Toxicity Effects of Polystyrene Nanoplastics with Different Sizes on Freshwater Microalgae Chlorella vulgaris
Abstract
:1. Introduction
2. Results and Discussion
2.1. PS-NPs Characterization
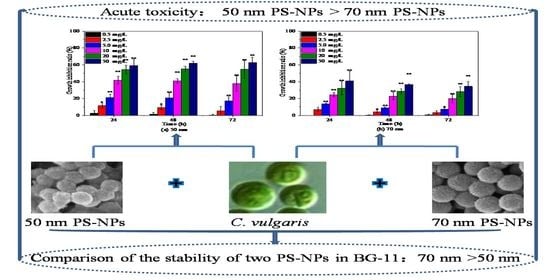
2.2. Effects of PS-NPs on C. vulgaris Growth
2.3. Effects of PS-NPs on Algae Photosynthesis
2.4. Effects of PS-NPs on Intracellular Oxidative Stress and Esterase Activities
2.5. Interactions between PS-NPs and C. vulgaris: The Variations of Algae Surface Macromolecular Composition and Cellular Structure
3. Materials and Methods
3.1. PS-NPs Characterization and Stability Testing
3.2. Algal Culture and Growth Inhibition Test
3.3. Quantification of Chlorophyll a
3.4. Oxidative Stress Analysis
3.5. Esterase Activity Analysis
3.6. Characterization of the Interactions between PS-NPs and Algae by FTIR
3.7. Analysis of Cell Morphology
3.8. Membrane Damage Analysis by Fluorescent Microscopy
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Ter Halle, A.; Jeanneau, L.; Martignac, M.; Jardé, E.; Pedrono, B.; Brach, L.; Gigault, J. Nanoplastic in the North Atlantic subtropical gyre. Environ. Sci. Technol. 2017, 51, 13689–13697. [Google Scholar] [CrossRef] [PubMed]
- Chao, F.; Zheng, R.; Zhang, Y.; Hong, F.; Mu, J.; Chen, M.; Song, P.; Lin, L.; Lin, H.; Le, F.; et al. Microplastic contamination in benthic organisms from the Arctic and sub-Arctic regions. Chemosphere 2018, 209, 298–306. [Google Scholar]
- Reed, S.; Clark, M.; Thompson, R.; Hughes, K.A. Microplastics in marine sediments near Rothera Research Station, Antarctica. Mar. Pollut. Bull. 2018, 133, 460–463. [Google Scholar] [CrossRef] [PubMed]
- Borrelle, S.B.; Ringma, J.; LavenderLaw, K.; Monnahan, C.C.; Lebreto, L.; McGivern, A.; Murphy, E.; Jambeck, J.; Leonard, G.H.; Hilleary, M.A.; et al. Predicted growth in plastic waste exceeds efforts to mitigate plastic pollution. Science 2020, 369, 1515–1518. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Gao, P.; Li, H.; Huang, J.; Zhang, W. Mechanism of the inhibition and detoxification effects of the interaction between nanoplastics and microalgae Chlorella pyrenoidosa. Sci. Total Environ. 2021, 783, 146919. [Google Scholar] [CrossRef]
- Piccardo, M.; Renzi, M.; Terlizzi, A. Nanoplastics in the oceans: Theory, experimental evidence and real world. Mar. Pollut. Bull. 2020, 157, 111317. [Google Scholar] [CrossRef]
- Pabortsava, K.; Lampitt, R.S. High concentrations of plastic hidden beneath the surface of the Atlantic Ocean. Nat. Commun. 2020, 11, 4073. [Google Scholar] [CrossRef]
- Rachael, M.Y.L.; Tony, H.; Adhi, Y.; Kuok, H.D.; Tang, M.H.K. Microplastic Occurrence in the Water and Sediment of Miri River Estuary, Borneo Island. Water Air Soil Poll. 2021, 232, 342. [Google Scholar]
- Lambert, S.; Wagner, M. Characterisation of nanoplastics during the degradation of polystyrene. Chemosphere 2015, 145, 265–268. [Google Scholar] [CrossRef]
- Bergami, E.; Pugnalini, S.; Vannuccini, M.L.; Manfra, L.; Faleri, C.; Savorelli, F.; Dawson, K.A.; Corsi, I. Long-term toxicity of surface-charged polystyrene nanoplastics to marineplanktonic species Dunaliella tertiolecta and Artemia franciscana. Aquat. Toxicol. 2017, 189, 159–169. [Google Scholar] [CrossRef]
- Besseling, E.; Redondo-Hasselerharm, P.; Foekema, E.M.; Koelmans, A.A. Quantifying ecological risks of aquatic micro-and nanoplastic. Crit. Rev. Environ. Sci. Technol. 2018, 49, 32–80. [Google Scholar] [CrossRef]
