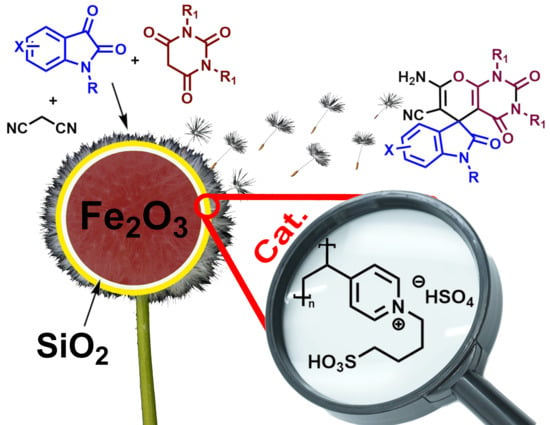
A Sulfonic Acid Polyvinyl Pyridinium Ionic Liquid Catalyzes the Multi-Component Synthesis of Spiro-indoline-3,5′-pyrano[2,3-d]-pyrimidines and -Pyrazines
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalyst Preparation and Characterization
2.2. Synthesis of Spiro-Indoline-3,5′-pyrano[2,3-d]pyrimidines—Optimization
2.3. Mechanistical Considerations
2.4. Synthesis of Spiro-Indoline-3,5′-pyrano[2,3-d]pyrimidine Derivatives—Substrate Scope
2.5. Synthesis of Spiro-Indoline-3,5′-pyrano[2,3-d]pyrazole Derivatives—Substrate Scope
3. Materials and Methods
3.1. Syntheses
3.1.1. Preparation of PVP IL-B-SO3H
3.1.2. Acidity Measurement Using the Barium Sulfate Test
3.1.3. General Procedure for the Preparation of the Pyrimidines (1–6)c
3.1.4. General Procedure for the Preparation of (1–8)d
3.1.5. Catalyst Recovery and Re-Use Tests
3.2. Instrumentation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Panda, S.S.; Girgis, A.S.; Aziz, M.N.; Bekheit, M.S. Spirooxindole: A Versatile Biologically Active Heterocyclic Scaffold. Molecules 2023, 28, 618. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, H.-F.; Ren, S.-Y.; He, M.-X.; Pan, Y.-M. Research Advances in Electrochemical Synthesis of Spirocyclic Skeleton Compounds. Synthesis 2023, 55. [Google Scholar] [CrossRef]
- Boddy, A.J.; Bull, J.A. Stereoselective synthesis and applications of spirocyclic oxindoles. Org. Chem. Front. 2021, 8, 1026–1084. [Google Scholar] [CrossRef]
- Nasri, S.; Bayat, M.; Mirzaei, F. Recent Strategies in the Synthesis of Spiroindole and Spirooxindole Scaffolds. Top. Curr. Chem. 2021, 379, 25. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Yu, D.-Q.; Liu, H.-M. Spirooxindoles: Promising scaffolds for anticancer agents. Eur. J. Med. Chem. 2015, 97, 673–698. [Google Scholar] [CrossRef]
- Ganesh, M.; Suraj, S. Expeditious entry into carbocyclic and heterocyclic spirooxindoles. Org. Biomol. Chem. 2022, 20, 5651–5693. [Google Scholar] [CrossRef]
- Youseftabar-Miri, L.; Hosseinjani-Pirdehi, H.; Akrami, A.; Hallajian, S. Recent investigations in the synthesis of spirooxindole derivatives by Iranian researchers. J. Iran. Chem. Soc. 2020, 17, 2179–2231. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, C.; Xie, H.; Xu, Y.; Wang, C.; Du, C.; Wang, Z.; Wang, L. Green Synthesis of Spirooxindoles via Lipase-Catalyzed One-Pot Tandem Reaction in Aqueous Media. Catalysts 2023, 13, 143. [Google Scholar] [CrossRef]
- Reddy, B.N.; Ramana, C.V. A concise approach for central core of trigolutes: Total synthesis of trigolute B and 3-epi-trigolute B and analogues. Tetrahedron 2017, 73, 888–899. [Google Scholar] [CrossRef]
- Ma, S.-S.; Mei, W.-L.; Guo, Z.-K.; Liu, S.-B.; Zhao, Y.-X.; Yang, D.-L.; Zeng, Y.-B.; Jiang, B.; Dai, H.-F. Two New Types of Bisindole Alkaloid from Trigonostemon lutescens. Org. Lett. 2013, 15, 1492–1495. [Google Scholar] [CrossRef]
- Emami, L.; Moezi, L.; Amiri-Zirtol, L.; Pirsalami, F.; Divar, M.; Solhjoo, A.; Khabnadideh, S. Anticonvulsant activity, molecular modeling and synthesis of spirooxindole-4H-pyran derivatives using a novel reusable organocatalyst. Mol. Divers. 2022, 26, 3129–3141. [Google Scholar] [CrossRef]
