Determining the Photoelectrical Behavior and Photocatalytic Activity of an h-YMnO3 New Type of Obelisk-like Perovskite in the Degradation of Malachite Green Dye
Abstract
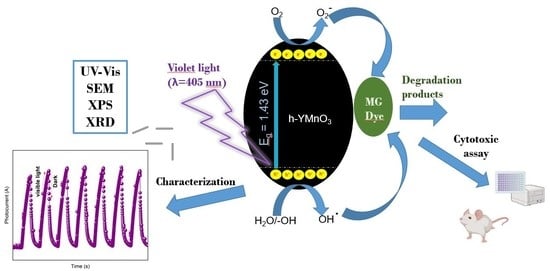
:1. Introduction
2. Results and Discussion
2.1. Characterization
2.2. Photocurrent Tests
2.3. Malachite Green Dye Degradation
2.3.1. Scavengers Test
2.3.2. pH Influence
2.3.3. Photodegradation Mechanism of the MG Dye Using h-YMnO3 as the Photocatalyst
2.4. Cytotoxic Properties
3. Materials and Methods
3.1. Synthesis Process
3.2. Characterization
3.2.1. Solid-State Techniques
3.2.2. Photoelectric and Photocurrent Assessments
3.3. Photocatalytic Activity
3.4. Cytotoxicity Assay
3.4.1. Protocol to Obtain Leukocytes
3.4.2. Cell Viability Test (Alamar Blue Assay)
3.4.3. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Li, M.; Tan, H.; Duan, W. Hexagonal rare-earth manganites and ferrites: A review of improper ferroelectricity, magnetoelectric coupling, and unusual domain walls. Phys. Chem. Chem. Phys. 2020, 22, 14415–14432. [Google Scholar] [CrossRef] [PubMed]
- Balli, M.; Roberge, B.; Fournier, P.; Jandl, S. Review of the Magnetocaloric Effect in RMnO3 and RMn2O5 Multiferroic Crystals. Crystals 2017, 7, 44. [Google Scholar] [CrossRef]
- Zhou, J.S.; Goodenough, J.B.; Gallardo-Amores, J.M.; Morán, E.; Alario-Franco, M.A.; Caudillo, R. Hexagonal versus perovskite phase of manganite RMnO3 (R = Y, Ho, Er, Tm, Yb, Lu). Phys. Rev. B 2006, 74, 014422. [Google Scholar] [CrossRef]
- Gibbs, A.S.; Knight, K.S.; Lightfoot, P. High-temperature phase transitions of hexagonal YMnO3. Phys. Rev. B 2011, 83, 094111. [Google Scholar] [CrossRef]
- Balamurugan, C.; Lee, D.W. Perovskite hexagonal YMnO3 nanopowder as p-type semiconductor gas sensor for H2S detection. Sens. Actuators B Chem. 2015, 221, 857–866. [Google Scholar] [CrossRef]
- Addabbo, T.; Bertocci, F.; Fort, A.; Gregorkiewitz, M.; Mugnaini, M.; Spinicci, R.; Vignoli, V. Gas sensing properties of YMnO3 based materials for the detection of NOx and CO. Sens. Actuators B Chem. 2017, 244, 1054–1070. [Google Scholar] [CrossRef]
- Bogusz, A.; Choudhary, O.S.; Skorupa, I.; Bürger, D.; Lawerenz, A.; Lei, Y.; Zeng, H.; Abendroth, B.; Stöcker, H.; Schmidt, O.G.; et al. Photocapacitive light sensor based on metal-YMnO3-insulator-semiconductor structures. Appl. Phys. Lett. 2016, 108, 052103. [Google Scholar] [CrossRef]
- Fujimura, N.; Ishida, T.; Yoshimura, T.; Ito, T. Epitaxially grown YMnO3 film: New candidate for nonvolatile memory devices. Appl. Phys. Lett. 1996, 69, 1011–1013. [Google Scholar] [CrossRef]
- Wang, Y.; Song, J. Synthesized and Photocatalytic Mechanism of the NiO Supported YMnO3 Nanoparticles for Photocatalytic Degradation of the Methyl Orange Dye. Z. Für Phys. Chem. 2020, 234, 153–170. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, H. Study on the construction of YMnO3/CeO2 composite photocatalyst heterostructure and photocatalytic degradation of methyl red. Optik 2020, 201, 163524. [Google Scholar] [CrossRef]
- Cao, Z.; Wang, C.; Chen, J. Synthesis and photocatalytic property of p–n junction YMnO3/SrTiO3 composites. Mater. Res. Express. 2018, 5, 115512. [Google Scholar] [CrossRef]
- You, J.; Zhan, S.; Wen, J.; Ma, Y.; Zhu, Z. Construction of heterojunction of Ag2S modified yttrium manganate visible photocatalyst and study on photocatalytic mechanism. Optik 2020, 217, 164900. [Google Scholar] [CrossRef]