- Nguyen, M.K.; Moon, J.Y.; Lee, Y.C. Microalgal ecotoxicity of nanoparticles: An updated review. Ecotox. Environ. Saf. 2020, 201, 110781. [Google Scholar] [CrossRef] [PubMed]
- Bergami, E.; Pugnalini, S.; Vannuccini, M.L.; Manfra, L.; Faleri, C.; Savorelli, F.; Cole, M.; Lindeque, P.; Fileman, E.; Halsband, C.; et al. The impact of polystyrene microplastics on feeding, function and fecundity in the marine copepod Calanus helgolandicus. Environ. Sci. Technol. 2015, 49, 1130–1137. [Google Scholar]
- Manfra, L.; Rotini, A.; Bergami, E.; Grassi, G.; Faleri, C.; Corsi, I. Comparative ecotoxicity of polystyrene nanoparticles in natural seawater and reconstituted seawater using the rotifer Brachionus plicatilis. Ecotox. Environ. Saf. 2017, 145, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wang, Z.; Wang, S.; Fang, H.; Wang, D. Aquatic behavior and toxicity of polystyrene nanoplastic particles with different functional groups: Complex roles of pH, dissolved organic carbon and divalent cations. Chemosphere 2019, 228, 195–203. [Google Scholar] [CrossRef]
- Shen, M.; Zhang, Y.; Zhu, Y.; Song, B.; Zeng, G.; Hu, D.; Wen, X.; Ren, X. Recent advances in toxicological research of nanoplastics in the environment: A review. Environ. Pollut. 2019, 252, 511–521. [Google Scholar] [CrossRef]
- Saavedra, J.; Stoll, S.; Slaveykova, V.I. Influence of nanoplastic surface charge on eco-corona formation, aggregation and toxicity to freshwater zooplankton. Environ. Pollut. 2019, 252, 715–722. [Google Scholar] [CrossRef]
- Gao, G.; Zhao, X.; Jin, P.; Gao, K.; Beardall, J. Current understanding and challenges for aquatic primary producers in aworld with rising micro- and nano-plastic levels. J. Hazard. Mater. 2021, 406, 124685. [Google Scholar] [CrossRef]
- Mao, Y.; Ai, H.; Yi, C.; Zhang, Z.; Zeng, P.; Li, K.; Li, W.; Gu, W.; Qiang, H.; Hong, L. Phytoplankton response to polystyrene microplastics: Perspective from an entire growth period. Chemosphere 2018, 208, 59–68. [Google Scholar] [CrossRef]
- Prata, J.C.; da Costa, J.P.; Lopes, I.; Duarte, A.C. Effects of microplastics on microalgae populations: A critical review. Sci. Total Environ. 2019, 665, 400–405. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Lin, S.; Turner, J.P.; Ke, P.C. Physical adsorption of charged plastic nanoparticles affects algal photosynthesis. J. Phys. Chem. C 2010, 114, 16556–16561. [Google Scholar] [CrossRef]
- Li, S.; Wang, P.; Zhang, C.; Zhou, X.; Yin, Z.; Hu, T.; Hu, D.; Liu, C.; Zhu, L. Influence of polystyrene microplastics on the growth, photosynthetic efficiency and aggregation of freshwater microalgae Chlamydomonas reinhardtii. Sci. Total. Environ. 2020, 714, 136767. [Google Scholar] [CrossRef] [PubMed]
- Sendra, M.; Staffieri, E.; Pilar Yeste, M.; Moreno-Garrido, I.; Manuel Gatica, J.; Corsi, I.; Blasco, J. Are the primary characteristics of polystyrene nanoplastics responsible for toxicity and ad/absorption in the marine diatom Phaeodactylum tricornutum? Environ. Pollut. 2019, 249, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Jiang, R.; You, J.; Muir, D.C.G.; Zeng, E.Y. Microplastic Impacts on Microalgae Growth: Effects of Size and Humic Acid. Environ. Sci. Technol. 2019, 54, 1782–1789. [Google Scholar] [CrossRef]
