Improvement of the In Vitro Cytotoxic Effect on HT-29 Colon Cancer Cells by Combining 5-Fluorouacil and Fluphenazine with Green, Red or Brown Propolis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Phenolic Compounds Characterization by LC/DAD/ESI-MSn
| Nr | tR (min) | λmax (nm) | [M − H]− m/z | MS2 (% Base Peak) | Compound | mg/g Extract |
|---|---|---|---|---|---|---|
| 1 | 4.9 | 294sh, 325 | 353 | 191 (100), 179 (8), 135 (1) | 5-O-Caffeoylquinic acid a,b | 1.35 ± 0.04 |
| 2 | 6.8 | 292, 323 | 179 | 135 | Caffeic acid a,b | 1.18 ± 0.04 |
| 3 | 9.7 | 310 | 163 | 119 | p-Coumaric acid a,b | 9.92 ± 0.01 |
| 4 | 10.8 | 294sh, 325 | 515 | 353 | Dicaffeoylquinic acid b,c | 6.04 ± 0.40 |
| 5 | 11.8 | 294sh, 325 | 515 | 353 | Dicaffeoylquinic acid (isomer) b,c | 8.81 ± 0.04 |
| 6 | 18.5 | 294sh, 325 | 677 | 515 | Tricaffeoylquinic acid b,c | 3.34 ± 0.01 |
| 7 | 24.9 | 292 | 301 | 283 (100), 151 (29) | Dihydrokaempferide b,c | 24.53 ± 0.06 |
| 8 | 27.7 | 267, 365 | 285 | 285 (100), 257 (13), 151 (20) | Kaempferol a,b | 1.47 ± 0.01 |
| 9 | 30.9 | 321 | 247 | 203 | 5-Isoprenyl caffeic acid b,d | 0.36 ± 0.02 |
| 10 | 36.7 | 315 | 231 | 187 | Drupanin b,c | 4.90 ± 0.02 |
| 11 | 39.8 | 310 | 327 | 283 | Dihydroconiferyl p-coumarate b,c | 0.44 ± 0.01 |
| 12 | 40.3 | 315 | 315 | 271 (100), 241 (70), 285 (59) | Cappilartimisin A b,c,d | 2.03 ± 0.01 |
| 13 | 45.6 | 315 | 315 | 271 (100), 241 (72), 285 (55) | Cappilartimisin A (isomer) b,d | 3.72 ± 0.02 |
| 14 | 49.2 | 266, 365 | 299 | 284 | Kaempferide b,c | 35.66 ± 0.13 |
| 15 | 50.1 | 266, 365 | 299 | 284 | Kaempferide (isomer) b,c | 14.95 ± 0.02 |
| 16 | 50.5 | 269, 363 | 329 | 314 | NI | |
| 17 | 53.2 | 316 | 393 | 349 (100), 163 (91), 145 (53) | 5-Isoprenyl caffeic acid-p-coumaric acid ester b,d | 4.05 ± 0.02 |
| 18 | 53.7 | 319 | 315 | 245 (100), 201 (41), 271 (11), 257 (11) | Cappilartimisin A (isomer) b,d | 1.57 ± 0.01 |
| 19 | 56.2 | 315 | 379 | 231 | Drupanin derivative b | 0.68 ± 0.01 |
| 20 | 57.4 | 284 | 377 | 245 (100), 319 (95), 349 (66) | E-Baccharin 5″-aldehyde b,e | 1.23 ± 0.01 |
| 21 | 61.3 | 314 | 299 | 255 | Artepillin C b,c | 24.03 ± 0.04 |
| 22 | 62.1 | 284 | 363 | 187 | Baccharin b,e | 2.10 ± 0.01 |
| 23 | 67.4 | 282 | 447 | 297 (100), 149 (10) | NI | |
| 24 | 68.2 | 277, 320 | 613 | 511 | NI |
| Nr | tR (min) | λmax (nm) | [M − H]− m/z | MS2 (% Base Peak) | Compound | mg/g Extract |
|---|---|---|---|---|---|---|
| 1 | 6.8 | 292, 323 | 179 | 135 | Caffeic acid a,b | 0.11 ± 0.00 |
| 2 | 17.4 | 276, 312 | 255 | 135 (100), 119 (10) | Liquiritigenin b,c | 5.20 ± 0.06 |
