Application of Photocatalysis and Sonocatalysis for Treatment of Organic Dye Wastewater and the Synergistic Effect of Ultrasound and Light
Abstract
:1. Introduction
2. Sonocatalytic and Photocatalytic Mechanisms
2.1. Sonocatalytic Mechanism
2.1.1. Heterogeneous Nucleation Mechanism
2.1.2. Photo-Excitation Mechanism
2.1.3. Thermal Excitation Mechanism
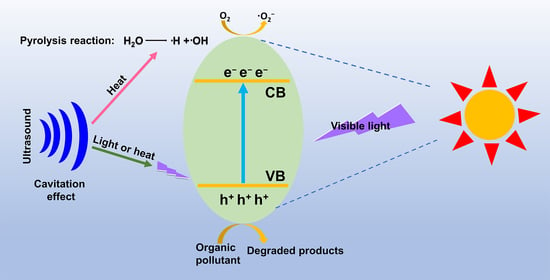
2.2. Photocatalytic Mechanism
2.3. Comparison of Sonocatalytic and Photocatalytic Mechanisms
2.3.1. Similarity
2.3.2. Difference
3. Sonophotocatalytic Process
3.1. Sonophotocatalytic Mechanism
3.2. Summary of the Synergistic Effect during Sonophotocatalytic Process
4. Degradation of Dyes Using TiO2-Based Semiconductor Catalysts
5. Further Research Trends
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, W.; Ding, K.; Chen, J.; Wang, H.; Deng, X. Synergistic Multisystem Photocatalytic Degradation of Anionic and Cationic Dyes Using Graphitic Phase Carbon Nitride. Molecules 2023, 28, 2796. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Feng, H.; Li, L.; Gong, J.; Jiang, K.; Xue, S.; Chu, P.K. Synthesis of tetragonal prismatic γ-In2Se3 nanostructures with predominantly {110} facets and photocatalytic degradation of tetracycline. Appl. Catal. B-Environ. 2020, 260, 118218. [Google Scholar] [CrossRef]
- Zou, X.; Zhang, J.; Zhao, X.; Zhang, Z. MoS2/RGO composites for photocatalytic degradation of ranitidine and elimination of NDMA formation potential under visible light. Chem. Eng. J. 2020, 383, 123084. [Google Scholar] [CrossRef]
- Huang, D.; Wang, H.; Wu, Y. Photocatalytic Aerobic Oxidation of Biomass-Derived 5-HMF to DFF over MIL-53(Fe)/g-C3N4 Composite. Molecules 2022, 27, 8537. [Google Scholar] [CrossRef] [PubMed]
- Heidari, S.; Haghighi, M.; Shabani, M. Sunlight-activated BiOCl/BiOBr–Bi24O31Br10 photocatalyst for the removal of pharmaceutical compounds. J. Clean. Prod. 2020, 259, 120679. [Google Scholar] [CrossRef]
- Zhou, Y.; Yu, M.; Liang, H.; Chen, J.; Xu, L.; Niu, J. Novel dual-effective Z-scheme heterojunction with g-C3N4, Ti3C2 MXene and black phosphorus for improving visible light-induced degradation of ciprofloxacin. Appl. Catal. B-Environ. 2021, 291, 120105. [Google Scholar] [CrossRef]
- Liu, K.; Tong, Z.; Muhammad, Y.; Huang, G.; Zhang, H.; Wang, Z.; Zhu, Y.; Tang, R. Synthesis of sodium dodecyl sulfate modified BiOBr/magnetic bentonite photocatalyst with Three-dimensional parterre like structure for the enhanced photodegradation of tetracycline and ciprofloxacin. Chem. Eng. J. 2020, 388, 124374. [Google Scholar] [CrossRef]
- Zhang, M.; Lai, C.; Li, B.; Huang, D.; Liu, S.; Qin, L.; Yi, H.; Fu, Y.; Xu, F.; Li, M. Ultrathin oxygen-vacancy abundant WO3 decorated monolayer Bi2WO6 nanosheet: A 2D/2D heterojunction for the degradation of Ciprofloxacin under visible and NIR light irradiation. J. Colloid Interface Sci. 2019, 556, 557–567. [Google Scholar] [CrossRef]
- Irshad, A.; Warsi, M.F.; Agboola, P.O.; Dastgeer, G.; Shahid, M. Sol-gel assisted Ag doped NiAl2O4 nanomaterials and their nanocomposites with g-C3N4 nanosheets for the removal of organic effluents. J. Alloys Compd. 2022, 902, 163805. [Google Scholar] [CrossRef]
- Gao, P.; Cui, J.; Deng, Y. Direct regeneration of ion exchange resins with sulfate radical-based advanced oxidation for enabling a cyclic adsorption–regeneration treatment approach to aqueous perfluorooctanoic acid (PFOA). Chem. Eng. J. 2021, 405, 126698. [Google Scholar] [CrossRef]
- Wu, J.; Wang, T.; Wang, J.; Zhang, Y.; Pan, W.-P. A novel modified method for the efficient removal of Pb and Cd from wastewater by biochar: Enhanced the ion exchange and precipitation capacity. Sci. Total Environ. 2021, 754, 142150. [Google Scholar] [CrossRef] [PubMed]
- Vapnik, H.; Elbert, J.; Su, X. Redox-copolymers for the recovery of rare earth elements by electrochemically regenerated ion-exchange. J. Mater. Chem. A 2021, 9, 20068–20077. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Li, M.; Shi, J.; Wang, L.; Luo, S.; Liu, H. Chemical Flocculation-Based Green Algae Materials for Photobiological Hydrogen Production. ACS Appl. Bio. Mater. 2022, 5, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Kurniawan, S.B.; Imron, M.F.; Sługocki, Ł.; Nowakowski, K.; Ahmad, A.; Najiya, D.; Abdullah, S.R.S.; Othman, A.R.; Purwanti, I.F.; Hasan, H.A. Assessing the effect of multiple variables on the production of bioflocculant by Serratia marcescens: Flocculating activity, kinetics, toxicity, and flocculation mechanism. Sci. Total Environ. 2022, 836, 155564. [Google Scholar] [CrossRef]
- Wang, X.; Wang, D.; Xu, J.; Fu, J.; Zheng, G.; Zhou, L. Modified chemical mineralization-alkali neutralization technology: Mineralization behavior at high iron concentrations and its application in sulfur acid spent pickling solution. Water Res. 2022, 218, 118513. [Google Scholar] [CrossRef]