- Mohamadpour, F.; Maghsoodlou, M.T.; Lashkari, M.; Heydari, R.; Hazeri, N. Synthesis of Quinolines, Spiro[4H-pyran-oxindoles] and Xanthenes Under Solvent-Free Conditions. Org. Prep. Proc. Int. 2019, 51, 456–476. [Google Scholar] [CrossRef]
- Borah, B.; Sharma, S.; Chowhan, L.R. Visible-Light-Mediated Direct Expeditious Photochemical Construction of Spirocyclic-oxindoles. Asian J. Org. Chem. 2023, 12, e202300020. [Google Scholar] [CrossRef]
- Gupta, N.K.; Haque, J.; Salghi, R.; Lgaz, H.; Mukherjee, A.K.; Quraishi, M.A. Spiro [indoline-3,4′-pyrano[2,3-c]pyrazole] Derivatives as Novel Class of Green Corrosion Inhibitors for Mild Steel in Hydrochloric Acid Medium: Theoretical and Experimental Approach. J. Bio. Tribo. Corr. 2018, 4, 16. [Google Scholar] [CrossRef]
- Ghadiri, S.; Bayat, M.; Hosseini, F.S. A simple and environmentally benign synthesis of novel spiro[indoline-3,5′-pyrano[2,3-d]pyrimidine] derivatives in water. Monatsh. Chem. 2019, 150, 1079–1084. [Google Scholar] [CrossRef]
- Jadidi, K.; Ghahremanzadeh, R.; Bazgir, A. Efficient synthesis of spiro[chromeno[2,3-d]pyrimidine-5,3′-indoline]-tetraones by a one-pot and three-component reaction. J. Comb. Chem. 2009, 11, 341–344. [Google Scholar] [CrossRef]
- Zhang, M.; Fu, Q.-Y.; Gao, G.; He, H.-Y.; Zhang, Y.; Wu, Y.-S.; Zhang, Z.-H. Catalyst-Free, Visible-Light Promoted One-Pot Synthesis of Spirooxindole-Pyran Derivatives in Aqueous Ethyl Lactate. ACS Sust. Chem. Eng. 2017, 5, 6175–6182. [Google Scholar] [CrossRef]
- Elinson, M.N.; Ryzhkov, F.V.; Korolev, V.A.; Egorov, M.P. Pot, atom and step-economic (PASE) synthesis of medicinally relevant spiro[oxindole-3,4′-pyrano[4,3-b]pyran] scaffold. Heterocycl. Commun. 2016, 22, 11–15. [Google Scholar] [CrossRef]
- Sadeghi, B.; Pirbaluti, M.G.; Nezhad, P.F.; Nezhad, R.A. A clean and expedient synthesis of spirooxindoles catalyzed by silica–sulfuric acid nanoparticles as an efficient and reusable reagent. Res. Chem. Intermed. 2015, 41, 4047–4055. [Google Scholar] [CrossRef]
- Rao, B.M.; Reddy, G.N.; Reddy, T.V.; Devi, B.L.A.P.; Prasad, R.B.N.; Yadav, J.S.; Reddy, B.V.S. Carbon–SO3H: A novel and recyclable solid acid catalyst for the synthesis of spiro[4H-pyran-3,3′-oxindoles]. Tetrahedron Lett. 2013, 54, 2466–2471. [Google Scholar] [CrossRef]
- Shanthi, G.; Subbulakshmi, G.; Perumal, P.T. A new InCl3-catalyzed, facile and efficient method for the synthesis of spirooxindoles under conventional and solvent-free microwave conditions. Tetrahedron 2007, 63, 2057–2063. [Google Scholar] [CrossRef]
- Ganesan, A.; Kothandapani, J.; Nanubolu, J.B.; Ganesan, S.S. Oleic acid: A benign Brønsted acidic catalyst for densely substituted indole derivative synthesis. RSC Adv. 2015, 5, 28597–28600. [Google Scholar] [CrossRef]
- Liju, W.; Ablajan, K.; Jun, F. Rapid and efficient one-pot synthesis of spiro[indoline-3,4′-pyrano[2, 3-c]pyrazole] derivatives catalyzed by L-proline under ultrasound irradiation. Ultrason. Sonochem. 2015, 22, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Wang, L.; Li, S.-S.; Liu, Y.; Xu, L. Divergent syntheses of spirooxindoles from oxindole-embedded four-membered synthon via cycloaddition reactions. Org. Chem. Front. 2020, 7, 747–755. [Google Scholar] [CrossRef]
