Remediation of Surfactants Used by VUV/O3 Techniques: Degradation Efficiency, Pathway and Toxicological Analysis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Degradation Rule of SDBS during Various Treatments
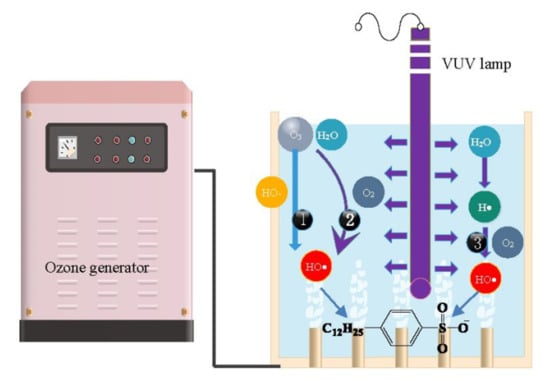
2.2. Types and Roles of Radicals Existing in VUV/O3 Process
2.3. Impacts of Varied Environmental Factors
2.3.1. Impacts of Varied Initial O3 Concentrations on VUV/O3
2.3.2. Impacts of Varied Initial SDBS Concentrations on VUV/O3
2.3.3. Performance of VUV/O3 in Varied Initial pH
2.3.4. Implications of Typical Anions on SDBS Degradation
2.4. Proposed Degradation Pathways of SDBS
2.5. Toxicological Analysis of Intermediates
2.6. Application of VUV/O3 for Real Laundry Wastewater Treatment
3. Materials and Methods
3.1. Chemicals
3.2. Experimental Procedures
3.3. Analytical Methods
4. Conclusions
- (1)
- VUV-activated O3 resulted in a synergistic effect and enhanced the oxidative effect of O3. VUV/O3 could convert SDBS to inorganic more efficiently, and DOCt/DOC0 dropped to 50.37% after 30 min treatment. With VUV alone and O3 alone, they only reached 10.63% and 29.60%. Advanced oxidation was effective to cleavage the S-O bond of SDBS, and the final concentrations of SO42− increased fastest in the VUV/O3 process.
- (2)
- VUV/O3 promoted the production of HO• compared to VUV alone and O3 alone, which was the major reactive species attacking SDBS molecules.
- (3)
- The performance of VUV/O3 was optimal at pH 9, with lower oxidation efficiency at more acidic levels. The addition of SO42− hardly affected the degradation of SDBS and that of Cl− and HCO3− slightly reduced the reaction rate, except that adding NO3− had a remarkable inhibitory effect on the process.
- (4)
- There were three isomers in SDBS, and the degradation modes of the three SDBS isomers as three pathways were very comparable. The degradation by-products of the VUV/O3 process decrease in harmfulness and toxicity compared to the SDBS parent.
- (5)
- VUV/O3 could effectively remove anionic surfactants from laundry wastewater. The removal efficiency of anionic surfactants in laundry wastewater was identified to be less than that depicted above in pure water.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Rebello, S.; Asok, A.K.; Mundayoor, S.; Jisha, M.S. Surfactants: Toxicity, remediation and green surfactants. Environ. Chem. Lett. 2014, 12, 275–287. [Google Scholar] [CrossRef]
- Ying, G. Fate, behavior and effects of surfactants and their degradation products in the environment. Environ. Int. 2006, 32, 417–431. [Google Scholar] [CrossRef] [PubMed]
- Koparal, A.S.; Önder, E.; Öütveren, B. Removal of linear alkylbenzene sulfonate from a model solution by continuous electrochemical oxidation. Desalination 2006, 197, 262–272. [Google Scholar] [CrossRef]
- Lewis, M.A. Chronic and sublethal toxicities of surfactants to aquatic animals: A review and risk assessment. Water Res. 1991, 25, 101–113. [Google Scholar] [CrossRef]
- Maksimov, V.N.; Parshikova, T.V. Influence of surfactants on the photosynthetic activity of algae. Hydrobiol. J. 2006, 42, 67–76. [Google Scholar] [CrossRef]