- Xie, L.; Du, T.; Wang, J.; Ma, Y.; Ni, Y.; Liu, Z.; Zhang, L.; Yang, C.; Wang, J. Recent advances on heterojunction-based photocatalysts for the degradation of persistent organic pollutants. Chem. Eng. J. 2021, 426, 130617. [Google Scholar] [CrossRef]
- Wang, L.; Bahnemann, D.W.; Bian, L.; Dong, G.; Zhao, J.; Wang, C. Two-dimensional layered zinc silicate nanosheets with excellent photocatalytic performance for organic pollutant degradation and CO2 conversion. Angew. Chem. Int. Ed. 2019, 131, 8187–8192. [Google Scholar] [CrossRef]
- Qutub, N.; Singh, P.; Sabir, S.; Sagadevan, S.; Oh, W.-C. Enhanced photocatalytic degradation of Acid Blue dye using CdS/TiO2 nanocomposite. Sci. Rep. 2022, 12, 5759. [Google Scholar] [CrossRef]
- Han, B.; Xie, A.; Yu, Q.; Huang, F.; Shen, Y.; Zhu, L. Synthesis of PbSO4 crystals by hydrogel template on postprocessing strategy for secondary pollution. Appl. Surf. Sci. 2012, 261, 623–627. [Google Scholar] [CrossRef]
- Bao, S.; Li, K.; Ning, P.; Peng, J.; Jin, X.; Tang, L. Highly effective removal of mercury and lead ions from wastewater by mercaptoamine-functionalised silica-coated magnetic nano-adsorbents: Behaviours and mechanisms. Appl. Surf. Sci. 2017, 393, 457–466. [Google Scholar] [CrossRef]
- Xin, S.; Zeng, Z.; Zhou, X.; Luo, W.; Shi, X.; Wang, Q.; Deng, H.; Du, Y. Recyclable Saccharomyces cerevisiae loaded nanofibrous mats with sandwich structure constructing via bio-electrospraying for heavy metal removal. J. Hazard. Mater. 2017, 324, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Mamba, G.; Mishra, A. Advances in magnetically separable photocatalysts: Smart, recyclable materials for water pollution mitigation. Catalysts 2016, 6, 79. [Google Scholar] [CrossRef]
- Wang, G.; Cheng, H. Facile synthesis of a novel recyclable dual Z-scheme WO3/NiFe2O4/BiOBr composite with broad-spectrum response and enhanced sonocatalytic performance for levofloxacin removal in aqueous solution. Chem. Eng. J. 2023, 461, 141941. [Google Scholar] [CrossRef]
- Vasseghian, Y.; Dragoi, E.-N.; Almomani, F. A comprehensive review on MXenes as new nanomaterials for degradation of hazardous pollutants: Deployment as heterogeneous sonocatalysis. Chemosphere 2022, 287, 132387. [Google Scholar] [CrossRef] [PubMed]
- Nas, M.S. AgFe2O4/MWCNT nanoparticles as novel catalyst combined adsorption-sonocatalytic for the degradation of methylene blue under ultrasonic irradiation. J. Environ. Chem. Eng. 2021, 9, 105207. [Google Scholar] [CrossRef]
- Dulta, K.; Koşarsoy Ağçeli, G.; Chauhan, P.; Jasrotia, R.; Chauhan, P.; Ighalo, J.O. Multifunctional CuO nanoparticles with enhanced photocatalytic dye degradation and antibacterial activity. Sustain. Environ. Res. 2022, 32, 1–15. [Google Scholar] [CrossRef]
- Ramamoorthy, S.; Das, S.; Balan, R.; Lekshmi, I. TiO2-ZrO2 nanocomposite with tetragonal zirconia phase and photocatalytic degradation of Alizarin Yellow GG azo dye under natural sunlight. Mater. Today Proc. 2021, 47, 4641–4646. [Google Scholar] [CrossRef]
- Wani, S.I.; Ganie, A.S. Ag2O incorporated ZnO-TiO2 nanocomposite: Ionic conductivity and photocatalytic degradation of an organic dye. Inorg. Chem. Commun. 2021, 128, 108567. [Google Scholar] [CrossRef]
- Vellingiri, K.; Vikrant, K.; Kumar, V.; Kim, K.-H. Advances in thermocatalytic and photocatalytic techniques for the room/low temperature oxidative removal of formaldehyde in air. Chem. Eng. J. 2020, 399, 125759. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, T.; Jiang, S.; Ma, X.; Shao, X.; Liu, Y.; Wang, D.; Li, X.; Li, B. Controllable self-assembly of BiOI/oxidized mesocarbon microbeads core-shell composites: A novel hierarchical structure facilitated photocatalytic activities. Chem. Eng. Sci. 2020, 221, 115653. [Google Scholar] [CrossRef]
- Lei, X.; Ouyang, C.; Huang, K. A first-principles investigation of Janus MoSSe as a catalyst for photocatalytic water-splitting. Appl. Surf. Sci. 2021, 537, 147919. [Google Scholar] [CrossRef]
- Majumder, S.; Chatterjee, S.; Basnet, P.; Mukherjee, J. ZnO based nanomaterials for photocatalytic degradation of aqueous pharmaceutical waste solutions–A contemporary review. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100386. [Google Scholar] [CrossRef]
- Costarramone, N.; Kartheuser, B.; Pecheyran, C.; Pigot, T.; Lacombe, S. Efficiency and harmfulness of air-purifying photocatalytic commercial devices: From standardized chamber tests to nanoparticles release. Catal. Today 2015, 252, 35–40. [Google Scholar] [CrossRef]
- Cushing, S.K.; Li, J.; Meng, F.; Senty, T.R.; Suri, S.; Zhi, M.; Li, M.; Bristow, A.D.; Wu, N. Photocatalytic activity enhanced by plasmonic resonant energy transfer from metal to semiconductor. J. Am. Chem. Soc. 2012, 134, 15033–15041. [Google Scholar] [CrossRef]