- Sjollema, S.B.; Redondo-Hasselerharm, P.; Leslie, H.A.; Kraak, M.H.S.; Vethaak, A.D. Do plastic particles affect microalgal photosynthesis and growth? Aquat. Toxicol. 2016, 170, 259–261. [Google Scholar] [CrossRef]
- Seoane, M.; Gonzalez-Fernandez, C.; Soudant, P.; Huvet, A.; Esperanza, M.; Cid, A.; Paul-Pont, I. Polystyrene microbeads modulate the energy metabolism of the marine diatom Chaetoceros neogracile. Environ. Pollut. 2019, 251, 363–371. [Google Scholar] [CrossRef]
- Hazeem, L.J.; Yesilay, G.; Bououdina, M.; Perna, S.; Cetin, D.; Suludere, Z.; Barras, A.; Boukherroub, R. Investigation of the toxic effects of different polystyrene micro-and nanoplastics on microalgae Chlorella vulgaris by analysis of cell viability, pigment content, oxidative stress and ultrastructural changes. Mar. Pollut. Bull. 2020, 156, 111278. [Google Scholar] [CrossRef]
- Wu, D.; Wang, T.; Wang, J.; Jiang, L.; Yin, Y.; Guo, H. Size-dependent toxic effects of polystyrene microplastic exposure on Microcystis aeruginosa growth and microcystin production. Sci. Total Environ. 2021, 761, 143265. [Google Scholar] [CrossRef]
- Huang, W.; Zhao, T.; Zhu, X.; Ni, Z.; Guo, X.; Tan, L.; Wang, J. The effects and mechanisms of polystyrene and polymethyl methacrylate with different sizes and concentrations on Gymnodinium aeruginosum. Environ. Pollut. 2021, 287, 117626. [Google Scholar] [CrossRef]
- Zhao, T.; Tan, L.; Zhu, X.; Huang, W.; Wang, J. Size-dependent oxidative stress effect of nano/micro-scaled polystyrene on Karenia mikimotoi. Mar. Pollut. Bull. 2020, 154, 111074. [Google Scholar] [CrossRef]
- Xi, J.; Shao, J.; Wang, Y.; Wang, X.; Yang, H.; Zhang, X.; Xiong, D. Acute toxicity of triflumizole to freshwater green algae Chlorella vulgaris. Pestic. Biochem. Phys. 2019, 158, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, M.; Wang, J.; Huang, J.; Wang, J. Polystyrene nanoplastics cause growth inhibition, morphological damage and physiological disturbance in the marine microalga Platymonas helgolandica. Mar. Pollut. Bull. 2020, 158, 111403. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Beadham, I.; Ren, H.Y.; Ji, M.M.; Ruan, W.Q. A study into the species sensitivity of green algae towards imidazolium-based ionic liquids using flow cytometry. Ecotox. Environ. Saf. 2020, 194, 110392. [Google Scholar] [CrossRef] [PubMed]
- Nolte, T.M.; Hartmann, N.B.; Kleijn, J.M.; Garnæs, J.; van de Meent, D.; Jan Hendriks, A.; Baun, A. The toxicity of plastic nanoparticles to green algae as influenced by surface modification, medium hardness and cellular adsorption. Aquat. Toxicol. 2017, 183, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Zhou, Q.; Chen, C.; Yang, F.; Cai, Z.; Li, D.; Geng, Q.; Feng, Y.; Wang, H. Role of extracellular polymeric substances on the behavior and toxicity of silver nanoparticles and ions to green algae Chlorella vulgaris. Sci. Total Environ. 2019, 660, 1182–1190. [Google Scholar] [CrossRef]
- Sadiq, I.M.; Pakrashi, S.; Chandrasekaran, N.; Mukherjee, A. Studies on toxicity of aluminum oxide (Al2O3) nanoparticles to microalgae species: Scenedesmus sp. and Chlorella sp. J. Nanopart. Res. 2011, 13, 3287–3299. [Google Scholar] [CrossRef]
- Kasibhatla, S.; Amarante-Mendes, G.P.; Finucane, D.; Brunner, T.; Green, D.R. Acridine Orange/Ethidium Bromide (AO/EB) Staining to Detect Apoptosis. Cold Spring Harb. Protoc. 2006, 21, pdb-prot4493. [Google Scholar] [CrossRef]
