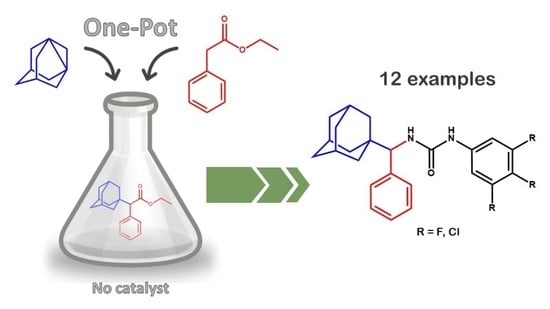
3.3. Synthesis
(±)-2-(3,5-Dimethyladamantane-1-yl)-2-phenylacetic acid ethyl ester (3b).
An amount of 2.5 g (0.015 mol) of 1,3-dehydro-5,7-dimethyladamantane
2b was added to 10 g (0.061 mol) of phenylacetic acid ethyl ester. The reaction mixture was exposed at the temperature of 55–60 °C for 4 h. The excess amount of phenylacetic acid ethyl ester was distilled, and the resulting product was recrystallized from diethyl ether. The yield is 4.3 g (85%), colorless viscous liquid. Mass spectrum, m/z (Irel. %): 326 (15% [M]
+), 253 (56% [(CH
3)
2-Ad-CH-Ph]
+), 163 (100% [(CH
3)
2-Ad]
+) (
Figures S1 and S2). Anal. Calc. for C
18H
22O
2: C 80.94; H 9.26. Found: C 80.92; H 9.28. M = 326.22.
(±)-2-(3,5-Dimethyladamantane-1-yl)-2-phenylacetic acid (
4b). An amount of 4.0 g (0.012 mol) of 2-(3,5-dimethyladamantane-1-yl)phenylacetic acid ethyl ester (
3b) was added to 7.0 g (0.125 mol) KOH in 70 mL of ethylene glycol. The reaction mixture was exposed at 190 °C for 16 h. The cooled reaction mass was diluted with 100 mL of H
2O and extracted with ethyl acetate. The aqueous layer was placed in a rotary evaporator to remove ethyl acetate residues and concentrated hydrochloric acid was added to it until pH = 3. The precipitated white residual matter was filtered and dried in vacuum. The yield is 3.6 g (73%), white powder, m.p. 251–252 °C. Mass spectrum, m/z (I
rel. %): 298 (14% [M]
+), 163 (2% [(CH
3)
2-Ad]
+), 135 (100% [(Ph)-CH-COOH]
+) (
Figures S3 and S4). Calc. for C
20H
26O
2: C 80.50; H 8.78. Found: C 80.48; H 8.80. M = 298.19.
(±)-1-(Isocyanato(phenyl)methyl)-3,5-dimethyladamantane (
5b). A mixture of 3.5 g (11.7 mmol) (3,5-dimethyladamantane-1-yl)phenylacetic acid
4b and 2.37 g (23.4 mmol) triethylamine in 40 mL of anhydrous toluene was treated dropwise with 3.22 g (11.7 mmol) diphenylphosphorylazide at room temperature for 30 min. Then the reaction mixture was heated to boil and exposed for 30 min until the nitrogen release was completely stopped. The toluene was evaporated, and the product was extracted from the reaction mass with anhydrous diethyl ether. The yield is 3.1 g (89%), colorless crystals, m.p. 110–111 °C. Mass spectrum, m/z (I
rel. %): 295 (3% [M]
+), 163 (100% [(CH
3)
2-Ad]
+) (
Figures S5 and S6). Calc. for C
20H
25NO: C 81.31; H 8.53; N 4.74. Found: 81.30; H 8.55.; N 4.71. M = 295.19.
(±)-1-[Adamantan-1-yl(phenyl)methyl]amine hydrochloride (6a).