- Sheikh, M.; Pazirofteh, M.; Dehghani, M.; Asghari, M.; Rezakazemi, M.; Valderrama, C.; Cortina, J.-L. Application of ZnO nanostructures in ceramic and polymeric membranes for water and wastewater technologies: A review. Chem. Eng. J. 2020, 391, 123475. [Google Scholar] [CrossRef]
- Zhou, D.; Wu, S.; Cheng, G.; Che, C.-M. A gold (iii)–TADF emitter as a sensitizer for high-color-purity and efficient deep-blue solution-processed OLEDs. J. Mater. Chem. C 2022, 10, 4590–4596. [Google Scholar] [CrossRef]
- Nemati, F.; Nikkhah, S.H.; Elhampour, A. An environmental friendly approach for the catalyst-free synthesis of highly substituted pyrazoles promoted by ultrasonic radiation. Chin. Chem. Lett. 2015, 26, 1397–1399. [Google Scholar] [CrossRef]
- Ali El-Remaily, M.A.E.A.A.; El-Dabea, T.; Alsawat, M.; Mahmoud, M.H.; Alfi, A.A.; El-Metwaly, N.; Abu-Dief, A.M. Development of new thiazole complexes as powerful catalysts for synthesis of pyrazole-4-carbonitrile derivatives under ultrasonic irradiation condition supported by DFT studies. ACS Omega 2021, 6, 21071–21086. [Google Scholar] [CrossRef]
- Wojcieszyńska, D.; Łagoda, K.; Guzik, U. Diclofenac Biodegradation by Microorganisms and with Immobilised Systems—A Review. Catalysts 2023, 13, 412. [Google Scholar]
- Zhu, Z.-H.; Liu, Y.; Song, C.; Hu, Y.; Feng, G.; Tang, B.Z. Porphyrin-Based Two-Dimensional Layered Metal–Organic Framework with Sono-/Photocatalytic Activity for Water Decontamination. ACS Nano 2021, 16, 1346–1357. [Google Scholar] [CrossRef]
- Guo, L.; Chen, Y.; Ren, Z.; Li, X.; Zhang, Q.; Wu, J.; Li, Y.; Liu, W.; Li, P.; Fu, Y. Morphology engineering of type-II heterojunction nanoarrays for improved sonophotocatalytic capability. Ultrason. Sonochem. 2021, 81, 105849. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, M.; Kahkha, M.R.R.; Fakhri, A.; Tahami, S.; Lariche, M.J. Degradation of macrolide antibiotics via sono or photo coupled with Fenton methods in the presence of ZnS quantum dots decorated SnO2 nanosheets. J. Photochem. Photobiol. B 2018, 185, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Preeyanghaa, M.; Vinesh, V.; Neppolian, B. Construction of S-scheme 1D/2D rod-like g-C3N4/V2O5 heterostructure with enhanced sonophotocatalytic degradation for Tetracycline antibiotics. Chemosphere 2022, 287, 132380. [Google Scholar] [CrossRef]
- Liu, J.; Ma, N.; Wu, W.; He, Q. Recent progress on photocatalytic heterostructures with full solar spectral responses. Chem. Eng. J. 2020, 393, 124719. [Google Scholar] [CrossRef]
- Rodríguez-González, V.; Obregón, S.; Patrón-Soberano, O.A.; Terashima, C.; Fujishima, A. An approach to the photocatalytic mechanism in the TiO2-nanomaterials microorganism interface for the control of infectious processes. Appl. Catal. B-Environ. 2020, 270, 118853. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.-J.; Shen, C.-H.; Fei, Z.-H.; Fang, D.; Liu, Z.-T.; Dai, J.-T.; Niu, C.-G. Recent developments on AgI based heterojunction photocatalytic systems in photocatalytic application. Chem. Eng. J. 2020, 383, 123083. [Google Scholar] [CrossRef]
- Theerthagiri, J.; Lee, S.J.; Karuppasamy, K.; Arulmani, S.; Veeralakshmi, S.; Ashokkumar, M.; Choi, M.Y. Application of advanced materials in sonophotocatalytic processes for the remediation of environmental pollutants. J. Hazard. Mater. 2021, 412, 125245. [Google Scholar] [CrossRef]
- Qiu, P.; Park, B.; Choi, J.; Thokchom, B.; Pandit, A.B.; Khim, J. A review on heterogeneous sonocatalyst for treatment of organic pollutants in aqueous phase based on catalytic mechanism. Ultrason. Sonochem. 2018, 45, 29–49. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Wu, Z.; Abramova, A.V.; Cravotto, G. Sonochemical processes for the degradation of antibiotics in aqueous solutions: A review. Ultrason. Sonochem. 2021, 74, 105566. [Google Scholar] [CrossRef]
- He, Y.; Ma, Z.; Junior, L.B. Distinctive binary g-C3N4/MoS2 heterojunctions with highly efficient ultrasonic catalytic degradation for levofloxacin and methylene blue. Ceram. Int. 2020, 46, 12364–12372. [Google Scholar] [CrossRef]
- Waheed, I.F.; Al-Janabi, O.Y.T.; Foot, P.J. Novel MgFe2O4-CuO/GO heterojunction magnetic nanocomposite: Synthesis, characterization, and batch photocatalytic degradation of methylene blue dye. J. Mol. Liq. 2022, 357, 119084. [Google Scholar] [CrossRef]
- Xu, X.; Xu, X.; Wang, T.; Xu, M.; Yang, X.; Hou, J.; Cao, D.; Wang, Q. Construction of Z-scheme CdS/Ag/TiO2 NTs photocatalysts for photocatalytic dye degradation and hydrogen evolution. Spectrochim. Acta A 2022, 276, 121215. [Google Scholar] [CrossRef]
- Abazari, R.; Sanati, S.; Morsali, A.; Kirillov, A.M. Instantaneous sonophotocatalytic degradation of tetracycline over NU-1000@ ZnIn2S4 core–shell nanorods as a robust and eco-friendly catalyst. Inorg. Chem. 2021, 60, 9660–9672. [Google Scholar] [CrossRef] [PubMed]