| 3 | 18.4 | 279, 310 | 285 | 270 | Vestitone b,d | 0.86 ± 0.03 |
| 4 | 19.1 | 289 | 283 | 268 | Calycosin b,c | 1.83 ± 0.02 |
| 5 | 21.3 | 276, 309 | 315 | 300 | Violanone b,e | 0.41 ± 0.02 |
| 6 | 22.0 | 280, 342 | 285 | 270 (100), 267 (17), 179 (4) | 3,4-Dihydroxy-9-methoxypterocarpan b,e | 1.29 ± 0.02 |
| 7 | 23.6 | 291 | 271 | 151 | Naringenin a,b | 2.64 ± 0.00 |
| 8 | 25.1 | 280 | 283 | 268 | Biochanin A b,d | 0.98 ± 0.00 |
| 9 | 30.1 | 281 | 299 | 284 | Sativanone b,f | 1.81 ± 0.02 |
| 10 | 32.7 | 282 | 271 | 227 (100), 109 (86), 135 (83) | Vestitol b,d | 26.16 ± 0.02 |
| 11 | 33.3 | 280, 320 | 267 | 252 | Formononetin b,d | 5.67 ± 0.04 |
| 12 | 33.6 | 240, 370 | 255 | 135 (100), 119 (25) | Isoliquiritigenin b,d | 2.42 ± 0.00 |
| 13 | 36.3 | 282 | 271 | 135 (100), 227 (74), 109 (62) | Neovestitol b,d | 17.01 ± 0.00 |
| 14 | 39.3 | 298, 325 | 247 | 179 (100), 135 (16) | Caffeic acid isoprenyl ester a,b | 20.95 ± 0.05 |
| 15 | 40.9 | 298, 325 | 247 | 179 (100), 135 (16) | Caffeic acid isoprenyl ester (isomer) a,b | 0.37 ± 0.01 |
| 16 | 41.7 | 298, 325 | 269 | 178 (100), 135 (96) | Caffeic acid benzyl ester b,g | 0.47 ± 0.00 |
| 17 | 43.3 | 289 | 255 | 213 (100), 211 (55), 151 (36) | Pinocembrin a,b | 2.14 ± 0.01 |
| 18 | 45.7 | 268, 313 | 253 | 209 | Chrysin a,b | 1.52 ± 0.01 |
| 19 | 46.3 | 294 | 313 | 253 (100), 271 (20) | Pinobanksin-3-O-acetate b,g | 1.97 ± 0.01 |
| 20 | 53.5 | 324 | 239 | 197 (100), 135 (36), 148 (19) | 7-Hydroxyflavanone b,d | 1.02 ± 0.02 |
| 21 | 54.3 | 283 | 397 | 123 (100), 167 (97), 351 (40) | NI | |
| 22 | 60.5 | 285, 481 | 521 | 397 (100), 491 (45) | Retusapurpurin B b,h | 0.47 ± 0.01 |
| 23 | 64.9 | 284, 481 | 521 | 397 (100), 491 (60) | Retusapurpurin A b,h | 0.94 ± 0.01 |
| 24 | 67.5 | 264, 327 | 425 | 410 (100), 367 (43), 355 (41) | Cycloartenol/α-amyrin/β-amyrin b,h | |
| 25 | 81.2 | 244, 351 | 617 | 465 (100), 481 (40), 521 (15) | 16-Hydroxyguttiferone b,h | 0.02 ± 0.00 |
| 26 | 83.8 | 244, 351 | 601 | 465 | Guttiferone E/Xanthochymol b,d | 27.95 ± 0.30 |
| 27 | 84.0 | 244, 351 | 601 | 327 (100), 273 (26), 271 (15) | Oblongifolin B b,d | 22.13 ± 0,21 |
| Nr | tR (min) | λmax (nm) | [M − H]− m/z | MS2 (% Base Peak) | Compound | mg/g Extract |
|---|---|---|---|---|---|---|
| 1 | 6.8 | 292, 323 | 179 | 135 | Caffeic acid a,b | 6.27 ± 0.09 |
| 2 | 9.7 | 310 | 163 | 119 | p-Coumaric acid a,b | 4.84 ± 0.03 |
| 3 | 10.6 | 295, 322 | 193 | 133 (100), 149 (49), 177 (15) | Ferulic acid a,b | 1.40 ± 0.01 |
| 4 | 11.1 | 298, 319 | 193 | 133 (100), 149 (49), 177 (15) | Isoferulic acid a,b | 5.25 ± 0.09 |
| 5 | 12.8 | 228 | 121 | Benzoic acid a,b | 1,07 ± 0.01 | |