To 1 g (3.75 mmol) of (±)-1-(isocyanato(phenyl)methyl)adamantane
5a in 20 mL of anhydrous toluene, 0.5 mL of concentrated hydrochloric acid (4.1 mmol of HCl) was added with stirring and the reaction mass was kept for 1 h. The resulting white precipitate was filtered off, washed with acetonitrile, and dried, then recrystallized from water. The yield is 1.03 g (99%), white powder m.p. 262–263 °C. Mass spectrum,
m/z (I rel. %): 241 (1% [M]
+), 135 (12% [Ad]
+), 106 (100% [Ph-CH
2-NH
2]
+) (
Figures S7 and S8).
1H NMR (300 MHz, DMSO-
d6, δ) ppm: 1.25–1.75 m (14H,
(Ad-CH(Ph)-
NH2), 1.91 s (3H, Ad), 7.12–7.35 m (5H, arom) (
Figure S9). Calc. for C
17H
23N: C 84.59; H 9.60; N 5.80. Found: 84.55; H 9.64.; N 5.75. M = 241.38.
Procedure for synthesis series of 1,3-disubstituted ureas
8a–
j and
10a–
c. Atom labels for
13C NMR presented on
Figure 2.
(±)-1-((Adamantyl)(phenyl)methyl)-3-phenyl urea (8a).
Method A. To 200 mg (0.75 mmol) 1-(isocyanato(phenyl)methyl)-3,5-dimethyl -adamantane (5a) in 5 mL of diethyl ether, 80 mg (0.79 mmol) of triethylamine and 70 mg (0.75 mmol) of aniline (7a) were added. The reaction mixture was exposed at room temperature for 12 h. After adding 5 mL of 1 N HCl, the mixture was stirred for 1 h. The precipitated white residual matter was filtered and washed with water. The product was purified by recrystallization from ethanol. Yield of 189 mg (70%), m.p. 253–254 °C.
Method B. To 200 mg (0.72 mmol) hydrochloride 1-[adamantyl(phenyl)methyl] -amine (6a) in 5 mL of diethyl ether, 160 mg (1.58 mmol) of triethylamine and 70 mg (0.75 mmol) of phenylisocyanate (9a) were added. The reaction mixture was exposed at room temperature for 12 h. After adding 5 mL of 1 N HCl, the mixture was stirred for 1 h. The precipitated white residual matter was filtered and washed with water. The product was purified by recrystallization from ethanol. The yield is 221 mg (85%), m.p. 253–254 °C.
1H NMR (300 MHz, DMSO-
d6, δ) ppm: 1.26–1.70 m (12H, Ad), 1.91 s (3H, Ad), 4.36 d (1H, Ad-C
H(Ph)-,
J = 9.2 Hz), 6.76–6.92 m (2H, Ad-CH(Ph)-
NH-C(O)-NH-
Ph-4H), 7.08–7.21 m (4H,
Ph-NH), 7.24–7.40 m (5H,
Ph-CH), 8.40 s (1H,
3NH) (
Figure S10).
13C NMR (75 MHz, DMSO-
d6, δ) ppm: 28.20 (
C3,
C5,
C7), 36.32 (
C1), 36.94 (
C4,
C6,
C10), 38.91 (
C2,
C8,
C9), 62.75 (
C11), 117.73 (
C20,
C24), 121.38 (
C15), 126.94 (
C22), 127.82 (
C14,
C16), 128.71 (
C21,
C23), 129.12 (
C13,
C17), 140.81 (
C19), 140.92 (
C12), 155.17 (
C18) (
Figure S11). Calc. for C
24H
28N
2O: C 79.96; H 7.83; N 7.77. Found: C 79.97; H 7.85; N 7.79. M = 360.22.
(±)-1-((Adamantan-1-yl)(phenyl)methyl)-3-(2-fluorophenyl) urea (8b).