- Hoo, D.Y.; Low, Z.L.; Low, D.Y.S.; Tang, S.Y.; Manickam, S.; Tan, K.W.; Ban, Z.H. Ultrasonic cavitation: An effective cleaner and greener intensification technology in the extraction and surface modification of nanocellulose. Ultrason. Sonochem. 2022, 90, 106176. [Google Scholar] [CrossRef] [PubMed]
- Moftakhari Anasori Movahed, S.; Calgaro, L.; Marcomini, A. Trends and characteristics of employing cavitation technology for water and wastewater treatment with a focus on hydrodynamic and ultrasonic cavitation over the past two decades: A Scientometric analysis. Sci. Total Environ. 2023, 858, 159802. [Google Scholar] [CrossRef]
- He, L.-L.; Zhu, Y.; Qi, Q.; Li, X.-Y.; Bai, J.-Y.; Xiang, Z.; Wang, X. Synthesis of CaMoO4 microspheres with enhanced sonocatalytic performance for the removal of Acid Orange 7 in the aqueous environment. Sep. Purif. Technol. 2021, 276, 119370. [Google Scholar] [CrossRef]
- Wang, G.; Ma, X.; Liu, J.; Qin, L.; Li, B.; Hu, Y.; Cheng, H. Design and performance of a novel direct Z-scheme NiGa2O4/CeO2 nanocomposite with enhanced sonocatalytic activity. Sci. Total Environ. 2020, 741, 140192. [Google Scholar] [CrossRef]
- Gao, J.; Jiang, R.; Wang, J.; Kang, P.; Wang, B.; Li, Y.; Li, K.; Zhang, X. The investigation of sonocatalytic activity of Er3+: YAlO3/TiO2-ZnO composite in azo dyes degradation. Ultrason. Sonochem. 2011, 18, 541–548. [Google Scholar] [CrossRef]
- Gao, H.; Pei, K.; Hu, G.; Liu, W.; Meng, A.; Wang, H.; Shao, H.; Li, W. The influence of pressure on the acoustic cavitation in saturated CO2-expanded N, N-dimethylformamide. Ultrason. Sonochem. 2022, 83, 105934. [Google Scholar] [CrossRef]
- Kozmus, G.; Zevnik, J.; Hočevar, M.; Dular, M.; Petkovšek, M. Characterization of cavitation under ultrasonic horn tip—Proposition of an acoustic cavitation parameter. Ultrason. Sonochem. 2022, 89, 106159. [Google Scholar] [CrossRef]
- Yao, C.; Zhao, S.; Liu, L.; Liu, Z.; Chen, G. Ultrasonic emulsification: Basic characteristics, cavitation, mechanism, devices and application. Front. Chem. Sci. Eng. 2022, 16, 1560–1583. [Google Scholar] [CrossRef]
- Zhang, H.; Qiao, J.; Li, G.; Li, S.; Wang, G.; Wang, J.; Song, Y. Preparation of Ce4+-doped BaZrO3 by hydrothermal method and application in dual-frequent sonocatalytic degradation of norfloxacin in aqueous solution. Ultrason. Sonochem. 2018, 42, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Li, S.; Ma, X.; Qiao, J.; Li, G.; Zhang, H.; Wang, J.; Song, Y. A novel Z-scheme sonocatalyst system, Er3+:Y3Al5O12@Ni(Fe0.05Ga0.95)2O4-Au-BiVO4, and application in sonocatalytic degradation of sulfanilamide. Ultrason. Sonochem. 2018, 45, 150–166. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, G.; Zhang, H.; Li, G.; Fang, D.; Wang, J.; Song, Y. Hydrothermal-precipitation preparation of CdS@(Er3+:Y3Al5O12/ZrO2) coated composite and sonocatalytic degradation of caffeine. Ultrason. Sonochem. 2017, 37, 222–234. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Huang, Y.; Li, G.; Zhang, H.; Wang, Y.; Li, B.; Wang, J.; Song, Y. Preparation of a novel sonocatalyst, Au/NiGa2O4-Au-Bi2O3 nanocomposite, and application in sonocatalytic degradation of organic pollutants. Ultrason. Sonochem. 2017, 38, 335–346. [Google Scholar] [CrossRef]
- Abdurahman, M.H.; Abdullah, A.Z.; Shoparwe, N.F. A comprehensive review on sonocatalytic, photocatalytic, and sonophotocatalytic processes for the degradation of antibiotics in water: Synergistic mechanism and degradation pathway. Chem. Eng. J. 2021, 413, 127412. [Google Scholar] [CrossRef]
- Hu, Y.; Wei, J.; Shen, Y.; Chen, S.; Chen, X. Barrier-breaking effects of ultrasonic cavitation for drug delivery and biomarker release. Ultrason. Sonochem. 2023, 94, 106346. [Google Scholar] [CrossRef]
- Li, S.; Wang, G.; Qiao, J.; Zhou, Y.; Ma, X.; Zhang, H.; Li, G.; Wang, J.; Song, Y. Sonocatalytic degradation of norfloxacin in aqueous solution caused by a novel Z-scheme sonocatalyst, mMBIP-MWCNT-In2O3 composite. J. Mol. Liq. 2018, 254, 166–176. [Google Scholar] [CrossRef]
- Hassandoost, R.; Kotb, A.; Movafagh, Z.; Esmat, M.; Guegan, R.; Endo, S.; Jevasuwan, W.; Fukata, N.; Sugahara, Y.; Khataee, A.; et al. Nanoarchitecturing bimetallic manganese cobaltite spinels for sonocatalytic degradation of oxytetracycline. Chem. Eng. J. 2022, 431, 133851. [Google Scholar] [CrossRef]
- Afzal, M.Z.; Zu, P.; Zhang, C.-M.; Guan, J.; Song, C.; Sun, X.-F.; Wang, S.-G. Sonocatalytic degradation of ciprofloxacin using hydrogel beads of TiO2 incorporated biochar and chitosan. J. Hazard. Mater. 2022, 434, 128879. [Google Scholar] [CrossRef]
- Jorfi, S.; Pourfadakari, S.; Kakavandi, B. A new approach in sono-photocatalytic degradation of recalcitrant textile wastewater using MgO@ Zeolite nanostructure under UVA irradiation. Chem. Eng. J. 2018, 343, 95–107. [Google Scholar] [CrossRef]