- Pietrowska, E.; Różalska, S.; Kaźmierczak, A.; Nawrocka, J.; Małolepsza, U. Reactive oxygen and nitrogen (ROS and RNS) species generation and cell deathin tomato suspension cultures Botrytis cinerea interaction. Protoplasma 2015, 252, 307–319. [Google Scholar] [CrossRef]
- Lin, Y.; Huang, J.J.; Dahms, H.U.; Zhen, J.J.; Ying, X.P. Cell damage and apoptosis in the hepatopancreas of Eriocheir sinensis induced by cadmium. Aquat. Toxicol. 2017, 190, 190–198. [Google Scholar] [CrossRef]
- Khoshnamvand, M.; Hanachi, P.; Ashtiani, S.; Walker, T.R. Toxic effects of polystyrene nanoplastics on microalgae Chlorella vulgaris: Changes in biomass, photosynthetic pigments and morphology. Chemosphere 2021, 280, 130725. [Google Scholar] [CrossRef]
- Long, M.; Moriceau, B.; Gallinari, M.; Lambert, C.; Huvet, A.; Raffray, J.; Soudant, P. Interactions between microplastics and phytoplankton aggregates: Impact on their respective fates. Mar. Chem. 2015, 175, 39–46. [Google Scholar] [CrossRef]
- Ritchie, R.J. Consistent Sets of Spectrophotometric Chlorophyll Equations for Acetone, Methanol and Ethanol Solvents. Photosynthe. Res. 2006, 89, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Tayemeh, M.B.; Esmailbeigi, M.; Shirdel, I.; Joo, H.S.; Johari, S.A.; Banan, A.; Nourani, H.; Mashhadi, H.; Jami, M.J.; Tabarrok, M. Perturbation of fatty acid composition, pigments, and growth indices of Chlorella vulgaris in response to silver ions and nanoparticles: A new holistic understanding of hidden ecotoxicological aspect of pollutants. Chemosphere 2020, 238, 124576. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Joseph, J.A. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radical Bio. Med. 1999, 27, 612–616. [Google Scholar] [CrossRef] [PubMed]
- Pikula, K.; Chaika, V.; Zakharenko, A.; Markina, Z.; Vedyagin, A.; Kuznetsov, V.; Gusev, A.; Park, S.; Golokhvast, K. Comparison of the Level and Mechanisms of Toxicity of Carbon Nanotubes, Carbon Nanofibers, and Silicon Nanotubes in Bioassay with Four Marine Microalgae. Nanomaterials 2020, 10, 485. [Google Scholar] [CrossRef]
- Zhang, L.; Lei, C.; Yang, K.; Jason, W.; Lin, D. Cellular response of Chlorella pyrenoidosa to oxidized multi-walled carbon nanotubes. Environ. Sci.-Nano 2018, 5, 2415–2425. [Google Scholar] [CrossRef]
- Déniel, M.; Lagarde, F.; Caruso, A.; Errien, N. Infrared spectroscopy as a tool to monitor interactions between nanoplastics and microalgae. Anal. Bioanal. Chem. 2020, 412, 4413–4422. [Google Scholar] [CrossRef]








| Material | Diameters (nm) | PDI | ζ Potential (mV) |
|---|---|---|---|
| 50 nm PS | 401.08 ± 15.62 | 0.27 ± 0.01 | −12.07 ± 0.65 |
| 70 nm PS | 99.73 ± 0.49 | 0.15 ± 0.12 | −35.28 ± 0.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, Q.; Zhou, Y.; Tan, C. Toxicity Effects of Polystyrene Nanoplastics with Different Sizes on Freshwater Microalgae Chlorella vulgaris. Molecules 2023, 28, 3958. https://doi.org/10.3390/molecules28093958
Xiang Q, Zhou Y, Tan C. Toxicity Effects of Polystyrene Nanoplastics with Different Sizes on Freshwater Microalgae Chlorella vulgaris. Molecules. 2023; 28(9):3958. https://doi.org/10.3390/molecules28093958
Chicago/Turabian StyleXiang, Qingqing, Ying Zhou, and Chengxia Tan. 2023. "Toxicity Effects of Polystyrene Nanoplastics with Different Sizes on Freshwater Microalgae Chlorella vulgaris" Molecules 28, no. 9: 3958. https://doi.org/10.3390/molecules28093958