Chiral Binaphthalene Building Blocks for Self-Assembled Nanoscale CPL Emitters
Abstract
:1. Introduction
2. Results
2.1. Synthesis
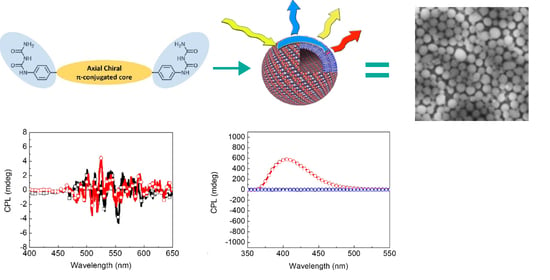
2.2. Characterization of Nanostructures
2.3. Photophysical Properties
| Compound | λabs (nm) | ε (104 M−1cm−1) | λem (THF, nm) | ΦTHF 2 (%) |
|---|---|---|---|---|
| BNPB | 307, 346 | 5.2, 4.1 | 402 | 81 |
| 7BBNPB | 346 | 3.7 | 432 | 72 |
| 8BBNPB | 335 | 5.3 | 396 | 66 |
| BNBTPB | 303, 344, 403 | 7.1, 3.3, 3.6 | 549 | 28 |
| 7BBNBTPB | 318, 404 | 6.3, 4.1 | 538 | 42 |
| BNCNCzPB | 314, 328, 403 | 5.8, 5.1, 0.56 | 525 | 21 (28) 3 |
2.4. Chiroptical Properties
3. Discussion
4. Materials and Methods
4.1. Preparation of Nanospheres
4.2. Techniques for Characterization
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Lehn, J.-M. Perspectives in Chemistry-Steps towards Complex Matter. Angew. Chem. Int. Ed. 2013, 52, 2836–2850. [Google Scholar] [CrossRef]
- Lehn, J.-M. Toward self-organization and complex matter. Science 2002, 295, 2400–2403. [Google Scholar] [CrossRef] [Green Version]
- Wong, K.-T.; Bassani, D.M. Energy transfer in supramolecular materials for new applications in photonics and electronics. NPG Asia Mater. 2014, 6, e116. [Google Scholar] [CrossRef] [Green Version]
- Aida, T.; Meijer, E.W.; Stupp, S.I. Functional Supramolecular Polymers. Science 2012, 335, 813–817. [Google Scholar] [CrossRef] [Green Version]
- Brunsveld, L.; Folmer, B.J.B.; Meijer, E.W.; Sijbesma, R.P. Supramolecular Polymers. Chem. Rev. 2001, 101, 4071–4097. [Google Scholar] [CrossRef] [PubMed]
- Tseng, K.-P.; Fang, F.-C.; Shyue, J.-J.; Wong, K.-T.; Raffy, G.; Del Guerzo, A.; Bassani, D.M. Spontaneous Generation of Highly Emissive RGB Organic Nanospheres. Angew. Chem. Int. Ed. 2011, 50, 7032. [Google Scholar] [CrossRef] [PubMed]
- Velu, S.K.P.; Yan, M.; Tseng, K.-P.; Wong, K.-T.; Bassani, D.M.; Terech, P. Spontaneous Formation of Artificial Vesicles in Organic Media through Hydrogen-Bonding Interactions. Macromolecules 2013, 46, 1591–1598. [Google Scholar] [CrossRef]
- Kuo, M.-C.; Chen, H.-F.; Shyue, J.-J.; Bassani, D.M.; Wong, K.-T. In situ reversible conversion of porphyrin aggregate morphology. Chem. Commun. 2012, 48, 8051–8053. [Google Scholar] [CrossRef] [PubMed]
- Tseng, K.-P.; Tsai, Y.-T.; Wu, C.-C.; Shyue, J.-J.; Bassani, D.M.; Wong, K.-T. Light- and solvent-driven morphological transformations of self-assembled hydrogen-bonded nanostructures. Chem. Commun. 2013, 49, 11536–11538. [Google Scholar] [CrossRef]
- Tsai, Y.-T.; Tseng, K.-P.; Chen, Y.-F.; Wu, C.-C.; Fan, G.-L.; Wong, K.-T.; Wantz, G.; Hirsch, L.; Raffy, G.; Del Guerzo, A.; et al. Electroluminescence from Spontaneously Generated Single-Vesicle Aggregates Using Solution-Processed Small Organic Molecules. ACS Nano 2016, 10, 998–1006. [Google Scholar] [CrossRef]
- Tsai, Y.-T.; Raffy, G.; Liu, H.-F.; Peng, B.-J.; Tseng, K.-P.; Hirsch, L.; Del Guerzo, A.; Bassani, D.M.; Wong, K.-T. Incorporation of narcissistic self-sorting supramolecular interactions for the spontaneous fabrication of multiple-color solid-state materials for OLED applications. Mater. Chem. Front. 2020, 4, 845–850. [Google Scholar] [CrossRef]