It was obtained similarly to the compound (
8a), by
method A, from 200 mg of the compound (
5a) and 83 mg of 2-fluorophenine (
7b). The yield is 71 mg (25%), m.p. 249–250 °C. It also was obtained by
method B, from 200 mg of the compound (
6a) and 98 mg of 2-fluorophenylisocyanate (
9b). The yield is 218 mg (80%), m.p. 249–250 °C.
1H NMR (300 MHz, DMSO-
d6, δ) ppm: 1.21–1.75 m (12H, Ad), 1.91 s (3H, Ad), 4.37 d (1H, Ad-
CH(Ph),
J = 8.9 Hz), 6.79–6.92 m (2H, N
H-C(O)-NH-
Ph-2F), 6.93–7.05 m (1H, NH-
Ph-2F), 7.07–7.22 m (4H, Ad-CH(
Ph)-NH-C(O)-NH-
Ph-2F), 7.23–7.38 m (2H,
Ph-CH), 8.01–8.20 m (1H, NH-
Ph-2F), 8.40 s (1H,
3NH) (
Figure S12).
13C NMR (75 MHz, DMSO-
d6, δ) ppm: 28.17 (
C3,
C5,
C7), 36.31 (
C1), 36.90 (
C4,
C6,
C10), 38.86 (
C2,
C8,
C9), 62.96 (
C11), 115.14 (d,
J = 18.9 Hz) (
C21), 119.96 (d,
J = 2.0 Hz) (
C23), 121.70 (d,
J = 7.3 Hz) (
C22), 124.81 (d,
J = 3.4 Hz) (
C24), 127.02 (
C15), 127.87 (
C14,
C16), 128.69 (
C13,
C17), 128.893 (d,
J = 10.1 Hz) (
C19), 140.61 (
C12), 151.77 (d,
J = 239.9 Hz) (
C20), 154.88 (
C18) (
Figure S13).
19F NMR (282 MHz, DMSO-
d6, δ) ppm: -133.85 (1F) (
Figure S14). Calc. for C
24H
27FN
2O: C 76.16; H 7.19; N 7.40. Found: C 76.18; H 7.17; N 7.42. M = 378.21.
(±)-1-((Adamantan-1-yl)(phenyl)methyl)-3-(3-fluorophenyl) urea (8c).
It was obtained similarly to the compound (
8a), by
method A, from 200 mg of compound (
5a) and 83 mg of 3-fluorophenine (
7c). The yield is 83 mg (29%), m.p. 233–234 °C.
1H NMR (300 MHz, DMSO-
d6, δ) ppm: 1.32–1.64 m (12H, Ad), 1.91 s (3H, Ad), 4.36 d (1H, Ad-C
H(Ph),
J = 9.3 Hz), 6.64 td (1H,
1NH,
J = 8.4, 2.6 Hz), 6.93-7.00 m (2H, arom), 7.12–7.39 m (7H, Ad-CH(
Ph)-NH-C(O)-NH-
Ph-3F), 7.41 dt (1H, NH-
Ph-3F,
J = 12.3, 2.3 Hz), 8.71 s (1H,
3NH) (
Figure S15).
13C NMR (75 MHz, DMSO-
d6, δ) ppm: 28.19 (
C3,
C5,
C7), 36.31 (
C1), 36.92 (
C4,
C6,
C10), 38.87 (
C2,
C8,
C9), 62.81 (
C11), 104.42 (d,
J = 26.7 Hz) (
C22), 107.62 (d,
J = 21.3 Hz) (
C20), 113.49 (
C24), 126.99 (
C15), 127.84 (
C14,
C16), 128.72 (
C13,
C17), 130.58 (d,
J = 9.9 Hz) (
C23), 140.63 (
C12), 142.82 (d,
J = 11.5 Hz) (
C19), 154.99 (
C18), 162.92 (d,
J = 240.3 Hz) (
C21) (
Figure S16).
19F NMR (282 MHz, DMSO-
d6, δ) ppm: -114.92 (1F) (
Figure S17). Calc. for C
24H
27FN
2O: C 76.16; H 7.19; N 7.40. Found: C 76.17; H 7.18; N 7.41. M = 378.21.
