L.; Liu, J.; Liu, J. Electrochemical sensor nanoarchitectonics for sensitive detection of uric acid in human whole blood based on screen-printed carbon electrode equipped with vertically-ordered mesoporous silica-nanochannel film. Nanomaterials 2022, 12, 1157. [Google Scholar] [CrossRef]
- Li, D.; Xu, S.; Jin, H.; Wang, J.; Yan, F. Copper nanoparticles confined in a silica nanochannel film for the electrochemical detection of nitrate ions in water samples. Molecules 2023, 28, 7515. [Google Scholar] [CrossRef]
- Guo, Q.; Fan, X.; Yan, F.; Wang, Y. Highly sensitive electrochemical immunosensor based on electrodeposited platinum nanostructures confined in silica nanochannels for the detection of the carcinoembryonic antigen. Front. Chem. 2023, 11, 1271556. [Google Scholar] [CrossRef]







| Sample | Spiked b (ng mL−1) | Found (ng mL−1) | RSD (%, n = 3) | Recovery (%) |
|---|---|---|---|---|
| Serum a | 0.0100 | 0.00963 | 1.3 | 96.3 |
| 0.100 | 0.0978 | 2.8 | 97.8 | |
| 1.00 | 1.01 | 2.9 | 101 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Wang, H.; Xi, F.; Lu, C. Sensitive Electrochemical Detection of Carcinoembryonic Antigen Based on Biofunctionalized Nanochannel Modified Carbonaceous Electrode. Molecules 2024, 29, 858. https://doi.org/10.3390/molecules29040858
Zhou Y, Wang H, Xi F, Lu C. Sensitive Electrochemical Detection of Carcinoembryonic Antigen Based on Biofunctionalized Nanochannel Modified Carbonaceous Electrode. Molecules. 2024; 29(4):858. https://doi.org/10.3390/molecules29040858
Chicago/Turabian StyleZhou, Yucheng, Hongxin Wang, Fengna Xi, and Chao Lu. 2024. "Sensitive Electrochemical Detection of Carcinoembryonic Antigen Based on Biofunctionalized Nanochannel Modified Carbonaceous Electrode" Molecules 29, no. 4: 858. https://doi.org/10.3390/molecules29040858