| 6 | 15.9 | 295sh, 322 | 207 | 192 (100), 163 (62) | 3,4-Dimethyl-caffeic acid a,b | 8.25 ± 0.04 |
| 7 | 19.2 | 287 | 285 | 267 (100), 239 (25), 252 (16) | Pinobanksin-5-methyl ether b,c | 23.95 ± 0.09 |
| 8 | 21.0 | 309 | 177 | 163 (100), 119 (16) | p-Coumaric acid methyl ester a,b | 3.22 ± 0.02 |
| 9 | 21.3 | 256, 355 | 315 | 300 | Quercetin-3-methyl ether b,c | 3.95 ± 0.11 |
| 10 | 23.8 | 292 | 271 | 253 (100), 225 (22), 151 (8) | Pinobanksin b,c | 19.79 ± 0.12 |
| 11 | 27.0 | 269, 337 | 269 | 225 (100), 151 (20) | Apigenin a,b | 5.06 ± 0.01 |
| 12 | 27.7 | 267, 365 | 285 | 285 (100), 257 (13), 151 (20) | Kaempferol a,b | 6.94 ± 0.04 |
| 13 | 29.3 | 253, 370 | 315 | 300 | Isorhamnetin a,b | 6.63 ± 0.09 |
| 14 | 30.1 | 267, 352 | 299 | 284 | Kaempferol-methyl ether b,c | 10.05 ± 0.07 |
| 15 | 32.6 | 311 | 173 | 129 | Cinnamylidenacetic acid b,c | 18.14 ± 0.12 |
| 16 | 35.9 | 256, 367 | 315 | 165 | Rhamnetin b,c | 2.76 ± 0.10 |
| 17 | 36.5 | 265, 300sh, 352 | 283 | 268 (100), 239 (76) | Galangin-5-methyl ether b,c | 3.66 ± 0.02 |
| 18 | 39.3 | 298, 325 | 247 | 179 (100), 135 (16) | Caffeic acid isoprenyl ester a,b | 12.49 ± 0.04 |
| 19 | 40.9 | 298, 325 | 247 | 179 (100), 135 (16) | Caffeic acid isoprenyl ester (isomer) a,b | 15.45 ± 0.20 |
| 20 | 41.7 | 298, 325 | 269 | 178 (100), 135 (96) | Caffeic acid benzyl ester b,c | 16.78 ± 0.03 |
| 21 | 43.3 | 289 | 255 | 213 (100), 211 (55), 151 (36) | Pinocembrin a,b | 140.6 ± 0.16 |
| 22 | 44.5 | 290 | 285 | 139 (100), 145 (42) | NI | |
| 23 | 45.7 | 268, 313 | 253 | 209 | Chrysin a,b | 66.93 ± 0.21 |
| 24 | 46.4 | 294 | 313 | 253 (100), 271 (20) | Pinobanksin-3-O-acetate b,c | 105.5 ± 0.05 |
| 25 | 47.1 | 266, 300sh, 359 | 269 | 269 (100), 241 (61) | Galangin a,b | 95.17 ± 0.19 |
| 26 | 48.9 | 268, 331 | 283 | 269 | Acacetin a,b | 4.72 ± 0.01 |
| 27 | 49.6 | 265, 300sh, 350sh | 283 | 269 | 6-Methoxychrysin b,c | 4.88 ± 0.02 |
| 28 | 51.1 | 250, 268sh, 343 | 313 | 298 | Chrysoeriol-methyl ether b,c | 5.48 ± 0.01 |
| 29 | 52.0 | 294, 310 | 231 | 163 (100), 119 (12) | p-Coumaric isoprenyl ester b,c | 4.72 ± 0.04 |
| 30 | 52.6 | 295, 324 | 295 | 178 (100), 135 (60) | Caffeic acid cinnamyl ester b,c | 11.74 ± 0.04 |
| 31 | 53.6 | 289 | 327 | 253 (100), 271 (10) | Pinobanksin-3-O-propionate b,c | 48.64 ± 0.11 |
| 32 | 56.5 | 289 | 269 | 254 (100), 251 (54), 165 (22) | 3-Hydroxy-5-methoxyflavanone b,c | 11.90 ± 0.02 |
| 33 | 58.2 | 292 | 417 | 297 (100), 402 (85), 267 (67) | Pinobanksin-methyl-ether-3-O-phenylpropionate b,d | 13.29 ± 0.01 |
| 34 | 59.1 | 292 | 475 | 415 | Pinobansin-3-O-acetate-5-O-hydroxyphenylpropionate b,c | 16.84 ± 0.10 |