(±)-1-((Adamantan-1-yl)(phenyl)methyl)-3-(4-fluorophenyl) urea (8d).
It was obtained similarly to the compound (
8a), by
method A, from 200 mg of compound (
5a) and 83 mg of 4-fluorophenine (
7d). The yield is 153 mg (54%), m.p. 270–271 °C.
1H NMR (300 MHz, DMSO-
d6, δ) ppm: 1.28–1.68 m (12H, Ad), 1.91 s (3H, Ad), 4.35 d (1H, Ad-C
H(Ph),
J = 9.2 Hz), 6.81 d (1H,
1NH,
J = 9.4 Hz), 7.0 t (2H, NH-
Ph-4F,
J = 8.7 Hz), 7.10–7.44 m (7H, Ad-CH(
Ph)-NH-C(O)-NH-
Ph-F), 8.42 s (1H,
3NH) (
Figure S18).
13C NMR (75 MHz, DMSO-
d6, δ) ppm: 28.16 (
C3,
C5,
C7), 36.29 (
C1), 36.88 (
C4,
C6,
C10), 38.87 (
C2,
C8,
C9), 62.80 (
C11), 115.59 (d,
J = 22.1 Hz) (
C22,
C23), 119.39 (d,
J = 7.6 Hz) (
C20,
C24), 127.01 (
C15), 127.86 (
C14,
C16), 128.68 (
C13,
C17), 137.15 (d,
J = 7.6 Hz) (
C19), 140.67 (
C12), 155.30 (
C18), 157.29 (d,
J = 237.0 Hz) (
C22) (
Figure S19). Calc. for C
24H
27FN
2O: C 76.16; H 7.19; N 7.40. Found: C 76.16; H 7.20; N 7.42. M = 378.21.
(±)-1-((Adamantan-1-yl)(phenyl)methyl)-3-(3-chlorophenyl) urea (8e).
It was obtained similarly to the compound (
8a), by
method A, from 200 mg of compound (
5a) and 96 mg of 3-chloraniline (
7e). The yield is 77 mg (26%), m.p. 244–245 °C.
1H NMR (300 MHz, DMSO-
d6, δ) ppm: 1.40–1.66 m (12H, Ad), 1.91 s (3H, Ad), 4.35 d (1H, Ad-C
H(Ph),
J = 9.3 Hz), 6.83–6.99 m (2H, Ad-CH(Ph)-N
H-C(O)-NH-
Ph-3Cl), 7.10–7.34 m (7H, Ad-CH(
Ph)-NH-C(O)-NH-
Ph-3Cl), 7.64 t (1H, NH-
Ph-3Cl,
J = 2.1 Hz), 8.63 s (1H,
3NH) (
Figure S20).
13C NMR (75 MHz, DMSO-
d6, δ) ppm: 27.72 (
C3,
C5,
C7), 35.72 (
C4,
C6,
C10), 36.46 (
C1), 38.41 (
C2,
C8,
C9), 62.39 (
C11), 115.71 (
C24), 116.66 (
C20), 120.55 (
C15), 126.57 (
C22), 127.41 (
C14,
C16), 128.25 (
C13,
C17), 130.26 (
C23), 133.19 (
C21), 140.13 (
C19), 141.95 (
C12), 154.48 (
C18) (
Figure S21). Calc. for C
24H
27ClN
2O: C 72.99; H 6.89; N 7.09. Found: C 72.98; H 6.92; N 7.11. M = 394.18.
(±)-1-((3,5-Dimethyladamantane-1-yl)(phenyl)methyl)-3-phenyl urea (
8f). Atom labels for
13C NMR presented on
Figure 3.
It was obtained similarly to the compound (
8a), by
method A, from 200 mg of compound (
5b) and 63 mg of aniline (
7a). The yield is 195 mg (74%), m.p. 69–70 °C.