- Martins, N.; Pereira, J.L.; Antunes, F.E.; Melro, E.; Duarte, C.M.G.; Dias, L.; Soares, A.M.V.M.; Lopes, I. Role of surfactant headgroups on the toxicity of SLEnS-LAS mixed micelles: A case study using microtox test. Sci. Total Environ. 2018, 643, 1366–1372. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Z.; Zhang, L.; Xu, S.; Guo, B.; Liu, Y.; Xia, S. Promoting waste activated sludge reduction by linear alkylbenzene sulfonates: Surfactant dose control extracellular polymeric substances solubilization and microbial community succession. J. Hazard. Mater. 2019, 374, 74–82. [Google Scholar] [CrossRef]
- Palmer, M.; Hatley, H. The role of surfactants in wastewater treatment: Impact, removal and future techniques: A critical review. Water Res. 2018, 147, 60–72. [Google Scholar] [CrossRef]
- Zhou, Y.; Guo, B.; Li, R.; Zhang, L.; Xia, S.; Liu, Y. Treatment of grey water (GW) with high linear alkylbenzene sulfonates (LAS) content and carbon/nitrogen (C/N) ratio in an oxygen-based membrane biofilm reactor (O2-MBfR). Chemosphere 2020, 258, 127363. [Google Scholar] [CrossRef]
- Ucevli, O.; Kaya, Y. A comparative study of membrane filtration, electrocoagulation, chemical coagulation and their hybrid processes for greywater treatment. J. Environ. Chem. Eng. 2021, 9, 104946. [Google Scholar] [CrossRef]
- Méndez-Díaz, J.D.; Sánchez-Polo, M.; Rivera-Utrilla, J.; Bautista-Toledo, M.I. Effectiveness of different oxidizing agents for removing sodium dodecylbenzenesulphonate in aqueous systems. Water Res. 2009, 43, 1621–1629. [Google Scholar] [CrossRef] [PubMed]
- Chin, W.H.; Roddick, F.A.; Harris, J.L. Greywater treatment by UVC/H2O2. Water Res. 2009, 43, 3940–3947. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Cui, Y.; Zhang, H.; Liu, Y.; Oinuma, G.; Yamauchi, T.; Mu, Z.; Yang, M. Degradation of SDBS in water solutions using plasma in gas-liquid interface discharge: Performance, byproduct formation and toxicity evaluation. Chemosphere 2019, 234, 471–477. [Google Scholar] [CrossRef]
- Gassie, L.W.; Englehardt, J.D. Advanced oxidation and disinfection processes for onsite net-zero greywater reuse: A review. Water Res. 2017, 125, 384–399. [Google Scholar] [CrossRef]
- Alrousan, D.M.A.; Dunlop, P.S.M. Evaluation of ozone-based oxidation and solar advanced oxidation treatment of greywater. J. Environ. Chem. Eng. 2020, 8, 104309. [Google Scholar] [CrossRef]
- Hassanshahi, N.; Karimi-Jashni, A. Comparison of photo-Fenton, O3/H2O2/UV and photocatalytic processes for the treatment of gray water. Ecotox. Environ. Saf. 2018, 161, 683–690. [Google Scholar] [CrossRef]
- Oh, K.S.; Poh, P.E.; Chong, M.N.; Gouwanda, D.; Lam, W.H.; Chee, C.Y. Optimizing the in-line ozone injection and delivery strategy in a multistage pilot-scale greywater treatment system: System validation and cost-benefit analysis. J. Environ. Chem. Eng. 2015, 3, 1146–1151. [Google Scholar] [CrossRef]
- Li, H.; Yang, Y.; Ren, J.; Zhou, Z.; Li, X.; Liu, Y.; Feng, J. Fate of organic fractions of greywater in combined process of vacuum-ultraviolet (VUV/UV)/ozone pre-oxidation with enhanced coagulation. J. Environ. Chem. Eng. 2022, 10, 107417. [Google Scholar] [CrossRef]
- Wang, Y.; Li, H.; Yi, P.; Zhang, H. Degradation of clofibric acid by UV, O3 and UV/O3 processes: Performance comparison and degradation pathways. J. Hazard. Mater. 2019, 379, 120771. [Google Scholar] [CrossRef]
- Hua, G.; Reckhow, D.A. Effect of pre-ozonation on the formation and speciation of DBPs. Water Res. 2013, 47, 4322–4330. [Google Scholar] [CrossRef]
- Sable, S.S.; Ghute, P.P.; Fakhrnasova, D.; Mane, R.B.; Rode, C.V.; Medina, F.; Contreras, S. Catalytic ozonation of clofibric acid over copper-based catalysts: In situ ATR-IR studies. Appl. Catal. B-Environ. 2017, 209, 523–529. [Google Scholar] [CrossRef]