- Tian, M.; Li, Y.; Wang, G.; Hao, X. Large photocurrent density in polycrystalline hexagonal YMnO3 thin film induced by ferroelectric polarization and the positive driving effect of grain boundary. Sol. Energy Mater. Sol. Cells 2021, 224, 111009. [Google Scholar] [CrossRef]
- Chen, H.; Liu, K.; Hu, L.; Al-Ghamdi, A.A.; Fang, X. New concept ultraviolet photodetectors. Mater. Today. 2015, 18, 493–502. [Google Scholar] [CrossRef]
- Yang, S.B.; Wang, C.A.; Li, Y.; Chen, Y.; Zhang, A.H.; Zeng, M.; Fan, Z.; Gao, X.S.; Lu, X.B.; Liu, J.-M. Hexagonal YMnO3 films as promising ultraviolet photodetectors. Ceram. Int. 2019, 45, 3239–3243. [Google Scholar] [CrossRef]
- Chen, L.; Zheng, G.; Yao, G.; Zhang, P.; Dai, S.; Jiang, Y.; Li, H.; Yu, B.; Ni, H.; Wei, S. Lead-Free Perovskite Narrow-Bandgap Oxide Semiconductors of Rare-Earth Manganates. ACS Omega 2020, 5, 8766–8776. [Google Scholar] [CrossRef]
- McBean, C.L.; Lewis, C.S.; Tiano, A.L.; Simonson, J.W.; Han, M.G.; Gannon, W.J.; Yue, S.; Patete, J.M.; Corrao, A.A.; Santuli, A.C.; et al. A Generalizable Multigram Synthesis and Mechanistic Investigation of YMnO3 Nanoplates. Ind. Eng. Chem. Res. 2017, 56, 5573–5585. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, Y.; Wang, Z.; Wang, X.; Liu, H.; Cheng, G.J. Molten salt synthesis of YMnO3 powder with high near-infrared reflectivity. Mater. Lett. 2018, 229, 171–173. [Google Scholar] [CrossRef]
- Branković, Z.; Branković, G.; Počuča-Nešić, M.; Marinković Stanojević, Z.; Žunić, M.; Luković Golić, D.; Tararam, R.; Cilense, M.; Zaghete, M.A.; Jagličić, Z.; et al. Hydrothermally assisted synthesis of YMnO3. Ceram. Int. 2015, 41 Pt B, 14293–14298. [Google Scholar] [CrossRef]
- Turut, A.; Coșkun, M.; Coșkun, F.M.; Polat, O.; Durmuș, Z.; Çağlar, M.; Efeoǧlu, H. The current-voltage characteristics of the ferroelectric p-YMnO3 thin film/bulk p-Si heterojunction over a broad measurement temperature range. J. Alloys Compd. 2019, 782, 566–575. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, Y.; Wang, Z.; Wang, X.; Liu, H. Magnetic characterization and charge state of nonmagnetic ion-substituted YMn0.8Fe0.2O3. J. Mater. Sci. Mater. Electron. 2018, 29, 7287–7293. [Google Scholar] [CrossRef]
- Wan, F.; Lin, X.; Bai, X.; Han, X.; Song, K.; Zheng, J.; Cao, C. Crystalline structure and dielectric properties of multiferroic Cr-doped YMnO3. J. Mater. Sci. Mater. Electron. 2016, 27, 3082–3087. [Google Scholar] [CrossRef]
- Ren, P.; Fan, H.; Wang, X. Bulk conduction and nonlinear behaviour in multiferroic YMnO3. Appl. Phys. Lett. 2013, 103, 152905. [Google Scholar] [CrossRef]
- Han, A.; Zhao, M.; Ye, M.; Liao, J.; Zhang, Z.; Li, N. Crystal structure and optical properties of YMnO3 compound with high near-infrared reflectance. Sol. Energy 2013, 91, 32–36. [Google Scholar] [CrossRef]
- Polat, O.; Coskun, F.M.; Coskun, M.; Durmus, Z.; Caglar, Y.; Caglar, M.; Turut, A. Tailoring the band gap of ferroelectric YMnO3 through tuning the Os doping level. J. Mater. Sci. Mater. Electron. 2019, 30, 3443–3451. [Google Scholar] [CrossRef]
- Yuan, Q.; Chen, L.; Xiong, M.; He, J.; Luo, S.L.; Au, C.T.; Yin, S.-F. Cu2O/BiVO4 heterostructures: Synthesis and application in simultaneous photocatalytic oxidation of organic dyes and reduction of Cr(VI) under visible light. Chem. Eng. J. 2014, 255, 394–402. [Google Scholar] [CrossRef]
- Mousavi, M.; Habibi-Yangjeh, A.; Abitorabi, M. Fabrication of novel magnetically separable nanocomposites using graphitic carbon nitride, silver phosphate and silver chloride and their applications in photocatalytic removal of different pollutants using visible-light irradiation. J. Colloid Interface Sci. 2016, 480, 218–231. [Google Scholar] [CrossRef]