- Khalaj, M.; Zarandi, M. A Cu(II) complex supported on Fe3O4@SiO2 as a magnetic heterogeneous catalyst for the reduction of environmental pollutants. RSC Adv. 2022, 12, 26527–26541. [Google Scholar] [CrossRef]
- Khalaj, M.; Taherkhani, M.; Kalhor, M. Preparation of some chromeno[4,3-d]pyrido[1,2-a]pyrimidine derivatives by ultrasonic irradiation using NiFe2O4@SiO2 grafted di(3-propylsulfonic acid) nanoparticles. New J. Chem. 2021, 45, 10718–10724. [Google Scholar] [CrossRef]
- Khalaj, M.; Mousavi-Safavi, S.M.; Farahani, N.; Lipkowski, J. MgO nanopowders catalyzed synthesis of pyrano[4,3-d]thiazolo[3,2-a]pyrimidine derivatives. Appl. Organomet. Chem. 2020, 34, e5865. [Google Scholar] [CrossRef]
- Khalaj, M. Preparation of benzo[4,5]thiazolo[3,2-a]chromeno[4,3-d]pyrimidin-6-one derivatives using MgO-MgAl2O4 composite nano-powder. Arab. J. Chem. 2020, 13, 6403–6411. [Google Scholar] [CrossRef]
- Varmazyar, A.; Sedaghat, S.; Goli-Kand, A.N.; Khalaj, M.; Arab-Salmanabadi, S. Domino ring opening/cyclization of oxiranes for synthesis of functionalized 2H-pyran-5-carboxylate. Mol. Divers. 2020, 24, 707–716. [Google Scholar] [CrossRef]
- Blackman, L.D.; Gunatillake, P.A.; Cass, P.; Locock, K.E.S. An introduction to zwitterionic polymer behavior and applications in solution and at surfaces. Chem. Soc. Rev. 2019, 48, 757–770. [Google Scholar] [CrossRef]
- Correia, D.M.; Fernandes, L.C.; Martins, P.M.; García-Astrain, C.; Costa, C.M.; Reguera, J.; Lanceros-Méndez, S. Ionic liquid–polymer composites: A new platform for multifunctional applications. Adv. Funct. Mater. 2020, 30, 1909736. [Google Scholar] [CrossRef]
- Eftekhari, A.; Saito, T. Synthesis and properties of polymerized ionic liquids. Eur. Polym. J. 2017, 90, 245–272. [Google Scholar] [CrossRef]
- Makvandi, P.; Iftekhar, S.; Pizzetti, F.; Zarepour, A.; Zare, E.N.; Ashrafizadeh, M.; Agarwal, T.; Padil, V.V.T.; Mohammadinejad. R.; Sillanpaa, M.; et al. Functionalization of polymers and nanomaterials for water treatment, food packaging, textile and biomedical applications: A review. Environ. Chem. Lett. 2021, 19, 583–611. [Google Scholar] [CrossRef]
- An, L.; Wei, C.; Lu, M.; Liu, H.; Chen, Y.; Scherer, G.G.; Fisher, A.C.; Xi, P.; Xu, Z.J.; Yan, C.-H. Recent Development of Oxygen Evolution Electrocatalysts in Acidic Environment. Adv. Mater. 2021, 33, 2006328. [Google Scholar] [CrossRef] [PubMed]
- Dandia, A.; Mahawar, D.K.; Saini, P.; Saini, S.; Gupta, S.L.; Rathore, K.S.; Parewa, V. Site–specific role of bifunctional graphitic carbon nitride catalyst for the sustainable synthesis of 3,3–spirocyclic oxindoles in aqueous media. RSC Adv. 2021, 11, 28452–28465. [Google Scholar] [CrossRef]
- Mohammadi Ziarani, G.; Faramarzi, S.; Lashgari, N.; Badiei, A. A simple and clean method for multicomponent synthesis of spiro[indole-tetrahydropyrano(2,3-d)pyrimidine] derivatives using SBA-Pr-SO3H as catalyst under solvent-free conditions. J. Iran. Chem. Soc. 2014, 11, 701–709. [Google Scholar] [CrossRef]