- Isari, A.A.; Mehregan, M.; Mehregan, S.; Hayati, F.; Kalantary, R.R.; Kakavandi, B. Sono-photocatalytic degradation of tetracycline and pharmaceutical wastewater using WO3/CNT heterojunction nanocomposite under US and visible light irradiations: A novel hybrid system. J. Hazard. Mater. 2020, 390, 122050. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-C.; Wang, J.-Q.; Wang, Y.; Chen, C.-L.; Wang, X.; Xiang, Z. Sonocatalytic degradation of ciprofloxacin by BiOBr/BiFeO3. Appl. Catal. A 2022, 643, 118776. [Google Scholar] [CrossRef]
- Xu, L.; Liu, N.-P.; An, H.-L.; Ju, W.-T.; Liu, B.; Wang, X.-F.; Wang, X. Preparation of Ag3PO4/CoWO4 S-scheme heterojunction and study on sonocatalytic degradation of tetracycline. Ultrason. Sonochem. 2022, 89, 106147. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.L.; Koe, A.Z.Y.; Chan, Y.Y.; Lim, S.; Chong, W.C. Enhanced Sonocatalytic Performance of Non-Metal Graphitic Carbon Nitride (g-C3N4)/Coconut Shell Husk Derived-Carbon Composite. Sustainability 2022, 14, 3244. [Google Scholar] [CrossRef]
- Sun, M.; Lin, X.; Meng, X.; Liu, W.; Ding, Z. Ultrasound-driven ferroelectric polarization of TiO2/Bi0.5Na0.5TiO3 heterojunctions for improved sonocatalytic activity. J. Alloys Compd. 2022, 892, 162065. [Google Scholar] [CrossRef]
- Li, S.; Zhang, M.; Ma, X.; Qiao, J.; Zhang, H.; Wang, J.; Song, Y. Preparation of ortho-symmetric double (OSD) Z-scheme SnO2\CdSe/Bi2O3 sonocatalyst by ultrasonic-assisted isoelectric point method for effective degradation of organic pollutants. J. Ind. Eng. Chem. 2019, 72, 157–169. [Google Scholar] [CrossRef]
- Lu, L.; Wang, T.; Fang, C.; Song, L.; Qian, C.; Lv, Z.; Fang, Y.; Liu, X.; Yu, X.; Xu, X.; et al. Oncolytic Impediment/Promotion Balance Disruption by Sonosensitizer-Free Nanoplatforms Unfreezes Autophagy-Induced Resistance to Sonocatalytic Therapy. ACS Appl. Mater. Interfaces 2022, 14, 36462–36472. [Google Scholar] [CrossRef]
- Haddadi, S.; Khataee, A.; Arefi-Oskoui, S.; Vahid, B.; Orooji, Y.; Yoon, Y. Titanium-based MAX-phase with sonocatalytic activity for degradation of oxytetracycline antibiotic. Ultrason. Sonochem. 2023, 92, 106255. [Google Scholar] [CrossRef]
- Wang, G.; Dou, K.; Cao, H.; Du, R.; Liu, J.; Tsidaeva, N.; Wang, W. Designing Z-scheme CdS/WS2 heterojunctions with enhanced photocatalytic degradation of organic dyes and photoreduction of Cr (VI): Experiments, DFT calculations and mechanism. Sep. Purif. Technol. 2022, 291, 120976. [Google Scholar] [CrossRef]
- Dharman, R.K.; Shejale, K.P.; Kim, S.Y. Efficient sonocatalytic degradation of heavy metal and organic pollutants using CuS/MoS2 nanocomposites. Chemosphere 2022, 305, 135415. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Ma, S.; Zhan, S. Superior photocatalytic disinfection effect of Ag-3D ordered mesoporous CeO2 under visible light. Appl. Catal. B-Environ. 2018, 224, 27–37. [Google Scholar] [CrossRef]
- Wan, L.; Zhou, Q.; Wang, X.; Wood, T.E.; Wang, L.; Duchesne, P.N.; Guo, J.; Yan, X.; Xia, M.; Li, Y.F. Cu2O nanocubes with mixed oxidation-state facets for (photo) catalytic hydrogenation of carbon dioxide. Nat. Catal. 2019, 2, 889–898. [Google Scholar] [CrossRef]
- Xu, L.; Wu, X.-Q.; Li, C.-Y.; Liu, N.-P.; An, H.-L.; Ju, W.-T.; Lu, W.; Liu, B.; Wang, X.-F.; Wang, Y.; et al. Sonocatalytic degradation of tetracycline by BiOBr/FeWO4 nanomaterials and enhancement of sonocatalytic effect. J. Clean. Prod. 2023, 394, 136275. [Google Scholar] [CrossRef]
- Xiang, W.; Ji, Q.; Xu, C.; Guo, Y.; Liu, Y.; Sun, D.; Zhou, W.; Xu, Z.; Qi, C.; Yang, S. Accelerated photocatalytic degradation of iohexol over Co3O4/g-C3N4/Bi2O2CO3 of pn/nn dual heterojunction under simulated sunlight by persulfate. Appl. Catal. B Environ. 2021, 285, 119847. [Google Scholar] [CrossRef]
- Qiu, J.; Li, M.; Xu, J.; Zhang, X.-F.; Yao, J. Bismuth sulfide bridged hierarchical Bi2S3/BiOCl@ZnIn2S4 for efficient photocatalytic Cr (VI) reduction. J. Hazard. Mater. 2020, 389, 121858. [Google Scholar] [CrossRef]
- Liu, J.; Wang, G.; Li, B.; Ma, X.; Hu, Y.; Cheng, H. A high-efficiency mediator-free Z-scheme Bi2MoO6/AgI heterojunction with enhanced photocatalytic performance. Sci. Total Environ. 2021, 784, 147227. [Google Scholar] [CrossRef]
- Vaiano, V.; Iervolino, G.; Sannino, D.; Murcia, J.J.; Hidalgo, M.C.; Ciambelli, P.; Navío, J.A. Photocatalytic removal of patent blue V dye on Au-TiO2 and Pt-TiO2 catalysts. Appl. Catal. B-Environ. 2016, 188, 134–146. [Google Scholar] [CrossRef]
- Li, S.; Zhang, M.; Qu, Z.; Cui, X.; Liu, Z.; Piao, C.; Li, S.; Wang, J.; Song, Y. Fabrication of highly active Z-scheme Ag/g-C3N4-Ag-Ag3PO4 (1 1 0) photocatalyst photocatalyst for visible light photocatalytic degradation of levofloxacin with simultaneous hydrogen production. Chem. Eng. J. 2020, 382, 122394. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, Z.; Hao, J.; Zhang, T.; Sun, Q.; Wang, Y. Boosted charge transfer in dual Z-scheme BiVO4@ ZnIn2S4/Bi2Sn2O7 heterojunctions: Towards superior photocatalytic properties for organic pollutant degradation. Chemosphere 2021, 276, 130226. [Google Scholar] [CrossRef]
