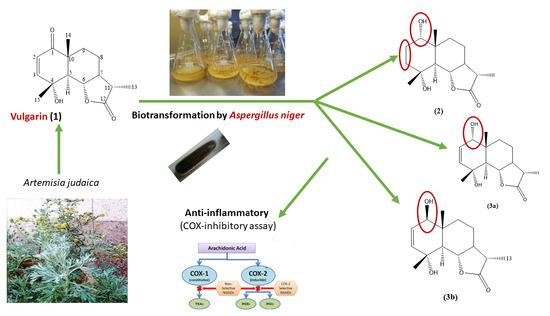
Microbial Transformation of the Sesquiterpene Lactone, Vulgarin, by Aspergillus niger
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structure Elucidation of Vulgarin Metabolites
2.1.1. Structural Elucidation of Metabolite (2)
2.1.2. Structural Elucidation of Metabolite (3a)
2.1.3. Structural Elucidation of Metabolite (3a, b)

2.2. Proposed Mechanism of Selective Reduction
2.3. COX-Inhibitory Activity of Vulgarin and Its Metabolites
3. Materials and Methods
3.1. General
3.2. Plant Material
3.3. Extraction and Isolation of Vulgarin
3.4. Microorganisms
3.5. Screening Procedures
3.6. Large Scale Fermentation
3.7. Isolation of Metabolites
3.8. In Vitro COX-1 and COX-2 Enzyme Inhibitory Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Lilly, M.D. Advances in biotransformation processes. Chem. Eng. Sci. 1994, 49, 151–159. [Google Scholar] [CrossRef]
- Hegazy, M.F.; Mohamed, T.A.; ElShamy, A.I.; Mohamed, A.H.; Mahalel, U.A.; Reda, E.H.; Shaheen, A.M.; Tawfik, W.A.; Shahat, A.A.; Shams, K.A.; et al. Microbial biotransformation as a tool for drug development based on natural products from mevalonic acid pathway: A review. J. Adv. Res. 2015, 6, 17–33. [Google Scholar] [CrossRef] [PubMed]
- Tkacz, J.S.; Lange, L. Advances in Fungal Biotechnology for Industry, Agriculture and Medicine; K1uwer Academic Plenum Publishers: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Parkinson, A.; Ogilvie, B.W. Biotransformation of xenobiotics. Casarett Doull Toxicol. 2008, 7, 161–304. [Google Scholar]
- Schuster, E.; Dunn-Coleman, N.; Frisvad, J.C.; van Dijck, P.W.M. On the safety of Aspergillus niger—A review. Appl. Microbial. Biotechnol. 2002, 59, 426–435. [Google Scholar]
- Geissman, T.A.; Ellestad, G.A. Vulgarin, a sesquiterpene lactone from Artemisia vulgaris L. J. Org. Chem. 1962, 27, 1855–1859. [Google Scholar] [CrossRef]
- Abegaz, B.; Camps, F.; Coll, J.; Feliz, M.; Jacobsson, U.; Miravitlles, C.; Molins, E.; Torramillans, J. The Structure of Vulgarin and its Isomers—A reinvestigation. Tetrahedron 1986, 42, 6003–6009. [Google Scholar] [CrossRef]
- Arias, J.M.; Bretón, J.L.; Gavin, J.A.; Granados, A.G.; Martinez, A.; Onorato, M.E. Microbial Transformation of Sesquiterpenenoid: Conversion of Deoxyvulgarin by Rhizopus nigrigans and Aspergillus ochraceous. J. Chem. Soc. Perkin Trans. 1987, 1, 471–474. [Google Scholar] [CrossRef]
- Al-Said, M.S.; Khalifa, S.I.; El-Feraly, F.S.; Hufford, C.D. Biogenetic-type Synthesis of Vulgarin and Peroxyvulgarin. Phytochemistry 1989, 28, 107–108. [Google Scholar] [CrossRef]
- Lee, K.-H.; Huang, E.-S.; Piantadosi, C.; Pagano, J.S.; Geissman, T.A. Cytotoxicity of Sesquiterpene Lactones. Cancer Res. 1971, 31, 1649–1654. [Google Scholar]
- Harborne, J.B.; Baxter, H. (Eds.) Phytochemical Dictionary: A Handbook of Bioactive Compounds from Plants; Taylor & Frances: London, UK, 1983. [Google Scholar]
- Pickman, A.K. Biological activity of sesquiterpene lactone. Bioch. Syst. Ecol. 1986, 14, 255. [Google Scholar] [CrossRef]
- De Vera, R.C.; Mesa, P.S.; de Diego, A.M.; del Fresno, V. A Preliminary study on the effects produced in the lipid fraction of various rat organs after administration of vulgarin (a new oral hypoglycemic agent of natural origin). Boll. Chim. Farm. 1976, 115, 445–456. [Google Scholar]
