Component and Content of Lipid Classes and Phospholipid Molecular Species of Eggs and Body of the Vietnamese Sea Urchin Tripneustes gratilla
Abstract
:1. Introduction
2. Results and Discussion
2.1. Total Lipid, Lipid Classes, and Fatty Acids Composition of T. gratilla
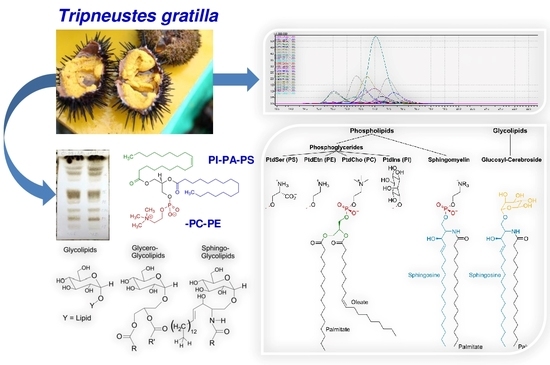
2.2. Polar Lipid Type and Phospholipid Classes
2.3. Molecular Species of Phosphatidylinositol (PI)
2.4. Molecular Species of Phosphatidylserine (PS)
2.5. Molecular Species of Phosphatidylethanolamine (PE)
2.6. Molecular Species of Phosphatidic Acids (PA)
2.7. Molecular Species of Phosphatidylcholine (PC)
3. Materials and Methods
3.1. Material
3.2. Extraction of Total Lipid
3.3. Analysis of Polar Lipid Classes
3.4. Analysis of Molecular Species of Phospholipids
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Imbs, A.B.; Ermolenko, E.V.; Grigorchuk, V.P.; Sikorskaya, T.V.; Velansky, P.V. Current Progress in Lipidomics of Marine Invertebrates. Mar. Drugs 2021, 19, 660. [Google Scholar] [CrossRef] [PubMed]
- Nomleni, A.; Widodo, M.S.; Kilawati, Y.; Valen, F.S. Contemporary records of sea urchin Tripneustes gratilla (Echinodermata: Echinoidea) in Timor Island, Indonesia. AACL Bioflux 2020, 13, 1899–1905. [Google Scholar]
- James, W.; Fetterman, J.R.; Martin, M.; Zdano, W.Z. Therapeutic potential of n-3 polyunsaturated fatty acids in disease. Am. J. Health-Syst. Pharm. 2009, 66, 1169–1179. [Google Scholar]
- Long, P.Q.; Minh, C.V. Lipids and Biologically Active Fatty Acids from Nuture; Science and Technology Publishing House: Hanoi, Vietnam, 2005; pp. 581–589. [Google Scholar]
- Kostetsky, E.Y.; Sanina, N.M.; Velansky, P.V. The thermotropic behavior and major molecular species composition of the phospholipids of echinoderms. Russ. J. Mar. Biol. 2014, 40, 131–139. [Google Scholar] [CrossRef]
- Dinh, T.H.K.; Long, P.Q.; Phuong, D.L. Research on the composition of lipids, fatty acids, and amino acids from egg and body of sea urchin Tripneustes gratilla. Vietnam J. Sci. Technol. 2018, 56, 30–38. [Google Scholar] [CrossRef]
- Pozharitskaya, O.N.; Shikov, A.N.; Laakso, I.; Seppänen-Laakso, T.; Makarenko, I.E.; Faustova, N.M.; Makarova, M.N.; Makarov, V.G. Bioactivity and chemical characterization of gonads of green sea urchin Strongylocentrotus droebachiensis from Barents Sea. J. Funct. Foods 2015, 17, 227–234. [Google Scholar] [CrossRef]