- Wang, G.; Ma, X.; Wang, C.; Li, S.; Qiao, J.; Zhang, H.; Li, G.; Wang, J.; Song, Y. Highly efficient visible-light driven photocatalytic hydrogen evolution over Er3+: YAlO3/Ta2O5/rGO/MoSe2 nanocomposite. J. Mol. Liq. 2018, 260, 375–385. [Google Scholar] [CrossRef]
- Wang, G.; Ma, X.; Wei, S.; Li, S.; Qiao, J.; Wang, J.; Song, Y. Highly efficient visible-light driven photocatalytic hydrogen production from a novel Z-scheme Er3+: YAlO3/Ta2O5-V5+|| Fe3+-TiO2/Au coated composite. J. Power Sources 2018, 373, 161–171. [Google Scholar] [CrossRef]
- Zhao, G.; Ding, J.; Zhou, F.; Chen, X.; Wei, L.; Gao, Q.; Wang, K.; Zhao, Q. Construction of a visible-light-driven magnetic dual Z-scheme BiVO4/g-C3N4/NiFe2O4 photocatalyst for effective removal of ofloxacin: Mechanisms and degradation pathway. Chem. Eng. J. 2021, 405, 126704. [Google Scholar] [CrossRef]
- Molla, A.; Kim, A.Y.; Woo, J.C.; Cho, H.S.; Youk, J.H. Study on preparation methodology of zero-valent iron decorated on graphene oxide for highly efficient sonocatalytic dye degradation. J. Environ. Chem. Eng. 2022, 10, 107214. [Google Scholar] [CrossRef]
- Wang, X.; He, X.-S.; Li, C.-Y.; Liu, S.-L.; Lu, W.; Xiang, Z.; Wang, Y. Sonocatalytic removal of tetracycline in the presence of S-scheme Cu2O/BiFeO3 heterojunction: Operating parameters, mechanisms, degradation pathways and toxicological evaluation. J. Water Process Eng. 2023, 51, 103345. [Google Scholar] [CrossRef]
- Dharman, R.K.; Palanisamy, G.; Oh, T.H. Sonocatalytic degradation of ciprofloxacin and organic pollutant by 1T/2H phase MoS2 in Polyvinylidene fluoride nanocomposite membrane. Chemosphere 2022, 308, 136571. [Google Scholar] [CrossRef]
- Akdağ, S.; Sadeghi Rad, T.; Keyikoğlu, R.; Orooji, Y.; Yoon, Y.; Khataee, A. Peroxydisulfate-assisted sonocatalytic degradation of metribuzin by La-doped ZnFe layered double hydroxide. Ultrason. Sonochem. 2022, 91, 106236. [Google Scholar] [CrossRef]
- Liu, C.; Mao, S.; Wang, H.; Wu, Y.; Wang, F.; Xia, M.; Chen, Q. Peroxymonosulfate-assisted for facilitating photocatalytic degradation performance of 2D/2D WO3/BiOBr S-scheme heterojunction. Chem. Eng. J. 2022, 430, 132806. [Google Scholar] [CrossRef]
- de Jesús Ruíz-Baltazar, Á. Sonochemical activation-assisted biosynthesis of Au/Fe3O4 nanoparticles and sonocatalytic degradation of methyl orange. Ultrason. Sonochem. 2021, 73, 105521. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, Y.; Zhang, K.; Zada, A.; Qi, K. Sonocatalytic degradation of tetracycline hydrochloride with CoFe2O4/g-C3N4 composite. Ultrason. Sonochem. 2023, 94, 106325. [Google Scholar] [CrossRef]
- Wang, X.; Yu, S.; Li, Z.-H.; He, L.-L.; Liu, Q.-L.; Hu, M.-Y.; Xu, L.; Wang, X.-F.; Xiang, Z. Fabrication Z-scheme heterojunction of Ag2O/ZnWO4 with enhanced sonocatalytic performances for meloxicam decomposition: Increasing adsorption and generation of reactive species. Chem. Eng. J. 2021, 405, 126922. [Google Scholar] [CrossRef]
- Sadeghi Rad, T.; Ansarian, Z.; Khataee, A.; Vahid, B.; Doustkhah, E. N-doped graphitic carbon as a nanoporous MOF-derived nanoarchitecture for the efficient sonocatalytic degradation process. Sep. Purif. Technol. 2021, 256, 117811. [Google Scholar] [CrossRef]
- Gote, Y.M.; Sinhmar, P.S.; Gogate, P.R. Sonocatalytic Degradation of Chrysoidine R Dye Using Ultrasonically Synthesized NiFe2O4 Catalyst. Catalysts 2023, 13, 597. [Google Scholar] [CrossRef]
- Joseph, C.G.; Puma, G.L.; Bono, A.; Krishnaiah, D. Sonophotocatalysis in advanced oxidation process: A short review. Ultrason. Sonochem. 2009, 16, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Malika, M.; Sonawane, S.S. The sono-photocatalytic performance of a Fe2O3 coated TiO2 based hybrid nanofluid under visible light via RSM. Colloids Surf. A 2022, 641, 128545. [Google Scholar] [CrossRef]
- Mosleh, S.; Rahimi, M.R.; Ghaedi, M.; Asfaram, A.; Jannesar, R.; Sadeghfar, F. A rapid and efficient sonophotocatalytic process for degradation of pollutants: Statistical modeling and kinetics study. J. Mol. Liq. 2018, 261, 291–302. [Google Scholar] [CrossRef]
- Karim, A.V.; Shriwastav, A. Degradation of amoxicillin with sono, photo, and sonophotocatalytic oxidation under low-frequency ultrasound and visible light. Environ. Res. 2021, 200, 111515. [Google Scholar] [CrossRef]
- Dinesh, G.K.; Anandan, S.; Sivasankar, T. Sonophotocatalytic treatment of Bismarck Brown G dye and real textile effluent using synthesized novel Fe (0)-doped TiO2 catalyst. RSC Adv. 2015, 5, 10440–10451. [Google Scholar] [CrossRef]