- Shaikh, S.; Yellapurkar, I.; Ramana, M.M.V. Mn2O3 NPs: A Versatile Catalyst for the Syntheses of Pyrano[3,2-c]Chromenes, Pyrano[3,2-b]Pyrans and Spirooxindoles. Chem. Africa 2021, 4, 812–834. [Google Scholar] [CrossRef]
- Nagaraju, S.; Paplal, B.; Sathish, K.; Giri, S.; Kashinath, D. Synthesis of functionalized chromene and spirochromenes using L-proline-melamine as highly efficient and recyclable homogeneous catalyst at room temperature. Tetrahedron Lett. 2017, 58, 4200–4204. [Google Scholar] [CrossRef]
- Safaei, H.R.; Shekouhy, M.; Shirinfeshan, A.; Rahmanpur, S. CaCl2 as a bifunctional reusable catalyst: Diversity-oriented synthesis of 4H-pyran library under ultrasonic irradiation. Mol. Divers. 2012, 16, 669–683. [Google Scholar] [CrossRef]








 | ||||
| Entry | Catalyst (g) b | Conditions c | Time (min) | Yield (%) d |
| 1 | - | US; EtOH; reflux | 70 | - |
| 2 | 0.04 | US; EtOH; r.t. | 70 | - |
| 3 | 0.04 | US; EtOH; 50 ℃ | 50 | 55 |
| 4 | 0.04 | US; EtOH; reflux | 50 | 95 |
| 5 | 0.02 | US; EtOH; reflux | 70 | 53 |
| 6 | 0.06 | US; EtOH; reflux | 45 | 94 |
| 7 | 0.04 | US; H2O; reflux | 60 | 45 |
| 8 | 0.04 | US; CH3CN; reflux | 60 | 48 |
| 9 | 0.04 | US; Et2O; reflux | 70 | - |
| 10 | 0.04 | US; n-hexane; reflux | 70 | - |
| 11 | 0.04 | US; EtOAc; reflux | 60 | - |
| 12 | 0.04 | US; toluene; 80 °C | 60 | - |
| 13 | 0.04 | US; DMF; 80 °C | 60 | 23 |
| 14 | 0.04 | US; CH2Cl2; reflux | 70 | - |
 | |||||
| Product | R | R1 | Time (min) | Yield (%) b | M.p. (°C) |
| 1c | CH3CH2 | CH3 | 50 | 95 | 289–291 |
| 2c | CH3CH2 | CH3CH2 | 50 | 94 | 285–287 |
| 3c | CH3CH2 | Ph–CH2 | 60 | 93 | ˃300 |
| 4c | CH3CH2CH2 | CH3 | 50 | 89 | 278–280 |
| 5c | Ph–CH2 | CH3 | 50 | 93 | ˃300 |
| 6c | Ph–CH2CH2 | CH3 | 60 | 97 | ˃300 |
 | |||||
| Product | R | X | Time (min) | Yield (%) b | M.p. (°C) |
| 1d | H | 5-CH3 | 90 | 95 | 269–271 |
| 2d | H | 5-OCH3 | 90 | 93 | 273–275 |
| 3d | H | 5-Cl | 70 | 96 | 286–288 |
| 4d | H | 5-Br | 80 | 94 | ˃300 |
| 5d | H | 5,7-CH3 | 90 | 95 | ˃300 |
| 6d | H | 5,7-Br | 60 | 97 | ˃300 |
| 7d | H | 5,7-Cl | 80 | 95 | ˃300 |
| 8d | Ph | 5,7-Cl | 75 | 89 | ˃300 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalaj, M.; Taherkhani, M.; Payen, L.; Klein, A. A Sulfonic Acid Polyvinyl Pyridinium Ionic Liquid Catalyzes the Multi-Component Synthesis of Spiro-indoline-3,5′-pyrano[2,3-d]-pyrimidines and -Pyrazines. Molecules 2023, 28, 3663. https://doi.org/10.3390/molecules28093663
Khalaj M, Taherkhani M, Payen L, Klein A. A Sulfonic Acid Polyvinyl Pyridinium Ionic Liquid Catalyzes the Multi-Component Synthesis of Spiro-indoline-3,5′-pyrano[2,3-d]-pyrimidines and -Pyrazines. Molecules. 2023; 28(9):3663. https://doi.org/10.3390/molecules28093663
Chicago/Turabian StyleKhalaj, Mehdi, Mahboubeh Taherkhani, Leo Payen, and Axel Klein. 2023. "A Sulfonic Acid Polyvinyl Pyridinium Ionic Liquid Catalyzes the Multi-Component Synthesis of Spiro-indoline-3,5′-pyrano[2,3-d]-pyrimidines and -Pyrazines" Molecules 28, no. 9: 3663. https://doi.org/10.3390/molecules28093663