| 35 | 59.4 | 308 | 431 | 281 | NI | |
| 36 | 59.8 | 292 | 417 | 267 (100), 281 (100) | Pinobanksin-methyl-ether-3-O-phenylpropionate (isomer) b,d | 8.93 ± 0.04 |
| 37 | 60.3 | 292 | 475 | 415 | Pinobansin-3-O-acetate-7-O-hydroxyphenylpropionate b,c | 30.23 ± 0.15 |
| 38 | 60.6 | 294, 320 | 413 | 161 | NI | |
| 39 | 63.7 | 292 | 355 | 253 | Pinobanksin-3-O-pentanoate or 2-methylbutyrate b,c | 15.87 ± 0.10 |
| 40 | 65.1 | 292, 322 | 315 | 179 (100), 135 (31) | Caffeic acid derivative | 3.00 ± 0.01 |
| 41 | 65.5 | 292 | 403 | 253 (100), 271 (21) | Pinobanksin-3-O-phenylpropionate b,c | 10.69 ± 0.01 |
| 42 | 67.0 | 292 | 369 | 253 (100), 271 (14) | Pinobanksin-3-O-hexanoate b,c | 23.90 ± 0.06 |
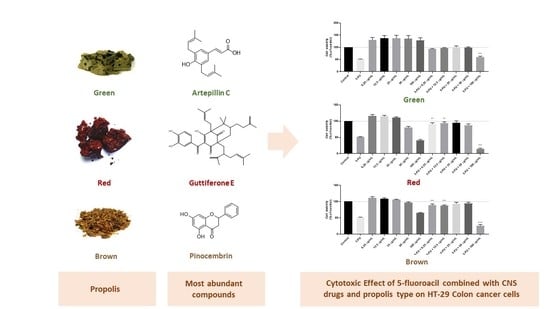
2.2. Evaluation of Propolis Extracts Effect on HT-29 Colon Cancer Cell Viability
2.3. Evaluation of 5-FU and Fluphenazine Effect on HT-29 Colon Cancer Cell Viability after Treatment with Propolis Extract
2.4. Evaluation of the Combination of 5-FU with Propolis Extracts on HT-29 Colon Cancer Cell Viability
2.5. Evaluation of the Combination of Fluphenazine with Propolis Extracts on HT-29 Colon Cancer Cell Viability
2.6. Evaluation of Drug Interaction in the Combinations of 5-FU/Fluphenazine with Propolis Extracts on HT-29 Colon Cancer Cells
3. Materials and Methods
3.1. Standards and Reagents
3.2. Propolis Samples
3.3. Phenolic Compounds Extraction
3.4. Phenolic Compounds Characterization by LC/DAD/ESI-MSn
3.5. Cell Culture
3.5.1. Cell Line and Cell Culture
3.5.2. Drug Treatment
3.5.3. Cell Viability Assay
3.5.4. Data Analysis
3.5.5. Analysis of Drug Interactions
3.5.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Bankova, V.S.; De Castro, S.L.; Marcucci, M.C. Propolis: Recent advances in chemistry and plant origin. Apidologie 2000, 31, 3–15. [Google Scholar] [CrossRef] [Green Version]
- Falcão, S.I.; Vale, N.; Gomes, P.; Domingues, M.R.M.; Freire, C.; Cardoso, S.M.; Vilas-Boas, M. Phenolic profiling of Portuguese propolis by LC-MS spectrometry: Uncommon propolis rich with flavonoid glycosides. Phytochem. Anal. 2013, 24, 309–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wieczorek, P.P.; Hudz, N.; Yezerska, O.; Horčinová-Sedláčková, V.; Shanaida, M.; Korytniuk, O.; Jasicka-Misiak, I. Chemical Variability and Pharmacological Potential of Propolis as a Source for the Development of New Pharmaceutical Products. Molecules 2022, 27, 1600. [Google Scholar] [CrossRef] [PubMed]
- ISO/DIS 24381; Bee Propolis—Specifications. International Organization for Standardization: Geneva, Switzerland, 2023.