1H NMR (300 MHz, DMSO-
d6, δ) ppm: 0.74 d (6H, (CH
3)
2,
J = 9.8 Hz), 1.00 dd (3H, Ad,
J = 24.6, 12.8 Hz), 1.20 s (10H, Ad), 1.42 d (1H, Ad,
J = 11.8 Hz), 1.98 d (1H, Ad,
J = 12.6 Hz), 4.39 d (1H, Ad-C
H(Ph),
J = 9.3 Hz), 6.84 t (1H,
1NH,
J = 7.6 Hz), 7.15 dd (3H, CH-
Ph,
J = 7.6, 4.4 Hz), 7.22 s (4H, arom), 7.15 dd (2H, NH-
Ph,
J = 11.2, 7.8 Hz), 8.36 s (1H,
3NH) (
Figure S22).
13C NMR (75 MHz, DMSO-
d6, δ) ppm: 29.16 (
C1), 31.03 (
C25,
C26), 31.05 (
C3), 31.09 (
C5), 31.11 (
C7), 38.14 (
C2), 43.09 (
C4,
C10), 45.02 (
C8), 45.22 (
C9), 51.03 (
C6), 62.23 (
C11), 117.63 (
C20,
C24), 121.32 (
C15), 126.90 (
C22), 127.82 (
C14,
C16), 128.62 (
C21,
C23), 129.07 (
C13,
C17), 140.82 (
C19), 140.89 (
C12), 155.02 (
C18) (
Figure S23). Calc. for C
26H
32N
2O: 80.37; H 8.30; N 7.21. Found: C 80.36; H 8.31; N 7.22. M = 388.56.
(±)-1-((3,5-Dimethyladamantane-1-yl)(phenyl)methyl)-3-(2-fluorophenyl) urea (8g).
It was obtained similarly to the compound (
8a), by
method A, from 200 mg of compound (
5b) and 75 mg of 2-fluorophenine (
7b). The yield is 80 mg (29%), m.p. 51–52 °C.
1H NMR (300 MHz, DMSO-
d6, δ) ppm: 0.72 td (6H, (CH
3)
2,
J = 8.9, 4.2 Hz), 0.89–1.32 m (13H, Ad), 4.26 td (1H, Ad-C
H(Ph),
J = 18.0, 9.8 Hz), 6.94–7.36 m (9H, arom), 8.39 s (1H,
3NH) (
Figure S24).
13C NMR (75 MHz, DMSO-
d6, δ) ppm: 29.22 (
C1), 31.08 (
C3), 31.13 (
C25,
C26), 31.15 (
C5), 31.17 (
C7), 38.41 (
C2), 43.14 (
C4,
C10), 45.05 (
C8), 45.22 (
C9), 51.08 (
C6), 63.14 (
C11), 115.13 (d,
J = 18.9 Hz) (
C21), 119.96 (d,
J = 2.0 Hz) (
C23), 121.70 (d,
J = 7.3 Hz) (
C22), 124.81 (d,
J = 3.4 Hz) (
C24), 127.06 (
C15), 127.80 (
C14,
C16), 127.81 (d,
J = 10.1 Hz) (
C19), 128.67 (
C13,
C17), 140.21 (
C12), 151.77 (d,
J = 239.9 Hz) (
C20), 154.76 (
C18) (
Figure S25). Calc. for C
26H
31FN
2O: C 76.81; H 7.69; N 6.89. Found: C 76.82; H 7.71; N 6.90. M = 406.24.
(±)-1-((3,5-Dimethyladamantane-1-yl)(phenyl)methyl)-3-(3-fluorophenyl) urea (8h).
It was obtained similarly to the compound (
8a), by
method A, from 200 mg of compound (
5b) and 75 mg of 3-fluorophenine (
7c). The yield is 85 mg (31%), m.p. 196–197 °C.