- Tsai, Y.-T.; Liu, H.-F.; Peng, B.-J.; Tseng, K.-P.; Kuo, M.-C.; Wong, K.-T.; Wantz, G.; Hirsch, L.; Raffy, G.; Del Guerzo, A.; et al. Frequency-Selective Photobleaching as a Route to Chromatic Control in Supramolecular OLED Devices. ACS Appl. Mater. Interfaces 2017, 9, 36045–36052. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, P.; Hu, D.; Ma, Y. Recent progress in hot exciton materials for organic light-emitting diodes. Chem. Soc. Rev. 2021, 50, 1030–1069. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Tsang, D.; Kuwabara, H.; Hatae, Y.; Li, B.; Takahashi, T.; Lee, S.Y.; Yasuda, T.; Adachi, C. Nearly 100% Internal Quantum Efficiency in Undoped Electroluminescent Devices Employing Pure Organic Emitters. Adv. Mater. 2015, 27, 2096–2100. [Google Scholar] [CrossRef]
- Cao, Z.; Hao, A.; Xing, P. Photoresponsive chiral vesicles as a light harvesting matrix with tunable chiroptical properties. Nanoscale 2021, 13, 700–707. [Google Scholar] [CrossRef]
- Hsieh, Y.-Y.; Sanchez, R.S.; Raffy, G.; Shyue, J.-J.; Hirsch, L.; Del Guerzo, A.; Wong, K.-T.; Bassani, D.M. Supramolecular gating of TADF process in self-assembled nano-spheres for high-resolution OLED applications. Chem. Commun. 2022, 58, 1163–1166. [Google Scholar] [CrossRef] [PubMed]
- Takaishi, K.; Maeda, C.; Ema, T. Circularly polarized luminescence in molecular recognition systems: Recent achievements. Chirality 2023, 35, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Eliseeva, S.V.; Bunzli, J.-C.G. Lanthanide luminescence for functional materials and bio-sciences. Chem. Soc. Rev. 2010, 39, 189–227. [Google Scholar] [CrossRef]
- Zhan, X.; Xu, F.F.; Zhou, Z.; Yan, Y.; Yao, J.; Zhao, Y.S. 3D laser displays based on circularly polarized lasing from cholesteric liquid crystal arrays. Adv. Mater. 2021, 33, 2104418. [Google Scholar] [CrossRef] [PubMed]
- Shuvaev, S.; Fox, M.A.; Parker, D. Monitoring of the ADP/ATP ratio by induced circularly polarised europium luminescence. Angew. Chem. 2018, 130, 7610–7614. [Google Scholar] [CrossRef] [Green Version]
- Sherson, J.F.; Krauter, H.; Olsson, R.K.; Julsgaard, B.; Hammerer, K.; Cirac, I.; Polzik, E.S. Quantum teleportation between light and matter. Nature 2006, 443, 557–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patil, Y.; Demangeat, C.; Favereau, L. Recent advances in room temperature phosphorescence of chiral organic materials. Chirality 2023. early view. [Google Scholar] [CrossRef]
- Feuillastre, S.; Pauton, M.; Gao, L.; Desmarchelier, A.; Riives, A.J.; Prim, D.; Tondelier, D.; Geffroy, B.; Muller, G.; Clavier, G. Design and synthesis of new circularly polarized thermally activated delayed fluorescence emitters. J. Am. Chem. Soc. 2016, 138, 3990–3993. [Google Scholar] [CrossRef] [Green Version]
- MacLean, M.W.A.; Wood, T.K.; Wu, G.; Lemieux, R.P.; Crudden, C.M. Chiral Periodic Mesoporous Organosilicas: Probing Chiral Induction in the Solid State. Chem. Mater. 2014, 26, 5852–5859. [Google Scholar] [CrossRef]
- Ng, M.-K.; Chow, H.-F.; Chan, T.-L.; Mak, T.C. Synthesis and chiroptical properties of axially chiral, binaphthol-based oligomers. Tetrahedron Lett. 1996, 37, 2979–2982. [Google Scholar] [CrossRef]
- Takaishi, K.; Hinoide, S.; Matsumoto, T.; Ema, T. Axially chiral peri-xanthenoxanthenes as a circularly polarized luminophore. J. Am. Chem. Soc. 2019, 141, 11852–11857. [Google Scholar] [CrossRef]
- Li, Y.; Xue, C.; Wang, M.; Urbas, A.; Li, Q. Photodynamic chiral molecular switches with thermal stability: From reflection wavelength tuning to handedness inversion of self-organized helical superstructures. Angew. Chem. Int. Ed. 2013, 52, 13703–13707. [Google Scholar] [CrossRef]
- Fang, F.-C.; Chu, C.-C.; Huang, C.-H.; Raffy, G.; Del Guerzo, A.; Wong, K.-T.; Bassani, D.M. Versatile one-step introduction of multiple hydrogen-bonding sites onto extended π-conjugated systems. Chem. Commun. 2008, 6369–6371. [Google Scholar] [CrossRef]