- Althurwi, H.N.; Soliman, G.A.; Abdel-Rahman, R.F.; Abd-Elsalam, R.M.; Ogaly, H.A.; Alqarni, M.H.; Albaqami, F.F.; Abdel-Kader, M.S. Vulgarin, a Sesquiterpene Lactone from Artemisia judaica, Improves the Antidiabetic Effectiveness of Glibenclamide in Streptozotocin-Induced Diabetic Rats via Modulation of PEPCK and G6Pase Genes Expression. Int. J. Mol. Sci. 2022, 23, 15856. [Google Scholar] [CrossRef] [PubMed]
- Orabi, K.Y.; El-Feraly, F.S.; Al-Sulmy, W.A.; Al Yahya, M.A. Biotransformation of vulgarin. Mini-Rev. Med. Chem. 2013, 13, 777–782. [Google Scholar] [CrossRef]
- Vane, J. Towards a better aspirin. Nature 1994, 367, 215–216. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, D.; Nissen, S.E.; Topol, E.J. Risk of cardiovascular events associated with selective COX-2 inhibitors. JAMA 2001, 286, 954–959. [Google Scholar] [CrossRef]
- Mansilla, H.; Palenzuela, J.A. Minor eudesmanolides from Artemisia canariensis. Phytochemistry 1999, 51, 995–997. [Google Scholar] [CrossRef]
- Marco, J.A.; Sanz, F.J.; Sancenon, F.; Rustataiyan, A.; Saberi, M. Sesquiterpene lactones from Artemsia species. Phytochemistry 1993, 32, 460–462. [Google Scholar] [CrossRef]
- Hollmann, F.; Opperman, D.J.; Paul, C.E. Biocatalytic Reduction Reactions from a Chemists Perspective. Angew. Chem. Int. Ed. 2021, 60, 5644–5665. [Google Scholar] [CrossRef]
- Zarghi, A.; Arfaei, S. Selective COX-2 Inhibitors: A Review of Their Structure-Activity Relationships. Iran J. Pharm Res. 2011, 10, 655–683. [Google Scholar]
- Riendeau, D.; Charleson, S.; Cromlish, W.; Mancini, J.A.; Wong, E.; Guay, J. Comparison of the cyclooxygenase-1 inhibitory properties of nonsteroidal anti-inflammatory drugs (NSAIDs) and selective COX-2 inhibitors, using sensitive microsomal and platelet assays. Candian J. Physiol. Pharmacol. 1997, 75, 1088–1095. [Google Scholar] [CrossRef]
- Pairet, M.; Van, R.J. Experimental models used to investigate the differential inhibition of cyclooxygenase-1 and cyclooxygenase-2 by non-steroidal-anti-inflammatory drugs. Inflamm. Res. 1998, 2, 93–101. [Google Scholar] [CrossRef]
- Betts, R.; Walters, D.; Rosazza, J. Microbial transformation of antitumor compounds. I. Conversion of acronycine to 9-hydroxyacronyine by Cunninghamella echinulate. J. Med. Chem. 1974, 17, 599–602. [Google Scholar] [CrossRef] [PubMed]
- El-Sharkawy, S. Microbial conversion of tamoxifen. Appl. Microb. Biotechnol. 1991, 35, 436–439. [Google Scholar] [CrossRef]
- Kargman, S.; Wong, E.; Greig, G.M.; Falgueyret, J.P.; Cromlish, W.; Ethier, D.; Yergey, J.A.; Riendeau, D.; Evans, J.F.; Kennedy, B.; et al. Mechanism of selective inhibition of human prostaglandin G/H synthase-1 and -2 in intact cells. Biochem. Pharmacol. 1996, 52, 1113–1125. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.J.; Zhang, Y.; Koboldt, C.M.; Muhammad, J.; Zweifel, B.S.; Shaffer, A.; Talley, J.J.; Masferrer, J.L.; Seibert, K.; Isakson, P.C. Pharmacological analysis of cyclooxygenase-1 in inflammation. Proc. Nat. Acad. Sci. USA 1998, 95, 13313–13318. [Google Scholar] [CrossRef]



| H | 1 | 2 | 3a | 3b (in 3a,b) |
|---|---|---|---|---|