- Falcão, S.I.; Tomás, A.; Vale, N.; Gomes, P.; Freire, C.; Vilas-Boas, M. Phenolic quantification and botanical origin of Portuguese propolis. Ind. Crops Prod. 2013, 49, 805–812. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, E.W.; Message, D.; Negri, G.; Salatino, A.; Stringheta, P.C. Seasonal variation, chemical composition and antioxidant activity of Brazilian propolis samples. Evid. Based Complement. Altern. Med. 2010, 7, 307–315. [Google Scholar] [CrossRef] [Green Version]
- Righi, A.A.; Alves, T.R.; Negri, G.; Marques, L.M.; Breyer, H.; Salatino, A. Brazilian red propolis: Unreported substances, antioxidant and antimicrobial activities. J. Sci. Food Agric. 2011, 91, 2363–2370. [Google Scholar] [CrossRef] [PubMed]
- Tavares, L.; Smaoui, S.; Lima, P.S.; de Oliveira, M.M.; Santos, L. Propolis: Encapsulation and application in the food and pharmaceutical industries. Trends Food Sci. Technol. 2022, 127, 169–180. [Google Scholar] [CrossRef]
- Rivera-Yañez, N.; Rivera-Yañez, C.R.; Pozo-Molina, G.; Méndez-Catalá, C.F.; Méndez-Cruz, A.R.; Nieto-Yañez, O. Biomedical Properties of Propolis on Diverse Chronic Diseases and Its Potential Applications and Health Benefits. Nutrients 2021, 13, 78. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Oun, R.; Moussa, Y.E.; Wheate, N.J. The side effects of platinum-based chemotherapy drugs: A review for chemists. Dalton Trans. 2018, 47, 6645–6653. [Google Scholar] [CrossRef]
- Entezar-Almahdi, E.; Mohammadi-Samani, S.; Tayebi, L.; Farjadian, F. Recent Advances in Designing 5-Fluorouracil Delivery Systems: A Stepping Stone in the Safe Treatment of Colorectal Cancer. Int. J. Nanomed. 2020, 15, 5445–5458. [Google Scholar] [CrossRef] [PubMed]
- Ianevski, A.; Giri, A.K.; Aittokallio, T. SynergyFinder 2.0: Visual analytics of multi-drug combination synergies. Nucleic Acids Res. 2021, 48, W488–W493. [Google Scholar] [CrossRef]
- Veschi, S.; De Lellis, L.; Florio, R.; Lanuti, P.; Massucci, A.; Tinari, N.; De Tursi, M.; di Sebastiano, P.; Marchisio, M.; Natoli, C.; et al. Effects of repurposed drug candidates nitroxoline and nelfinavir as single agents or in combination with erlotinib in pancreatic cancer cells. J. Exp. Clin. Cancer Res. 2018, 37, 236. [Google Scholar] [CrossRef] [PubMed]
- Turner, T.H.; Alzubi, M.A.; Harrell, J.C. Identification of synergistic drug combinations using breast cancer patient-derived xenografts. Sci. Rep. 2020, 10, 1493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmer, A.C.; Sorger, P.K. Combination Cancer Therapy Can Confer Benefit via Patient-to-Patient Variability without Drug Additivity or Synergy. Cell 2017, 171, 1678–1691. [Google Scholar] [CrossRef] [Green Version]
- Hatem, E.; Azzi, S.; El Banna, N.; He, T.; Heneman-Masurel, A.; Vernis, L.; Baïlle, D.; Masson, V.; Dingli, F.; Loew, D.; et al. Auranofin/Vitamin C: A Novel Drug Combination Targeting Triple-Negative Breast Cancer. JNCI J. Natl. Cancer Inst. 2019, 111, 597–608. [Google Scholar] [CrossRef]