- Imbs, A.B.; Dang, L.P.T.; Viacheslav, G.R.; Vasily, I.S. Fatty acid, Lipid class, and Phospholipid Molecular Species Composition of the Soft Coral Xenia sp. (Nha Trang Bay, the South China Sea, Vietnam). Lipids 2015, 50, 575–589. [Google Scholar] [CrossRef] [PubMed]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Ermolenko, E.V.; Sikorskaya, T.V.; Grigorchuk, V.P. The Phospholipid Molecular Species Profile of Apostichopus japonicus Tissues Modifies through Exposure to n-3 Polyunsaturated Fatty Acid-Deficient Diet. Mar. Drugs 2022, 20, 578. [Google Scholar] [CrossRef] [PubMed]
- Imbs, A.B.; Dang, L.P.T.; Nguyen, K.B. Comparative lipidomic analysis of phospholipids of hydrocorals and corals from tropical and cold-water regions. PLoS ONE 2019, 14, e0215759. [Google Scholar] [CrossRef] [PubMed]
- Harrabi, S.; Herchi, W.; Kallel, H.; Mayer, P.M.; Boukhchina, S. Liquid chromatographic–mass spectrometric analysis of glycerophospholipids in corn oil. Food Chem. 2009, 114, 712–716. [Google Scholar] [CrossRef]













| No. | Fatty Acids | Eggs of T. gratilla (%) | Body of T. gratilla (%) | No. | Fatty Acids | Eggs of T. gratilla (%) | Body of T. gratilla (%) |
|---|---|---|---|---|---|---|---|
| 1 | 12:0 | 0.08 | - | 15 | 18:0 | 1.39 | 1.57 |
| 2 | 14:0 | 14.50 | 3.59 | 16 | 20:0 | 0.23 | 0.12 |
| 3 | 14:1 (n-7) | 2.03 | 0.33 | 17 | 20:3 (n-3) | 0.32 | 0.68 |
| 4 | 15:0 | 0.44 | 0.20 | 18 | 20:2 (n-6) | 0.67 | - |
| 5 | 16:1 (n-9) | 8.66 | 3.59 | 19 | 20:1 (n-9) | 2.50 | 6.25 |
| 6 | 16:2 (n-4) | 0.32 | - | 20 | 20:4 (n-6) | 10.95 | 30.96 |
| 7 | 16:1 (n-7) | 3.08 | 2.04 | 21 | 20:5 (n-3) | 6.42 | 13.39 |
| 8 | 16:0 | 25.10 | 11.74 | 22 | 20:3 (n-6) | 2.46 | 5.55 |
| 9 | 18:4 (n-3) | 3.67 | 2.64 | 23 | 20:4 (n-3) | 1.15 | 1.46 |
| 10 | 18:2 (n-6) | 1.86 | 1.81 | 24 | 22:6 (n-3) | 0.22 | 0.31 |
| 11 | 18:1 (n-9) | 8.87 | 4.62 | 25 | 22:1 (n-9) | 0.55 | 0.27 |
| 12 | 18:1 (n-7) | 0.87 | 0.91 | 26 | 22:6 (n-6) | - | 0.30 |
| 13 | 18:3 (n-3) | 2.19 | 2.19 | 27 | 22:4 (n-6) | - | 0.57 |
| 14 | 18:3 (n-6) | 0.85 | 0.64 | 28 | others | 0.65 | 4.27 |
| SFA | 41.74 | 17.22 | Omega-6 | 16.79 | 39.83 | ||
| MUFA | 26.53 | 18.01 | Omega-9 | 20.55 | 14.73 | ||