- Al-Musawi, T.J.; Rajiv, P.; Mengelizadeh, N.; Mohammed, I.A.; Balarak, D. Development of sonophotocatalytic process for degradation of acid orange 7 dye by using titanium dioxide nanoparticles/graphene oxide nanocomposite as a catalyst. J. Environ. Manag. 2021, 292, 112777. [Google Scholar] [CrossRef]
- Rameshbabu, R.; Kumar, N.; Pecchi, G.; Delgado, E.J.; Karthikeyan, C.; Mangalaraja, R. Ultrasound-assisted synthesis of rGO supported NiO-TiO2 nanocomposite: An efficient superior sonophotocatalyst under diffused sunlight. J. Environ. Chem. Eng. 2022, 10, 107701. [Google Scholar] [CrossRef]
- Gokul, P.; Vinoth, R.; Neppolian, B.; Anandhakumar, S. Binary metal oxide nanoparticle incorporated composite multilayer thin films for sono-photocatalytic degradation of organic pollutants. Appl. Surf. Sci. 2017, 418, 119–127. [Google Scholar] [CrossRef]
- Ding, Z.; Sun, M.; Liu, W.; Sun, W.; Meng, X.; Zheng, Y. Ultrasonically synthesized N-TiO2/Ti3C2 composites: Enhancing sonophotocatalytic activity for pollutant degradation and nitrogen fixation. Sep. Purif. Technol. 2021, 276, 119287. [Google Scholar] [CrossRef]
- Wang, S.; Gong, Q.; Liang, J. Sonophotocatalytic degradation of methyl orange by carbon nanotube/TiO2 in aqueous solutions. Ultrason. Sonochem. 2009, 16, 205–208. [Google Scholar] [CrossRef]
- Ahmad, R.; Ahmad, Z.; Khan, A.U.; Mastoi, N.R.; Aslam, M.; Kim, J. Photocatalytic systems as an advanced environmental remediation: Recent developments, limitations and new avenues for applications. J. Environ. Chem. Eng. 2016, 4, 4143–4164. [Google Scholar] [CrossRef]
- Sathishkumar, P.; Mangalaraja, R.V.; Mansilla, H.D.; Gracia-Pinilla, M.; Anandan, S. Sonophotocatalytic (42 kHz) degradation of Simazine in the presence of Au–TiO2 nanocatalysts. Appl. Catal. B-Environ. 2014, 160, 692–700. [Google Scholar] [CrossRef]
- Hapeshi, E.; Fotiou, I.; Fatta-Kassinos, D. Sonophotocatalytic treatment of ofloxacin in secondary treated effluent and elucidation of its transformation products. Chem. Eng. J. 2013, 224, 96–105. [Google Scholar] [CrossRef]
- Mosleh, S.; Rahimi, M.; Ghaedi, M.; Dashtian, K. Sonophotocatalytic degradation of trypan blue and vesuvine dyes in the presence of blue light active photocatalyst of Ag3PO4/Bi2S3-HKUST-1-MOF: Central composite optimization and synergistic effect study. Ultrason. Sonochem. 2016, 32, 387–397. [Google Scholar] [CrossRef]
- Babu, S.G.; Karthik, P.; John, M.C.; Lakhera, S.K.; Ashokkumar, M.; Khim, J.; Neppolian, B. Synergistic effect of sono-photocatalytic process for the degradation of organic pollutants using CuO-TiO2/rGO. Ultrason. Sonochem. 2019, 50, 218–223. [Google Scholar] [CrossRef]
- Benomara, A.; Guenfoud, F.; Mokhtari, M.; Boudjemaa, A. Sonolytic, sonocatalytic and sonophotocatalytic degradation of a methyl violet 2B using iron-based catalyst. React. Kinet. Mech. Catal. 2021, 132, 513–528. [Google Scholar] [CrossRef]
- Ahmad, M.; Ahmed, E.; Hong, Z.; Ahmed, W.; Elhissi, A.; Khalid, N. Photocatalytic, sonocatalytic and sonophotocatalytic degradation of Rhodamine B using ZnO/CNTs composites photocatalysts. Ultrason. Sonochem. 2014, 21, 761–773. [Google Scholar] [CrossRef]
- Abdullah, A.Z.; Ling, P.Y. Heat treatment effects on the characteristics and sonocatalytic performance of TiO2 in the degradation of organic dyes in aqueous solution. J. Hazard. Mater. 2010, 173, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Mahanta, U.; Khandelwal, M.; Deshpande, A.S. TiO2@SiO2 nanoparticles for methylene blue removal and photocatalytic degradation under natural sunlight and low-power UV light. Appl. Surf. Sci. 2022, 576, 151745. [Google Scholar] [CrossRef]
- Rajagopal, S.; Paramasivam, B.; Muniyasamy, K. Photocatalytic removal of cationic and anionic dyes in the textile wastewater by H2O2 assisted TiO2 and micro-cellulose composites. Sep. Purif. Technol. 2020, 252, 117444. [Google Scholar] [CrossRef]
- Li, S.; Wang, J.; Xia, Y.; Li, P.; Wu, Y.; Yang, K.; Song, Y.; Jiang, S.; Zhang, T.; Li, B. Boosted electron-transfer by coupling Ag and Z-scheme heterostructures in CdSe-Ag-WO3-Ag for excellent photocatalytic H2 evolution with simultaneous degradation. Chem. Eng. J. 2021, 417, 129298. [Google Scholar] [CrossRef]
- Hunge, Y.M.; Yadav, A.A.; Dhodamani, A.G.; Suzuki, N.; Terashima, C.; Fujishima, A.; Mathe, V.L. Enhanced photocatalytic performance of ultrasound treated GO/TiO2 composite for photocatalytic degradation of salicylic acid under sunlight illumination. Ultrason. Sonochem. 2020, 61, 104849. [Google Scholar] [CrossRef]
- Ribao, P.; Corredor, J.; Rivero, M.J.; Ortiz, I. Role of reactive oxygen species on the activity of noble metal-doped TiO2 photocatalysts. J. Hazard. Mater. 2019, 372, 45–51. [Google Scholar] [CrossRef]
- Gogoi, D.; Namdeo, A.; Golder, A.K.; Peela, N.R. Ag-doped TiO2 photocatalysts with effective charge transfer for highly efficient hydrogen production through water splitting. Int. J. Hydrogen Energy 2020, 45, 2729–2744. [Google Scholar] [CrossRef]