- Duarte, D.; Cardoso, A.; Vale, N. Synergistic Growth Inhibition of HT-29 Colon and MCF-7 Breast Cancer Cells with Simultaneous and Sequential Combinations of Antineoplastics and CNS Drugs. Int. J. Mol. Sci. 2021, 22, 7408. [Google Scholar] [CrossRef] [PubMed]
- Duarte, D.; Vale, N. New trends for antimalarial drugs: Synergism between antineoplastics and antimalarials on breast cancer cells. Biomolecules 2020, 10, 1623. [Google Scholar] [CrossRef]
- Fong, W.; To, K.K.W. Drug repurposing to overcome resistance to various therapies for colorectal cancer. Cell. Mol. Life Sci. 2019, 76, 3383–3406. [Google Scholar] [CrossRef]
- Antoszczak, M.; Markowska, A.; Markowska, J.; Huczyński, A. Old wine in new bottles: Drug repurposing in oncology. Eur. J. Pharmacol. 2020, 866, 172784. [Google Scholar] [CrossRef]
- Fluphenazine|DrugBank Online. Available online: https://go.drugbank.com/drugs/DB00623 (accessed on 9 April 2021).
- Guo, W.; Song, Y.; Song, W.; Liu, Y.; Liu, Z.; Zhang, D.; Tang, Z.; Bai, O. Co-delivery of Doxorubicin and Curcumin with Polypeptide Nanocarrier for Synergistic Lymphoma Therapy. Sci. Rep. 2020, 10, 7832. [Google Scholar] [CrossRef] [PubMed]
- Calaf, G.M.; Ponce-Cusi, R.; Carrión, F. Curcumin and paclitaxel induce cell death in breast cancer cell lines. Oncol. Rep. 2018, 40, 2381–2388. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.B.; Sengupta, R.; Qazi, S.; Vachhani, H.; Yu, Y.; Rishi, A.K.; Majumdar, A.P.N. Curcumin enhances the effects of 5-fluorouracil and oxaliplatin in mediating growth inhibition of colon cancer cells by modulating EGFR and IGF-1R. Int. J. Cancer 2008, 122, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Dent, P.; Booth, L.; Roberts, J.L.; Poklepovic, A.; Hancock, J.F. (Curcumin+sildenafil) enhances the efficacy of 5FU and anti-PD1 therapies in vivo. J. Cell. Physiol. 2020, 235, 6862–6874. [Google Scholar] [CrossRef]
- Suzuki, I.; Hayashi, I.; Takaki, T.; Groveman, D.S.; Fujimiya, Y. Antitumor and Anticytopenic Effects of Aqueous Extracts of Propolis in Combination with Chemotherapeutic Agents. Cancer Biother. Radiopharm. 2002, 17, 553–562. [Google Scholar] [CrossRef]
- Barary, M.; Hosseinzadeh, R.; Kazemi, S.; Liang, J.J.; Mansoori, R.; Sio, T.T.; Hosseine, M.; Moghadamnia, A.A. The effect of propolis on 5-fluorouracil-induced cardiac toxicity in rats. Sci. Rep. 2022, 12, 8661–8677. [Google Scholar] [CrossRef]
- Sameni, H.R.; Yosefi, S.; Alipour, M.; Pakdel, A.; Torabizadeh, N.; Semnani, V.; Bandegi, A.R. Co-administration of 5FU and Propolis on AOM/DSS Induced Colorectal Cancer in BALB-C Mice. Life Sci. 2021, 276, 119390. [Google Scholar] [CrossRef]
- Coelho, J.; Falcão, S.I.; Vale, N.; Almeida-Muradian, L.B.; Vilas-Boas, M. Phenolic composition and antioxidant activity assessment of southeastern and south Brazilian propolis. J. Apic. Res. 2017, 56, 21–31. [Google Scholar] [CrossRef] [Green Version]
- Vieira de Morais, D.; Rosalen, P.L.; Ikegaki, M.; de Souza Silva, A.P.; Massarioli, A.P.; de Alencar, S.M. Active Antioxidant Phenolics from Brazilian Red Propolis: An Optimization Study for Their Recovery and Identification by LC-ESI-QTOF-MS/MS. Antioxidants 2021, 10, 297. [Google Scholar] [CrossRef]