- Setareh, P.; Khezri, S.M.; Hossaini, H.; Pirsaheb, M. Coupling effect of ozone/ultrasound with coagulation for improving NOM and turbidity removal from surface water. J. Water Process. Eng. 2020, 37, 101340. [Google Scholar] [CrossRef]
- Chang, E.; Liu, T.; Huang, C.; Liang, C.; Chiang, P. Degradation of mefenamic acid from aqueous solutions by the ozonation and O3/UV processes. Sep. Purif. Technol. 2012, 98, 123–129. [Google Scholar] [CrossRef]
- Zoschke, K.; Börnick, H.; Worch, E. Vacuum-UV radiation at 185 nm in water treatment—A review. Water Res. 2014, 52, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Oppenländer, T. Photochemical Purification of Water and Air; Wiley-VCH: Hoboken, NJ, USA, 2007; ISBN 9783527305636; 3527305637; 352761088X; 9783527610884. [Google Scholar]
- Gonzalez, M.G.; Oliveros, E.; Wörner, M.; Braun, A.M. Vacuum-ultraviolet photolysis of aqueous reaction systems. J. Photochem. Photobiol. C 2004, 5, 225–246. [Google Scholar] [CrossRef]
- Crapulli, F.; Santoro, D.; Sasges, M.R.; Ray, A.K. Mechanistic modeling of vacuum UV advanced oxidation process in an annular photoreactor. Water Res. 2014, 64, 209–225. [Google Scholar] [CrossRef]
- Moussavi, G.; Hossaini, H.; Jafari, S.J.; Farokhi, M. Comparing the efficacy of UVC, UVC/ZnO and VUV processes for oxidation of organophosphate pesticides in water. J. Photochem. Photobiol. A 2014, 290, 86–93. [Google Scholar] [CrossRef]
- Jiang, F.; Qiu, B.; Sun, D. Degradation of refractory organics from biologically treated incineration leachate by VUV/O3. Chem. Eng. J. 2019, 370, 346–353. [Google Scholar] [CrossRef]
- Fu, P.; Feng, J.; Yang, H.; Yang, T. Degradation of sodium n-butyl xanthate by vacuum UV-ozone (VUV/O3) in comparison with ozone and VUV photolysis. Process Saf. Environ. Protect. 2016, 102, 64–70. [Google Scholar] [CrossRef]
- Ratpukdi, T.; Siripattanakul, S.; Khan, E. Mineralization and biodegradability enhancement of natural organic matter by ozone–VUV in comparison with ozone, VUV, ozone–UV, and UV: Effects of pH and ozone dose. Water Res. 2010, 44, 3531–3543. [Google Scholar] [CrossRef]
- Krakkó, D.; Illés, Á.; Licul-Kucera, V.; Dávid, B.; Dobosy, P.; Pogonyi, A.; Demeter, A.; Mihucz, V.G.; Dóbé, S.; Záray, G. Application of (V)UV/O3 technology for post-treatment of biologically treated wastewater: A pilot-scale study. Chemosphere 2021, 275, 130080. [Google Scholar] [CrossRef]
- Liu, H.; Gao, Y.; Wang, J.; Ma, D.; Wang, Y.; Gao, B.; Yue, Q.; Xu, X. The application of UV/O3 process on ciprofloxacin wastewater containing high salinity: Performance and its degradation mechanism. Chemosphere 2021, 276, 130220. [Google Scholar] [CrossRef] [PubMed]
- Wen, D.; Wu, Z.; Tang, Y.; Li, M.; Qiang, Z. Accelerated degradation of sulfamethazine in water by VUV/UV photo-Fenton process: Impact of sulfamethazine concentration on reaction mechanism. J. Hazard. Mater. 2018, 344, 1181–1187. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wu, Y.; Zhang, W.; Fan, X.; Wang, Y.; Zhang, H. Performance of artificial sweetener sucralose mineralization via UV/O3 process: Kinetics, toxicity and intermediates. Chem. Eng. J. 2018, 353, 626–634. [Google Scholar] [CrossRef]