- Wang, R.; Tang, T.; Wei, Y.; Dang, D.; Huang, K.; Chen, X.; Yin, H.; Tao, X.; Lin, Z.; Dang, Z.; et al. Photocatalytic debromination of polybrominated diphenyl ethers (PBDEs) on metal doped TiO2 nanocomposites: Mechanisms and pathways. Environ. Int. 2019, 127, 5–12. [Google Scholar] [CrossRef]
- Zhang, H.; Tang, P.; Yang, K.; Wang, Q.; Feng, W.; Tang, Y. PAA/TiO2@C composite hydrogels with hierarchical pore structures as high efficiency adsorbents for heavy metal ions and organic dyes removal. Desalination 2023, 558, 116620. [Google Scholar] [CrossRef]
- Nuengmatcha, P.; Chanthai, S.; Mahachai, R.; Oh, W.-C. Sonocatalytic performance of ZnO/graphene/TiO2 nanocomposite for degradation of dye pollutants (methylene blue, texbrite BAC-L, texbrite BBU-L and texbrite NFW-L) under ultrasonic irradiation. Dyes Pigm. 2016, 134, 487–497. [Google Scholar] [CrossRef]
- Yao, Y.; Sun, M.; Yuan, X.; Zhu, Y.; Lin, X.; Anandan, S. One-step hydrothermal synthesis of N/Ti3+ co-doping multiphasic TiO2/BiOBr heterojunctions towards enhanced sonocatalytic performance. Ultrason. Sonochem. 2018, 49, 69–78. [Google Scholar] [CrossRef]
- Sriramoju, J.B.; Muniyappa, M.; Marilingaiah, N.R.; Sabbanahalli, C.; Shetty, M.; Mudike, R.; Chitrabanu, C.; Shivaramu, P.D.; Nagaraju, G.; Rangappa, K.S. Carbon-based TiO2-x heterostructure nanocomposites for enhanced photocatalytic degradation of dye molecules. Ceram. Int. 2021, 47, 10314–10321. [Google Scholar] [CrossRef]
- Mousavi, M.; Ghasemi, J.B. Novel visible-light-responsive Black-TiO2/CoTiO3 Z-scheme heterojunction photocatalyst with efficient photocatalytic performance for the degradation of different organic dyes and tetracycline. J. Taiwan Inst. Chem. Eng. 2021, 121, 168–183. [Google Scholar] [CrossRef]
- May-Lozano, M.; Lopez-Medina, R.; Escamilla, V.M.; Rivadeneyra-Romero, G.; Alonzo-Garcia, A.; Morales-Mora, M.; González-Díaz, M.; Martinez-Degadillo, S. Intensification of the Orange II and Black 5 degradation by sonophotocatalysis using Ag-graphene oxide/TiO2 systems. Chem. Eng. Process. 2020, 158, 108175. [Google Scholar] [CrossRef]
- Sun, M.; Yao, Y.; Ding, W.; Anandan, S. N/Ti3+ co-doping biphasic TiO2/Bi2WO6 heterojunctions: Hydrothermal fabrication and sonophotocatalytic degradation of organic pollutants. J. Alloys Compd. 2020, 820, 153172. [Google Scholar] [CrossRef]




| TiO2-Based Catalyst | Dye | Catalytic Conditions | Experiment Conditions | Result (Kinetic Constant (k) or Degradation Efficiency (%)) | Ref. |
|---|---|---|---|---|---|
| ZnO/graphene/TiO2 (ZGT) | Methylene blue | Bath sonicator Power = 750 W Frequency = 20 kHz | [Catalyst] = 1.00 g/L [Pollutant] = 20 mg/L | 1.97 × 10−2 min−1 | [130] |
| N/Ti3+ TiO2/BiOBr0.3 | Methylene blue, rhodamine B | Bath sonicator Power = 180 W Frequency = 30 kHz | [Catalyst] = 7.5 mg [Pollutant] = 5 mg/L Time = 50 min | 98.2% | [131] |
| Er3+: YAlO3/TiO2-ZnO | Acid red B | Bath sonicator Power = 50 W Frequency = 40 kHz | [Catalyst] = 1.0 g/L [Pollutant] = 10 mg/L Time = 60 min | 76.84% | [58] |
| RGO-TiO2−x | Methylene blue | Light power = 150 W | [Catalyst] = 20 mg [Pollutant] = 5 ppm | 0.075 min−1 | [132] |
| Black-TiO2/CoTiO3 | Rhodamine B, methylene blue, and methyl orange | Light power = 50 W | [Catalyst] = 100 mg [Pollutant] = 5 ppm Time = 60 min | 99% | [133] |
| Au-TiO2 | Patent blue V | Light power = 570 W/m2 | [Catalyst] = 23 g/L [Pollutant] = 7 mg/L Time = 180 min | 93% | [88] |
| TiO2_Ag_Graphene | Black 5 | Bath sonicator Power = 30 W/L Frequency = 40 kHz UV light power = 5 W | [Catalyst] = 0.03 g [Pollutant] = 5 mg/L | 0.05 min−1 | [134] |
| NT-TBWx | Methylene blue (MB) | Bath sonicator Power = 180 W Frequency = 35 kHz UV light power = 100 mW/cm2 | [Catalyst] = 7.5 mg [Pollutant] = 5 mg/L Time = 50 min | 99% | [135] |
| CNTs/TiO2 | methyl orange (MO) | Bath sonicator Power = 50 W Frequency = 20kHz UV light Power = 30 W | [Catalyst] = 50 mg [Pollutant] = 25 ppm | 0.01118 min−1 | [113] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, G.; Cheng, H. Application of Photocatalysis and Sonocatalysis for Treatment of Organic Dye Wastewater and the Synergistic Effect of Ultrasound and Light. Molecules 2023, 28, 3706. https://doi.org/10.3390/molecules28093706
Wang G, Cheng H. Application of Photocatalysis and Sonocatalysis for Treatment of Organic Dye Wastewater and the Synergistic Effect of Ultrasound and Light. Molecules. 2023; 28(9):3706. https://doi.org/10.3390/molecules28093706
Chicago/Turabian StyleWang, Guowei, and Hefa Cheng. 2023. "Application of Photocatalysis and Sonocatalysis for Treatment of Organic Dye Wastewater and the Synergistic Effect of Ultrasound and Light" Molecules 28, no. 9: 3706. https://doi.org/10.3390/molecules28093706