1H NMR (300 MHz, DMSO-
d6, δ) ppm: 0.66–0.79 m (6H, (CH
3)
2), 0.84–1.32 m (13H, Ad), 4.21–4.44 m (1H, Ad-C
H(Ph)), 6.93 t (1H,
1NH,
J = 8.0 Hz), 6.97–7.34 m (7H, arom), 7.71 d (1H, Ph-F,
J = 10.0 Hz), 8.59 s (1H,
3NH) (
Figure S26).
13C NMR (75 MHz, DMSO-
d6, δ) ppm: 29.22 (
C1), 31.08 (
C3), 31.12 (
C25,
C26), 31.15 (
C5), 31.17 (
C7), 38.40 (
C2), 43.14 (
C4,
C10), 45.04 (
C8), 45.25 (
C9), 51.08 (
C6), 63.13 (
C11), 104.42 (d,
J = 26.7 Hz) (
C22), 107.62 (d,
J = 21.3 Hz) (
C20), 113.49 (
C24), 127.04 (
C15), 127.80 (
C14,
C16), 128.68 (
C13,
C17), 130.58 (d,
J = 9.9 Hz) (
C23), 140.76 (
C12), 142.82 (d,
J = 11.5 Hz) (
C19), 154.88 (
C18), 162.92 (d,
J = 240.3 Hz) (
C21) (
Figure S27). Calc. for C
26H
31FN2O: C 76.81; H 7.69; N 6.89. Found: C 76.82; H 7.70; N 6.88. M = 406.24.
(±)-1-((3,5-Dimethyladamantane-1-yl)(phenyl)methyl)-3-(4-fluorophenyl) urea (8i).
It was obtained similarly to the compound (
8a), by
method A, from 200 mg of compound (
5b) and 75 mg of 4-fluorine (
7d). The yield is 168 mg (61%), m.p. 103–104 °C.
1H NMR (300 MHz, DMSO-
d6, δ) ppm: 0.74 d (6H, (CH
3)
2,
J = 9.1 Hz), 0.85–1.47 m (13H, Ad), 4.39 d (1H, Ad-C
H(Ph),
J = 9.4 Hz), 6.81 d (2H,
Ph-F,
J = 9.3 Hz), 7.00 t (1H, arom,
J = 8.9 Hz), 7.14 d (2H,
Ph-F,
J = 7.6 Hz), 7.16–7.38 m (4H, arom), 8.39 s (1H,
3NH) (
Figure S28).
13C NMR (75 MHz, DMSO-
d6, δ) ppm: 29.23 (
C1), 31.09 (
C3), 31.12 (
C25,
C26), 31.16 (
C5), 31.18 (
C7), 38.20 (
C2), 43.15 (
C4,
C10), 45.08 (
C8), 45.27 (
C9), 51.09 (
C6), 62.32 (
C11), 115.58 (d,
J = 22.1 Hz) (
C21,
C23), 119.22 (d,
J = 7.6 Hz) (
C20,
C24), 126.98 (
C15), 127.90 (
C14,
C16), 128.68 (
C13,
C17), 137.25 (
C19), 140.92 (
C12), 155.14 (
C18), 157.29 (d,
J = 237.0 Hz) (
C22) (
Figure S29). Calc. for C
26H
31FN
2O: C 76.81; H 7.69; N 6.89. Found: C 76.83; H 7.71; N 6.90. M = 406.24.
(±)-1-((3,5-Dimethyladamantane-1-yl)(phenyl)methyl)-3-(3-chlorophenyl) urea (8j).
It was obtained similarly to the compound (
8a), by method A, from 200 mg of compound (
5b) and 99 mg of 3-chloraniline (
7e). The yield is 158 mg (53%), m.p. 111–112 °C.
1H NMR (300 MHz, DMSO-
d6, δ) ppm: 0.73 t (6H, (CH
3)
2,
J = 2.8 Hz), 0.76–1.46 m (13H, Ad), 4.38 d (1H, Ad-C
H(Ph),
J = 9.3 Hz), 6.83–6.97 m (1H, Ph-Cl), 6.99–7.35 m (7H, arom), 7.63 t (1H, Ph-Cl,
J = 2.0 Hz), 8.58 s (1H,
3NH) (
Figure S30).