| PUFA | 31.08 | 60.50 | PUFA/SFA | 0.74 | 3.51 | ||
| Omega-3 | 13.97 | 20.67 | n3/n6 | 0.83 | 0.52 | ||
| No. | Molecular Species | Molecular Weight [M − H]− | Molecular Formula (MF) | Retention Time (Rt, min) | Content in Total PI (%) | |
|---|---|---|---|---|---|---|
| Egg | Body | |||||
| 1. | PI 16:0/20:5 | 855.5122 | C45H77O13P | 18.308 | 0.77 | 0.07 |
| 2. | PI 16:0/20:4 | 857.5291 | C45H79O13P | 17.893 | 3.36 | 1.44 |
| 3. | PI 18:0e/20:5 | 869.5626 | C47H83O12P | 16.239 | 0.69 | 0.54 |
| 4. | PI 17:0/20:4 | 871.5443 | C46H81O13P | 17.568 | 0.90 | 0.46 |
| 5. | PI 18:0e/20:4 | 871.5786 | C47H85O12P | 15.748 | 4.82 | 4.71 |
| 6. | PI 18:1/20:5 | 881.5264 | C47H79O13P | 18.156 | 0.57 | 0.17 |
| 7. | PI 18:0/20:5 | 883.5401 | C47H81O13P | 17.661 | 9.36 | 6.96 |
| 8. | PI 18:0/20:4 | 885.5562 | C47H83O13P | 17.212 | 38.65 | 48.19 |
| 9. | PI 18:0/20:3 | 887.5741 | C47H85O13P | 16.938 | 2.17 | 2.19 |
| 10. | PI 20:1e/20:4 | 897.5883 | C49H87O12P | 15.551 | 0.69 | 0.40 |
| 11. | PI 19:0/20:5 | 897.5507 | C48H83O13P | 17.327 | 1.23 | 1.08 |
| 12. | PI 19:0/20:4 | 899.5699 | C48H85O13P | 16.873 | 5.92 | 6.43 |
| 13. | PI 40:8 | 905.5219 | C49H79O13P | 18.195 | 0.44 | 0.55 |
| 14. | PI 20:3/20:4 | 907.5339 | C49H81O13P | 17.870 | 0.72 | 0.50 |
| 15. | PI 20:1/20:5 | 909.5489 | C49H83O13P | 17.444 | 2.91 | 2.21 |
| 16. | PI 20:0/20:5 | 911.5645 | C49H85O13P | 16.959 | 13.37 | 12.64 |
| 17. | PI 20:0/20:4 | 913.5814 | C49H87O13P | 16.573 | 11.49 | 9.89 |
| 18. | PI 20:0/20:3 | 915.5966 | C49H89O13P | 16.273 | 0.69 | 0.46 |
| 19. | PI 21:1/20:4 | 925.5774 | C50H87O13P | 16.684 | 0.71 | 0.87 |
| 20. | PI 21:0/20:4 | 927.5927 | C50H89O13P | 16.243 | 0.42 | 0.24 |
| No. | Molecular Species | Molecular Weight [M – H]− | Molecular Formula (MF) | Retention Time (Rt, min) | Content in Total PS (%) | |
|---|---|---|---|---|---|---|
| Eggs | Body | |||||
| 1. | PS 38:5 | 808.5111 | C44H76NO10P | 15.524 | 1.27 | 0.50 |
| 2. | PS 18:0/20:4 | 810.5312 | C44H78NO10P | 15.067 | 5.47 | 3.88 |
| 3. | PS 20:1/18:1 | 814.5532 | C44H82NO10P | 13.959 | 5.87 | 0.60 |
| 4. | PS 20:1/20:4 | 836.5447 | C46H80NO10P | 14.747 | 9.67 | 5.30 |
| 5. | PS 40:4 | 838.5545 | C46H82NO10P | 13.907 | 2.68 | 1.53 |
| 6. | PS 20:1/20:1 | 842.5962 | C46H86NO10P | 12.900 | 42.48 | 44.41 |