| 1 | - | 3.41, br s | 3.49, br s | 3.41, br s |
| 2 | 5.86, d (10.4) | 1.60 m 1.91 m | 5.94, dd (10.1, 5.6) | 5.94, dd (10.1, 5.6) |
| 3 | 6.58, d (10.4) | 1.33 m 1.97 m | 5.70, d (10.1) | 5.70, d (10.1) |
| 5 | 2.40, d (11.5) | 2.18, d (11.7) | 2.29, d (11.4) | 2.18, d (11.6) |
| 6 | 4.15, dd (10.9, 10.9) | 4.07, dd (10.7, 10.6) | 4.05, dd (11.4, 10.7) | 4.12, dd (8.1, 4.6) |
| 7 | 1.67, dddd (12.6, 12.6, 12.6, 3.5) | 1.69, m | 1.71, m | 1.71, m |
| 8 | 1.46, dddd (12.9, 12.9, 12.9, 3.2) 1.96, m | 1.51, m 1.90, m | 8α = 1.92, m 8β = 1.49, m | 8α = 1.92, m 8β = 1.49, m |
| 9 | 1.56, ddd (13.6, 13.6, 3.5) 1.99, m | 1.59, m 1.95, m | 9α = 2.14, m 9β = 1.32, m | 9α = 1.95, m 9β = 1.25, m |
| 11 | 2.34, dq (13.7, 6.9) | 2.27, dq (12.6, 7.0) | 2.33, dq (12.5, 6.9) | 2.33, dq (12.5, 6.9) |
| 13 | 1.22, d (6.9) | 1.23, d (6.9) | 1.25, d (6.9) | 1.22, d (6.9) |
| 14 | 1.19, s | 1.00, s | 0.97, s | 1.00, s |
| 15 | 1.53, s | 1.36, s | 1.39, s | 1.36, s |
| C | 1 | 2 | 3a | 3b (in 3a,b) |
|---|---|---|---|---|
| 1 | 202.1 C | 73.3 CH | 71.3 CH | 73.4 CH |
| 2 | 125.9 CH | 33.0 CH2 | 127.3 CH | 127.3 CH |
| 3 | 152.2 CH | 36.4 CH2 | 136.2 CH | 136.2 CH |
| 4 | 70.4 C | 71.5 C | 70.4 C | 71.3 C |
| 5 | 54.9 CH | 52.9 CH | 48.9 CH | 50.7 CH |
| 6 | 79.9 CH | 81.4 CH | 81.3 CH | 81.5 CH |
| 7 | 52.7 CH | 50.5 CH | 52.5 CH | 53.0 CH |
| 8 | 23.0 CH2 | 23.2 CH2 | 23.2 CH2 | 23.2 CH2 |
| 9 | 34.6 CH2 | 26.6 CH2 | 35.5 CH2 | 36.5 CH2 |
| 10 | 46.6 C | 41.6 C | 40.3 C | 41.7 C |
| 11 | 40.9 CH | 40.7 CH | 40.9 CH | 41.7 CH |
| 12 | 178.7 C | 178.7 C | 179.0 C | 179.0 C |
| 13 | 12.8 CH3 | 12.5 CH3 | 12.6 CH3 | 12.6 CH3 |
| 14 | 20.1 CH3 | 20.0 CH3 | 20.2 CH3 | 20.1 CH3 |
| 15 | 24.1 CH3 | 24.3 CH3 | 24.6 CH3 | 24.4 CH3 |
| Compounds | COX-1% | COX-2% |
|---|---|---|
| Vulgarin (1) | 45.61% | 60.69% |
| 2 | 9.43% | 6.80% |
| 3a | 53.60% | 22.7% |
| 3a,b (epimeric mixture) | 48.40% | 26.17% |
| Indomethacin | 85.68 | 14.89% |
| Celecoxib | 8.7% | 92% |
| Compounds | COX-1 IC50 * (μM/mL) | COX-2 IC50 * (μM/mL) | Selectivity Index: (IC50 COX-1/ IC50COX-2) | Selectivity Index: (IC50 COX-2/IC50 COX-1) |
|---|---|---|---|---|
| Vulgarin (1) | 11.32 ± 0.24 | 07.21 ± 0.10 | 18.53 | 0.64 |
| 2 | 92.33 ± 0.10 | 98.02 ± 0.10 | 0.94 | 1.06 |
| 3a | 15.70 ± 0.51 | 60.66 ± 0.16 | 0.26 | 3.86 |
| 3a,b | 10.12 ± 0.01 | 64.22 ± 1.05 | 0.16 | 6.35 |
| Indomethacin | 0.24 ± 0.05 | 3.28 ± 0.09 | 0.07 | 13.67 |
| Celecoxib | 29.19 ± 0.33 | 0.08 ± 0.44 | 364.88 | 4.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
ElGamal, R.A.; Galala, A.A.; Abdel-Kader, M.S.; Badria, F.A.; Soliman, A.F. Microbial Transformation of the Sesquiterpene Lactone, Vulgarin, by Aspergillus niger. Molecules 2023, 28, 3729. https://doi.org/10.3390/molecules28093729
ElGamal RA, Galala AA, Abdel-Kader MS, Badria FA, Soliman AF. Microbial Transformation of the Sesquiterpene Lactone, Vulgarin, by Aspergillus niger. Molecules. 2023; 28(9):3729. https://doi.org/10.3390/molecules28093729
Chicago/Turabian StyleElGamal, Reem A., Amal A. Galala, Maged S. Abdel-Kader, Farid A. Badria, and Amal F. Soliman. 2023. "Microbial Transformation of the Sesquiterpene Lactone, Vulgarin, by Aspergillus niger" Molecules 28, no. 9: 3729. https://doi.org/10.3390/molecules28093729