13C NMR (75 MHz, DMSO-
d6, δ) ppm: 29.18 (
C1), 31.04 (
C3), 31.08 (
C25,
C26), 31.11 (
C5), 31.13 (
C7), 38.14 (
C2), 43.09 (
C4,
C10), 45.03 (
C8), 45.23 (
C9), 51.04 (
C6), 62.41 (
C11), 116.17 (
C24), 117.11 (
C20), 121.08 (
C15), 127.07 (
C22), 127.79 (
C14,
C16), 128.63 (
C13,
C17), 130.73 (
C23), 133.62 (
C21), 140.13 (
C19), 142.26 (
C12), 154.89 (
C18) (
Figure S31). Calc. for C
26H
31ClN
2O: C 73.83; H 7.39; N 6.62. Found: C 73.85; H 7.41; N 6.63. M = 406.24.
(±)-(4-((4-(3-((3,5-Dimethyladamantan-1-yl)(phenyl)methyl)ureido)cyclohexyl)oxy) benzoic acid (
8k). Atom labels for
13C NMR presented on
Figure 4.
It was obtained similarly to the compound (
8a), by method A, from 200 mg of compound (
5b) and 160 mg of trans-4-(cyclohexyloxy)benzoic acid (
7f). The yield is 184 mg (51%), m.p. 174–175 °C.
1H NMR (300 MHz, DMSO-
d6, δ) ppm: 0.65–0.79 m (6H, (CH
3)
2), 0.87–1.03 m (2H, CH
2 cyclohex), 0.98–1.24 m (13H, Ad), 1.25–1.53 m (2H, CH
2 cyclohex), 4.05 s (1H, Ad-C
H(Ph)), 4.15–4.43 m (1H, CH cyclohex), 6.98 m (2H, arom), 7.08–7.15 m (1H, arom), 7.16–7.35 m (4H, arom), 7.71 d (2H, arom,
J = 10.0 Hz), 7.81–7.87 m (2H, N
H-C(O)-N
H), 8.00 br.s (1H, COOH) (
Figure S32).
13C NMR (75 MHz, DMSO-
d6, δ) ppm: 26.30 (
C20), 29.23 (
C1), 29.35 (
C24), 31.05 (
C3), 31.12 (
C32,
C33), 31.13 (
C5), 31.17 (
C7), 37.27 (
C23), 38.14 (
C21), 38.41 (
C2), 43.14 (
C4,
C10), 45.03 (
C8), 45.23 (
C9), 51.08 (
C6), 63.11 (
C11), 64.63 (
C19), 74.28 (
C22), 115.60 (
C26,
C30), 120.32 (
C28), 120.38 (
C15), 127.80 (
C14,
C16), 128.68 (
C13,
C17), 131.84 (
C27,
C29), 140.21 (
C12), 156.48 (
C18), 161.35 (
C25), 167.40 (
C31) (
Figure S33). Calc. for C
33H
42N
2O
4: C 74.69; H 7.98; N 5.28. Found, %: C 74.68; H 7.99; N 5.30. M = 530.71.
(±)-1-((Adamantan-1-yl)(phenyl)methyl)-3-(4-(trifluoromethoxy)phenyl) urea (
10a). Atom labels for
13C NMR presented on
Figure 5.
It was obtained similarly to the compound (
8a) according to
method B from 200 mg of compound (
5b) and 146 mg of 4-(trifluoromethoxy)phenylisocyanate (
9c). The yield is 181 mg (76%), m.p. 268–269 °C.
1H NMR (300 MHz, DMSO-
d6, δ) ppm: 1.11–1.77 m (12H, Ad), 1.91 s (3H, Ad), 4.36 d (1H, Ad-C
H(Ph),
J = 8.5 Hz), 6.87 m (1H,
1NH), 7.10–7.49 m (9H, Ad-CH(
Ph)-NH-C(O)-NH-
Ph), 8.59 s (1H,
3NH) (
Figure S34).