| 7. | PS 20:1/21:1 | 856.6115 | C47H88NO10P | 12.646 | 10.43 | 14.60 |
| 8. | PS 20:1/22:4 | 864.5818 | C48H84NO10P | 12.874 | 7.71 | 6.03 |
| 9. | PS 20:1/22:2 | 868.6151 | C48H88NO10P | 12.815 | 5.43 | 8.09 |
| 10. | PS 20:1/22:1 | 870.6207 | C48H90NO10P | 12.338 | 7.31 | 11.32 |
| 11. | PS 21:1/22:4 | 878.5876 | C49H86NO10P | 12.629 | 2.07 | 2.70 |
| No. | Molecular Species | Molecular Weight [M – H]− | Molecular Formula (MF) | Retention Time (Rt, min) | Content in Total PE (%) | |
|---|---|---|---|---|---|---|
| Egg | Body | |||||
| 1. | PE 16:1e/20:4 | 722.5249 | C41H74NO7P | 5.931 | 2.83 | 3.58 |
| 2. | PE 36:3e | 726.5584 | C41H78NO7P | 5.619 | 1.15 | 1.80 |
| 3. | PE 37:6e | 734.5102 | C42H74NO7P | 5.986 | 0.62 | 0.41 |
| 4. | PE 17:1e/20:4 | 736.5281 | C42H76NO7P | 5.724 | 2.11 | 2.47 |
| 5. | PE 37:3e | 740.5585 | C42H80NO7P | 5.250 | 0.42 | 0.93 |
| 6. | PE 18:1e/20:5 | 748.5244 | C43H76NO7P | 5.811 | 10.56 | 6.60 |
| 7. | PE 18:1e/20:4 | 750.5424 | C43H78NO7P | 5.531 | 41.21 | 40.03 |
| 8. | PE 18:0e/20:4 | 752.5572 | C43H80NO7P | 5.365 | 6.88 | 5.28 |
| 9. | PE18:1e/20:2 | 754.5742 | C43H82NO7P | 5.141 | 7.98 | 13.68 |
| 10. | PE 19:1e/20:4 | 764.5616 | C44H80NO7P | 5.304 | 1.85 | 1.63 |
| 11. | PE 18:1/20:4 | 764.5205 | C43H76NO8P | 6.382 | 1.41 | 2.95 |
| 12. | PE 18:0/20:4 | 766.5372 | C43H78NO8P | 6.161 | 2.52 | 3.41 |
| 13. | PE 20:2e/20:5 | 774.5364 | C45H78NO7P | 5.662 | 1.62 | 0.97 |
| 14. | PE 20:2e/20:4 | 776.5542 | C45H80NO7P | 5.381 | 6.58 | 4.41 |
| 15. | PE 20:1e/20:4 | 778.5565 | C45H82NO7P | 5.205 | 6.56 | 5.25 |
| 16. | PE 39:5 | 778.5317 | C44H78NO8P | 6.057 | 0.31 | 0.14 |
| 17. | PE 20:2e/20:2 | 780.5863 | C45H84NO7P | 5.075 | 2.25 | 2.34 |
| 18. | PE 39:4 | 780.5457 | C44H80NO8P | 5.085 | 0.14 | 0.11 |
| 19. | PE 40:8e | 790.5334 | C45H78NO8P | 6.204 | 0.53 | 0.59 |
| 20. | PE 20:1/20:4 | 792.5579 | C45H80NO8P | 4.945 | 1.07 | 1.12 |
| 21. | PE 20:1/20:4 isomer | 792.5498 | C45H80NO8P | 5.996 | 1.44 | 1.77 |
| 22. | PE 20:1/20:1 | 798.5944 | C45H86NO8P | 5.237 | 0.27 | 0.54 |
| No. | Molecular Species | Molecular Weight [M – H]− | Molecular Formula (MF) | Retention Time (Rt, min) | Content in Total PA (%) | |
|---|---|---|---|---|---|---|
| Egg | Body | |||||