- Takaishi, K.; Kawamoto, M.; Tsubaki, K. Multibridged chiral naphthalene oligomers with continuous extreme-cisoid conformation. Org. Lett. 2010, 12, 1832–1835. [Google Scholar] [CrossRef]
- Suzuki, S.; Fujii, T.; Baba, H. Interpretation of electronic spectra by configuration analysis: Absorption spectra of monosubstituted naphthalenes. J. Mol. Spectrosc. 1973, 47, 243–251. [Google Scholar] [CrossRef]
- Takaishi, K.; Yamamoto, T.; Hinoide, S.; Ema, T. Helical Oligonaphthodioxepins Showing Intense Circularly Polarized Luminescence (CPL) in Solution and in the Solid State. Chem. Eur. J. 2017, 23, 9249–9252. [Google Scholar] [CrossRef] [PubMed]
- Uejima, M.; Sato, T.; Yokoyama, D.; Tanaka, K.; Park, J.-W. Quantum yield in blue-emitting anthracene derivatives: Vibronic coupling density and transition dipole moment density. Phys. Chem. Chem. Phys. 2014, 16, 14244–14256. [Google Scholar] [CrossRef] [PubMed]
- Im, Y.; Kim, M.; Cho, Y.J.; Seo, J.-A.; Yook, K.S.; Lee, J.Y. Molecular design strategy of organic thermally activated delayed fluorescence emitters. Chem. Mater. 2017, 29, 1946–1963. [Google Scholar] [CrossRef]
- Nawara, K.; Waluk, J. Goodbye to quinine in sulfuric acid solutions as a fluorescence quantum yield standard. Anal. Chem. 2019, 91, 5389–5394. [Google Scholar] [CrossRef]
- Kimoto, T.; Tajima, N.; Fujiki, M.; Imai, Y. Control of Circularly Polarized Luminescence by Using Open-and Closed-Type Binaphthyl Derivatives with the Same Axial Chirality. Chem.–Asian J. 2012, 7, 2836–2841. [Google Scholar] [CrossRef] [PubMed]
- Berova, N.; Nakanishi, K.; Woody, R.W. Circular Dichroism: Principles and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2000. [Google Scholar]
- Harada, N.; Nakanishi, K. Exciton chirality method and its application to configurational and conformational studies of natural products. Acc. Chem. Res. 1972, 5, 257–263. [Google Scholar] [CrossRef]
- Rosini, C.; Superchi, S.; Peerlings, H.; Meijer, E. Enantiopure dendrimers derived from the 1,1′-binaphthyl moiety: A correlation between chiroptical properties and conformation of the 1,1′-binaphthyl template. Eur. J. Org. Chem. 2000, 2000, 61–71. [Google Scholar] [CrossRef]
- Van Es, J.; Biemans, H.; Meijer, E. Synthesis and characterization of optically active cyclic 6,6′-dinitro-1,1′-binaphthyl-2,2′-diethers. Tetrahedron-Asymmetry 1997, 8, 1825–1831. [Google Scholar] [CrossRef]
- Deuβen, H.; Shibaev, P.; Vinokur, R.; Bjørnholm, T.; Schaumburg, K.; Bechgaard, K.; Shibaev, V. New 6,6′-disubstituted-binaphthol derivatives as chiral dopants: Synthesis and temperature dependence of molecular conformations. Liq. Cryst. 1996, 21, 327–340. [Google Scholar] [CrossRef]
- Arrico, L.; Di Bari, L.; Zinna, F. Quantifying the Overall Efficiency of Circularly Polarized Emitters. Chem. Eur. J. 2021, 27, 2920–2934. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsieh, Y.-Y.; Shyue, J.-J.; Chao, Y.-C.; Wong, K.-T.; Bassani, D.M. Chiral Binaphthalene Building Blocks for Self-Assembled Nanoscale CPL Emitters. Molecules 2023, 28, 3382. https://doi.org/10.3390/molecules28083382
Hsieh Y-Y, Shyue J-J, Chao Y-C, Wong K-T, Bassani DM. Chiral Binaphthalene Building Blocks for Self-Assembled Nanoscale CPL Emitters. Molecules. 2023; 28(8):3382. https://doi.org/10.3390/molecules28083382
Chicago/Turabian StyleHsieh, Yu-Yu, Jing-Jong Shyue, Yu-Chiang Chao, Ken-Tsung Wong, and Dario M. Bassani. 2023. "Chiral Binaphthalene Building Blocks for Self-Assembled Nanoscale CPL Emitters" Molecules 28, no. 8: 3382. https://doi.org/10.3390/molecules28083382