- Moise, A.R.; Bobiş, O. Baccharis dracunculifolia and Dalbergia ecastophyllum, Main Plant Sources for Bioactive Properties in Green and Red Brazilian Propolis. Plants 2020, 9, 1619. [Google Scholar] [CrossRef]
- Xu, X.; Yang, B.; Wang, D.; Zhu, Y.; Miao, X.; Yang, W. The Chemical Composition of Brazilian Green Propolis and Its Protective Effects on Mouse Aortic Endothelial Cells against Inflammatory Injury. Molecules 2020, 25, 4612. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, D.M.; De Souza, M.C.; Arruda, C.; Pereira, R.A.S.; Bastos, J.K. The Role of Baccharis dracunculifolia and its Chemical Profile on Green Propolis Production by Apis mellifera. J. Chem. Ecol. 2020, 46, 150–162. [Google Scholar] [CrossRef]
- Ccana-Ccapatinta, G.V.; Mejía, J.A.A.; Tanimoto, M.H.; Groppo, M.; Carvalho, J.C.A.S.d.; Bastos, J.K. Dalbergia ecastaphyllum (L.) Taub. and Symphonia globulifera L.f.: The Botanical Sources of Isoflavonoids and Benzophenones in Brazilian Red Propolis. Molecules 2020, 25, 2060. [Google Scholar] [CrossRef]
- Omar, R.M.K.; Igoli, J.; Gray, A.I.; Ebiloma, G.U.; Clements, C.; Fearnley, J.; Edrada Ebel, R.A.; Zhang, T.; De Koning, H.P.; Watson, D.G. Chemical characterisation of Nigerian red propolis and its biological activity against Trypanosoma Brucei. Phytochem. Anal. 2016, 27, 107–115. [Google Scholar] [CrossRef] [Green Version]
- Falcão, S.I.; Lopes, M.; Vilas-Boas, M. A First Approach to the Chemical Composition and Antioxidant Potential of Guinea-Bissau Propolis. Nat. Prod. Commun. 2019, 14, 1–5. [Google Scholar] [CrossRef]
- Silva, V.C.; Silva, A.M.G.S.; Basílio, J.A.D.; Xavier, J.A.; do Nascimento, T.G.; Naal, R.M.Z.G.; del Lama, M.P.; Leonelo, L.A.D.; Mergulhão, N.L.O.N.; Maranhão, F.C.A.; et al. New Insights for Red Propolis of Alagoas—Chemical Constituents, Topical Membrane Formulations and Their Physicochemical and Biological Properties. Molecules 2020, 25, 5811. [Google Scholar] [CrossRef] [PubMed]
- Falcão, S.I.; Vilas-Boas, M.; Estevinho, L.M.; Barros, C.; Domingues, M.R.; Cardoso, S.M. Phenolic characterization of Northeast Portuguese propolis: Usual and unsual compounds. Anal. Bioanal. Chem. 2010, 396, 887. [Google Scholar] [CrossRef] [Green Version]
- Dantas Silva, R.P.; Machado, B.A.S.; Barreto, G.d.A.; Costa, S.S.; Andrade, L.N.; Amaral, R.G.; Carvalho, A.A.; Padilha, F.F.; Barbosa, J.D.V.; Umsza-Guez, M.A. Antioxidant, antimicrobial, antiparasitic, and cytotoxic properties of various Brazilian propolis extracts. PLoS ONE 2017, 12, e0172585. [Google Scholar] [CrossRef]
- Dos Santos, F.F.; Morais-Urano, R.P.; Cunha, W.R.; de Almeida, S.G.; Cavallari, P.S.D.S.R.; Manuquian, H.A.; Pereira, H.A.; Furtado, R.; Santos, M.F.C.; Andrade, E.; et al. A review on the anti-inflammatory activities of Brazilian green, brown and red propolis. J. Food Biochem. 2022, 46, e14350. [Google Scholar] [CrossRef]