| 1. | PA 18:1e/20:4 | 707.4949 | C41H73O7P | 4.131 | 3.09 | 2.64 |
| 2. | PA 20:1/18:1 | 727.5227 | C41H77O8P | 4.162 | 19.18 | 1.72 |
| 3. | PA 38:1 | 729.5479 | C41H79O8P | 2.064 | 1.07 | 1.70 |
| 4. | PA 39:2 | 741.5406 | C42H79O8P | 4.074 | 2.17 | 3.43 |
| 5. | PA 40:5 | 749.5069 | C43H75O8P | 4.329 | 7.95 | 6.29 |
| 6. | PA 40:4 | 751.5273 | C43H77O8P | 4.200 | 8.76 | 3.45 |
| 7. | PA 40:3 | 753.5372 | C43H79O8P | 4.105 | 6.07 | 4.84 |
| 8. | PA 20:1/20:1 | 755.5570 | C43H81O8P | 3.904 | 52.42 | 52.60 |
| 9. | PA 20:1/21:1 | 769.5727 | C44H83O8P | 3.953 | 6.69 | 10.75 |
| 10. | PA 20:1/22:2 | 781.5719 | C45H81O8P | 3.963 | 3.96 | 7.06 |
| 11. | PA 42:2 | 783.5871 | C45H85O8P | 3.915 | 2.83 | 5.52 |
| No. | Molecular Species | Molecular Weight [M + H]+ | Molecular Formula (MF) | Retention Time (Rt, min) | Content in Total PC (%) | |
|---|---|---|---|---|---|---|
| Egg | Body | |||||
| 1. | PC 30:0e | 692.5549 | C38H78NO7P | 8.461 | 0.61 | 0.85 |
| 2. | PC 14:0/16:1 | 704.5201 | C38H74NO8P | 10.767 | 1.05 | 0.69 |
| 3. | PC 31:0e | 706.5763 | C39H80NO7P | 4.094 | 0.21 | 0.27 |
| 4. | PC 14:0/16:0 | 706.5381 | C38H76NO8P | 10.214 | 1.33 | 1.95 |
| 5. | PC 16:0e/16:1 | 718.5753 | C40H80NO7P | 8.261 | 0.67 | 1.38 |
| 6. | PC 31:1 | 718.5373 | C39H76NO8P/4 | 10.245 | 0.12 | 0.15 |
| 7. | PC 32:0e | 720.5907 | C40H82NO7P | 7.923 | 0.69 | 1.27 |
| 8. | PC 31:0 | 720.5506 | C39H78NO8P | 9.700 | 0.20 | 0.28 |
| 9. | PC 32:2 | 730.5374 | C40H76NO8P | 10.409 | 0.78 | 0.68 |
| 10. | PC 33:1e | 732.5879 | C41H82NO7P/3 | 7.898 | 0.22 | 0.41 |
| 11. | PC 16:0/16:1 | 732.5615 | C40H78NO8P | 9.851 | 3.08 | 3.16 |
| 12. | PC 33:0e | 734.6039 | C41H84NO7P/2 | 7.650 | 0.35 | 0.21 |
| 13. | PC 16:0/16:0 | 734.5714 | C40H80NO8P | 9.651 | 1.46 | 2.25 |
| 14. | PC 34:4e | 740.563 | C42H78NO7P | 8.625 | 0.17 | 0.28 |
| 15. | PC 34:3e | 742.5725 | C42H80NO7P | 8.365 | 0.18 | 0.26 |
| 16. | PC 34:2e | 744.5921 | C42H82NO7P | 8.022 | 0.23 | 0.31 |
| 17. | PC 33:2 | 744.5538 | C41H78NO8P | 10.034 | 0.08 | 0.15 |
| 18. | PC 16:0e/18:1 | 746.6071 | C42H84NO7P | 7.637 | 0.87 | 1.20 |
| 19. | PC 17:0/16:1 | 746.5754 | C41H80NO8P | 9.389 | 0.26 | 0.46 |
| 20. | PC 34:0e | 748.6226 | C42H86NO7P | 7.420 | 0.41 | 0.41 |
| 21. | PC 33:0 | 748.5921 | C41H82NO8P/3 | 9.003 | 0.27 | 0.18 |