- Michel, C.R.; Lopez-Alvarez, M.A.; Martínez-Preciado, A.H.; Carbajal-Arízaga, G.G. Novel UV Sensing and Photocatalytic Properties of DyCoO3. J. Sens. 2019, 2019, e5682645. [Google Scholar] [CrossRef]
- Michel, C.R.; López-Alvarez, M.A.; Martínez-Preciado, A.H.; Oleinikov, V. Ultraviolet Detection and Photocatalytic Activity of Nanostructured LaCoO3 Prepared by Solution-Polymerization. ECS J. Solid State Sci. Technol. 2019, 8, Q9. [Google Scholar] [CrossRef]
- Renita, A.A.; Vardhan, K.H.; Kumar, P.S.; Ngueagni, P.T.; Abilarasu, A.; Nath, S.; Kumari, P.; Saravanan, R. Effective removal of malachite green dye from aqueous solution in hybrid system utilizing agricultural waste as particle electrodes. Chemosphere 2021, 273, 129634. [Google Scholar] [CrossRef]
- Lin, Y.R.; Hu, Y.F.; Huang, C.Y.; Huang, H.T.; Liao, Z.H.; Lee, A.T.; Wu, Y.-S.; Nan, F.-H. Removing Malachite Green and Leucomalachite Green From Freshwater and Seawater With Four Water Treatment Agents. 2022. Available online: https://scholars.ntou.edu.tw/handle/123456789/22059 (accessed on 19 February 2023).
- Mukhlish, M.Z.B.; Najnin, F.; Rahman, M.M.; Uddin, M.J. Photocatalytic Degradation of Different Dyes Using TiO2 with High Surface Area: A Kinetic Study. J. Sci. Res. 2013, 5, 301–314. [Google Scholar] [CrossRef]
- Simonsen, M.E.; Li, Z.; Søgaard, E.G. Influence of the OH groups on the photocatalytic activity and photoinduced hydrophilicity of microwave assisted sol–gel TiO2 film. Appl. Surf. Sci. 2009, 255, 8054–8062. [Google Scholar] [CrossRef]
- Riss, T.L.; Moravec, R.A.; Niles, A.L.; Duellman, S.; Benink, H.A.; Worzella, T.J.; Minor, L. Cell Viability Assays. In Assay Guidance Manual; Markossian, S., Grossman, A., Brimacombe, K., Arkin, M., Auld, D., Austin, C., Baell, J., Chung, T.D.Y., Coussens, N.P., Dahlin, J.L., et al., Eds.; Eli Lilly & Company and the National Center for Advancing Translational Sciences: Bethesda, MD, USA, 2004. Available online: http://www.ncbi.nlm.nih.gov/books/NBK144065/ (accessed on 1 May 2023).



















Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Alvarez, M.Á.; Silva-Jara, J.M.; Silva-Galindo, J.G.; Reyes-Becerril, M.; Velázquez-Carriles, C.A.; Macías-Rodríguez, M.E.; Macías-Lamas, A.M.; García-Ramírez, M.A.; López de Alba, C.A.; Reynoso-García, C.A. Determining the Photoelectrical Behavior and Photocatalytic Activity of an h-YMnO3 New Type of Obelisk-like Perovskite in the Degradation of Malachite Green Dye. Molecules 2023, 28, 3932. https://doi.org/10.3390/molecules28093932
López-Alvarez MÁ, Silva-Jara JM, Silva-Galindo JG, Reyes-Becerril M, Velázquez-Carriles CA, Macías-Rodríguez ME, Macías-Lamas AM, García-Ramírez MA, López de Alba CA, Reynoso-García CA. Determining the Photoelectrical Behavior and Photocatalytic Activity of an h-YMnO3 New Type of Obelisk-like Perovskite in the Degradation of Malachite Green Dye. Molecules. 2023; 28(9):3932. https://doi.org/10.3390/molecules28093932
Chicago/Turabian StyleLópez-Alvarez, Miguel Ángel, Jorge Manuel Silva-Jara, Jazmín Guadalupe Silva-Galindo, Martha Reyes-Becerril, Carlos Arnulfo Velázquez-Carriles, María Esther Macías-Rodríguez, Adriana Macaria Macías-Lamas, Mario Alberto García-Ramírez, Carlos Alberto López de Alba, and César Alberto Reynoso-García. 2023. "Determining the Photoelectrical Behavior and Photocatalytic Activity of an h-YMnO3 New Type of Obelisk-like Perovskite in the Degradation of Malachite Green Dye" Molecules 28, no. 9: 3932. https://doi.org/10.3390/molecules28093932