13C NMR (75 MHz, DMSO-
d6, δ) ppm: 28.19 (
C3,
C5,
C7), 36.33 (
C1), 36.92 (
C4,
C6,
C10), 38.888 (
C2,
C8,
C9), 62.82 (
C11), 118.82 (
C21,
C23), 120.67 (d,
J = 253.5 Hz) (
C25), 122.04 (
C22,
C24), 126.99 (
C15), 127.84 (
C14,
C16), 128.69 (
C13,
C17), 140.19 (
C19), 140.64 (
C12), 142.45 (
C22), 155.03 (
C18) (
Figure S35).
19F NMR (282 MHz, DMSO-
d6, δ) ppm: -59.80 (3F) (
Figure S36). Calc. for C
25H
27F
3N
2O
2: C 67.55; H 6.12; N 12.82. Found, %: C 67.57; H 6.14; N 12.83. M = 444.20.
(±)-1-((Adamantan-1-yl)(phenyl)methyl)-3-(2-chlorophenyl) urea (10b).
It was obtained similarly to the compound (
8a) by
method B from 200 mg of the compound (
5b) and 92 mg of 3-chlorophenylisocyanate (
9d). The yield is 176 mg (83%), m.p. 265–266°C.
1H NMR (300 MHz, DMSO-
d6, δ) ppm: 1.55 s (12H, Ad), 1.94 s (3H, Ad), 4.41 s (1H, Ad-C
H(Ph)), 7.23 s (9H, arom), 8.16 s (2H,
NH-C(O)-
NH) (
Figure S37).
13C NMR (75 MHz, DMSO-
d6, δ) ppm: 28.18 (
C3,
C5,
C7), 36.34 (
C1), 36.91 (
C4,
C6,
C10), 38.95 (
C2,
C8,
C9), 63.13 (
C11), 120.91 (
C15), 121.22 (
C20), 122.72 (
C23), 122.82 (
C24), 127.08 (
C21), 127.90 (
C14,
C16), 128.78 (
C13,
C17), 129.54 (
C22), 137.21 (
C19), 140.58 (
C12), 154.89 (
C18) (
Figure S38). Calc. for C
24H
27ClN
2O: C 72.99; H 6.89; N 8.98. Found, %: C 72.98; H 6.88; N 8.99. M = 394.18.
(±)-1-((Adamantan-1-yl)(phenyl)methyl)-3-(cyclohexyl) urea (10c).
It was obtained similarly to the compound (
8a) by
method B from 200 mg of compound (
5b) and 90 mg of cyclohexylisocyanate (
9e). The yield is 156 mg (79%), m.p. 263–264 °C.
1H NMR (300 MHz, DMSO-
d6, δ) ppm: 1.55 s (23H, Ad, 4-CH
2), 1.89 es (2H, CH
2), 4.29 s (1H, Ad-C
H(Ph)-NH), 5.73 s (1H,
1NH), 6.37 s (1H, N
H-C
6H
11), 7.23 s (5H, arom) (
Figure S39).
13C NMR (75 MHz, DMSO-
d6, δ) ppm: 24.79 (
C21,
C23), 25.77 (
C22), 28.21 (
C3,
C5,
C7), 33.76 (
C20,
C24), 36.42 (
C1), 37.00 (
C4,
C6,
C10), 38.91 (
C2,
C8,
C9), 48.05 (
C19), 62.65 (
C11), 126.69 (
C15), 127.67 (
C14,
C16), 128.74 (
C13,
C17), 141.37 (
C12), 157.48 (
C18) (
Figure S40). Calc. for C
24H
34N
2O: C 78.64; H 9.35; N 7.64. Found: C 78.66; H 9.36; N 7.65. M = 366.27.