| 22. | PC 35:4e | 754.5794 | C43H80NO7P | 8.230 | 0.08 | 0.11 |
| 23. | PC 34:4 | 754.5339 | C42H76NO8P | 10.397 | 1.62 | 1.33 |
| 24. | PC 34:3 | 756.5537 | C42H78NO8P | 10.019 | 1.66 | 1.37 |
| 25. | PC 16:1/18:1 | 758.5755 | C42H80NO8P | 9.515 | 1.55 | 1.33 |
| 26. | PC 16:0/18:1 | 760.5882 | C42H82NO8P | 9.020 | 3.59 | 3.37 |
| 27. | PC 34:0 | 762.6052 | C42H84NO8P | 8.757 | 0.86 | 0.55 |
| 28. | PC 16:0e/20:5 | 766.5762 | C44H80NO7P | 8.282 | 2.06 | 2.17 |
| 29. | PC 16:0e/20:4 | 768.5954 | C44H82NO7P | 7.904 | 3.60 | 4.36 |
| 30. | PC 16:0e/20:3 | 770.6110 | C44H84NO7P | 7.759 | 0.77 | 1.05 |
| 31. | PC 36:2e | 772.6268 | C44H80NO7P | 7.416 | 0.50 | 0.63 |
| 32. | PC 35:2 | 772.5811 | C43H82NO8P | 9.076 | 0.20 | 0.28 |
| 33. | PC 36:1e | 774.6345 | C44H88NO7P | 7.076 | 0.49 | 0.52 |
| 34. | PC 35:1 | 774.5959 | C43H84NO8P | 8.695 | 0.19 | 0.33 |
| 35. | PC 36:6 | 778.5337 | C44H76NO8P | 10.421 | 0.66 | 0.50 |
| 36. | PC 37:5e | 780.5907 | C45H82NO7P | 7.992 | 0.62 | 0.53 |
| 37. | PC 16:0/20:5 | 780.5498 | C44H78NO8P | 9.905 | 3.46 | 2.77 |
| 38. | PC 17:0e/20:4 | 782.6112 | C45H84NO7P | 7.605 | 0.98 | 1.01 |
| 39. | PC 16:0/20:4 | 782.5703 | C44H80NO8P | 9.409 | 4.76 | 4.21 |
| 40. | PC 16:0/20:3 | 784.5843 | C44H82NO8P | 9.175 | 2.63 | 2.66 |
| 41. | PC 16:0/20:2 | 786.6031 | C44H84NO8P | 8.702 | 3.60 | 3.42 |
| 42. | PC 16:0/20:1 | 788.6177 | C44H86NO8P | 8.446 | 1.12 | 1.66 |
| 43. | PC 38:6e | 792.5874 | C46H82NO7P | 8.067 | 0.70 | 0.62 |
| 44. | PC 18:0e/20:5 | 794.6091 | C46H84NO7P | 7.697 | 4.26 | 3.85 |
| 45. | PC 18:0e/20:4 | 796.6243 | C46H86NO7P | 7.320 | 7.77 | 7.18 |
| 46. | PC 18:0e/20:3 | 798.6404 | C46H88NO7P | 7.009 | 2.01 | 2.55 |
| 47. | PC 37:3 | 798.6045 | C45H84NO8P/6 | 8.783 | 0.17 | 0.16 |
| 48. | PC 18:0e/20:2 | 800.6484 | C46H90NO7P | 6.837 | 1.32 | 1.37 |
| 49. | PC 37:2 | 800.6126 | C45H86NO8P/5 | 8.352 | 0.21 | 0.24 |
| 50. | PC 38:7 | 804.5497 | C46H78NO8P | 10.103 | 0.87 | 0.62 |
| 51. | PC 38:6 | 806.5599 | C46H80NO8P | 9.579 | 2.48 | 1.82 |
| 52. | PC 39:5e | 808.6155 | C47H86NO7P | 7.474 | 0.21 | 0.21 |
| 53. | PC 18:1/20:4 | 808.5769 | C46H82NO8P | 9.086 | 4.00 | 3.05 |
| 54. | PC 39:4e | 810.6348 | C47H88NO7P | 7.047 | 0.34 | 0.31 |
| 55. | PC 18:0/20:4 | 810.6025 | C46H84NO8P | 8.690 | 3.88 | 3.60 |