- Frión-Herrera, Y.; Gabbia, D.; Scaffidi, M.; Zagni, L.; Cuesta-Rubio, O.; De Martin, S.; Carrara, M. Cuban Brown Propolis Interferes in the Crosstalk between Colorectal Cancer Cells and M2 Macrophages. Nutrients 2020, 12, 2040. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.C. Drug combination studies and their synergy quantification using the choutalalay method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Drug | IC50 |
|---|---|
| 5-FU | 3.79 µM |
| Fluphenazine | 1.86 µM |
| Green Propolis | >100 µg/mL |
| Red Propolis | 53.03 µg/mL |
| Brown Propolis | 56.54 µg/mL |
| Drug A | Dose A (µM) | Sample B | Dose B (µg/mL) | Effect (Fa) | CI Value | Interaction |
|---|---|---|---|---|---|---|
| Fluphenazine | 1.86 | Green Propolis | 6.25 | 0.00001 | >100 | Antagonism |
| 12.5 | 0.0289 | >100 | Antagonism | |||
| 25 | 0.0555 | 46.59 | Antagonism | |||
| 50 | 0.1733 | 7.92 | Antagonism | |||
| 100 | 0.6569 | 0.36 | Synergism | |||
| Red Propolis | 6.25 | 0.00001 | >100 | Antagonism | ||
| 12.5 | 0.0001 | >100 | Antagonism | |||
| 25 | 0.001 | >100 | Antagonism | |||
| 50 | 0.2458 | 4.86 | Antagonism | |||
| 100 | 0.849 | 0.73 | Synergism | |||
| Brown Propolis | 6.25 | 0.0076 | >100 | Antagonism | ||
| 12.5 | 0.0316 | >100 | Antagonism | |||
| 25 | 0.1036 | 18.50 | Antagonism | |||
| 50 | 0.2319 | 5.31 | Antagonism | |||
| 100 | 0.6549 | 1.05 | Antagonism | |||
| 5-FU | 3.78 | Green Propolis | 6.25 | 0.0635 | 0.49 | Synergism |
| 12.5 | 0.0236 | 1.23 | Antagonism | |||
| 25 | 0.00001 | >100 | Antagonism | |||
| 50 | 0.0165 | 1.71 | Antagonism | |||
| 100 | 0.3949 | 0.06 | Synergism | |||
| Red Propolis | 6.25 | 0.0842 | 0.47 | Synergism | ||
| 12.5 | 0.0606 | 0.72 | Synergism | |||
| 25 | 0.0409 | 1.19 | Additivity | |||
| 50 | 0.1236 | 0.95 | Synergism | |||
| 100 | 0.8457 | 0.66 | Synergism | |||
| Brown Propolis | 6.25 | 0.1100 | 0.37 | Synergism | ||
| 12.5 | 0.1238 | 0.42 | Synergism | |||
| 25 | 0.0606 | 0.92 | Additivity | |||
| 50 | 0.0496 | 1.47 | Antagonism | |||
| 100 | 0.7379 | 0.64 | Synergism |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falcão, S.I.; Duarte, D.; Diallo, M.; Santos, J.; Ribeiro, E.; Vale, N.; Vilas-Boas, M. Improvement of the In Vitro Cytotoxic Effect on HT-29 Colon Cancer Cells by Combining 5-Fluorouacil and Fluphenazine with Green, Red or Brown Propolis. Molecules 2023, 28, 3393. https://doi.org/10.3390/molecules28083393
Falcão SI, Duarte D, Diallo M, Santos J, Ribeiro E, Vale N, Vilas-Boas M. Improvement of the In Vitro Cytotoxic Effect on HT-29 Colon Cancer Cells by Combining 5-Fluorouacil and Fluphenazine with Green, Red or Brown Propolis. Molecules. 2023; 28(8):3393. https://doi.org/10.3390/molecules28083393
Chicago/Turabian StyleFalcão, Soraia I., Diana Duarte, Moustapha Diallo, Joana Santos, Eduarda Ribeiro, Nuno Vale, and Miguel Vilas-Boas. 2023. "Improvement of the In Vitro Cytotoxic Effect on HT-29 Colon Cancer Cells by Combining 5-Fluorouacil and Fluphenazine with Green, Red or Brown Propolis" Molecules 28, no. 8: 3393. https://doi.org/10.3390/molecules28083393