| 56. | PC 38:3 | 812.6167 | C46H86NO8P | 8.377 | 1.69 | 1.55 |
| 57. | PC 18:1/20:1 | 814.6241 | C46H88NO8P | 8.101 | 1.54 | 0.86 |
| 58. | PC 40:6e | 820.6119 | C48H86NO7P | 7.522 | 1.35 | 1.18 |
| 59. | PC 39:6 | 820.5864 | C47H82NO8P | 9.069 | 0.19 | 0.17 |
| 60. | PC 20:1e/20:4 | 822.6367 | C48H88NO7P | 7.138 | 3.11 | 3.03 |
| 61. | PC 39:5 | 822.5991 | C47H84NO8P | 8.680 | 0.39 | 0.38 |
| 62 | PC 40:4e | 824.6498 | C48H90NO7P | 6.862 | 1.98 | 1.82 |
| 63. | PC 39:4 | 824.6192 | C47H86NO8P | 8.355 | 0.21 | 0.21 |
| 64. | PC 40:9 | 828.5505 | C48H78NO8P | 10.116 | 0.84 | 0.91 |
| 65. | PC 40:8 | 830.5639 | C48H80NO8P | 9.650 | 1.28 | 1.37 |
| 66. | PC 40:7 | 832.5771 | C48H82NO8P | 9.214 | 1.66 | 1.69 |
| 67. | PC 20:1/20:5 | 834.5997 | C48H84NO8P | 8.722 | 3.38 | 3.15 |
| 68. | PC 41:5e | 836.6457 | C49H90NO7P | 6.924 | 0.30 | 0.42 |
| 69. | PC 20:1/20:4 | 836.6159 | C48H86NO8P | 8.320 | 3.74 | 3.23 |
| 70. | PC 20:2/20:2 | 838.6321 | C48H88NO8P | 8.023 | 1.95 | 1.82 |
| 71. | PC 40:3 | 840.6399 | C48H90NO8P | 7.753 | 0.78 | 0.83 |
| 72. | PC 40:5e | 850.6595 | C50H92NO7P/7 | 6.748 | 0.45 | 0.61 |
| 73. | PC 41:5 | 850.6241 | C49H88NO8P | 8.121 | 0.19 | 0.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dinh, T.-K.-H.; Nguyen, P.-H.; Phuong, D.L.; Dang, T.-P.-L.; Quan, P.M.; Dao, T.-K.-D.; Grigorchuk, V.P.; Long, P.Q. Component and Content of Lipid Classes and Phospholipid Molecular Species of Eggs and Body of the Vietnamese Sea Urchin Tripneustes gratilla. Molecules 2023, 28, 3721. https://doi.org/10.3390/molecules28093721
Dinh T-K-H, Nguyen P-H, Phuong DL, Dang T-P-L, Quan PM, Dao T-K-D, Grigorchuk VP, Long PQ. Component and Content of Lipid Classes and Phospholipid Molecular Species of Eggs and Body of the Vietnamese Sea Urchin Tripneustes gratilla. Molecules. 2023; 28(9):3721. https://doi.org/10.3390/molecules28093721
Chicago/Turabian StyleDinh, Thi-Kim-Hoa, Phi-Hung Nguyen, Doan Lan Phuong, Thi-Phuong-Ly Dang, Pham Minh Quan, Thi-Kim-Dung Dao, Valeria P. Grigorchuk, and Pham Quoc Long. 2023. "Component and Content of Lipid Classes and Phospholipid Molecular Species of Eggs and Body of the Vietnamese Sea Urchin Tripneustes gratilla" Molecules 28, no. 9: 3721. https://doi.org/10.3390/molecules28093721