- Karimian, S.; Moussavi, G.; Fanaei, F.; Mohammadi, S.; Shekoohiyan, S.; Giannakis, S. Shedding light on the catalytic synergies between Fe(II) and PMS in vacuum UV (VUV/Fe/PMS) photoreactors for accelerated elimination of pharmaceuticals: The case of metformin. Chem. Eng. J. 2020, 400, 125896. [Google Scholar] [CrossRef]
- Li, H.; Yang, Y.; Gao, J.; Li, X.; Zhou, Z.; Wang, N.; Du, P.; Zhang, T.; Feng, J. Degradation of sodium dodecyl benzenesulfonate by vacuum ultraviolet irradiation. J. Water Process. Eng. 2020, 34, 101172. [Google Scholar] [CrossRef]
- Dai, Q.; Wang, J.; Chen, J.; Chen, J. Ozonation catalyzed by cerium supported on activated carbon for the degradation of typical pharmaceutical wastewater. Sep. Purif. Technol. 2014, 127, 112–120. [Google Scholar] [CrossRef]
- Staehelin, J.; Hoigne, J. Decomposition of ozone in water in the presence of organic solutes acting as promoters and inhibitors of radical chain reactions. Environ. Sci. Technol. 1985, 19, 1206–1213. [Google Scholar] [CrossRef]
- Koppenol, W.H.; Liebman, J.F. The oxidizing nature of the hydroxyl radical. A comparison with the Ferryl Ion (FeO2+). J. Phys. Chem. 1984, 88, 99–101. [Google Scholar] [CrossRef]
- Duca, C.; Imoberdorf, G.; Mohseni, M. Effects of inorganics on the degradation of micropollutants with vacuum UV (VUV) advanced oxidation. J. Environ. Sci. Health Part a-Toxic/Hazard. Subst. Environ. Eng. 2017, 52, 524–532. [Google Scholar] [CrossRef]
- Tan, C.; Gao, N.; Zhou, S.; Xiao, Y.; Zhuang, Z. Kinetic study of acetaminophen degradation by UV-based advanced oxidation processes. Chem. Eng. J. 2014, 253, 229–236. [Google Scholar] [CrossRef]
- Zhao, Q.; Mao, Q.; Zhou, Y.; Wei, J.; Liu, X.; Yang, J.; Luo, L.; Zhang, J.; Chen, H.; Chen, H.; et al. Metal-free carbon materials-catalyzed sulfate radical-based advanced oxidation processes: A review on heterogeneous catalysts and applications. Chemosphere 2017, 189, 224–238. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Cao, Y.; Jiang, J.; Lu, X.; Ma, J.; Pang, S.; Li, J.; Liu, Y.; Zhou, Y.; Guan, C. Comparative study on degradation of propranolol and formation of oxidation products by UV/H2O2 and UV/persulfate (PDS). Water Res. 2019, 149, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, M.; Li, W.; Jiang, Y.; Qiang, Z. Bench- and pilot-scale studies on the removal of pesticides from water by VUV/UV process. Chem. Eng. J. 2018, 342, 155–162. [Google Scholar] [CrossRef]
- Zhou, C.; Gao, N.; Deng, Y.; Chu, W.; Rong, W.; Zhou, S. Factors affecting ultraviolet irradiation/hydrogen peroxide (UV/H2O2) degradation of mixed n-nitrosamines in water. J. Hazard. Mater. 2012, 231–232, 43–48. [Google Scholar] [CrossRef]
- Zhu, H.; Jia, R.; Sun, S.; Feng, G.; Wang, M.; Zhao, Q.; Xin, X.; Zhou, A. Elimination of trichloroanisoles by UV/H2O2: Kinetics, degradation mechanism, water matrix effects and toxicity assessment. Chemosphere 2019, 230, 258–267. [Google Scholar] [CrossRef]
- Furatian, L.; Mohseni, M. Inuence of major anions on the 185 nm advanced oxidation process—Sulphate, bicarbonate, and chloride. Chemosphere 2018, 201, 503–510. [Google Scholar] [CrossRef]
- Yi, X.; Ji, H.; Wang, C.; Li, Y.; Li, Y.; Zhao, C.; Wang, A.; Fu, H.; Wang, P.; Zhao, X.; et al. Photocatalysis-activated SR-AOP over PDINH/MIL-88A(Fe) composites for boosted chloroquine phosphate degradation: Performance, mechanism, pathway and DFT calculations. Appl. Catal. B-Environ. 2021, 293, 120229. [Google Scholar] [CrossRef]
- Ji, H.; Du, P.; Zhao, D.; Li, S.; Sun, F.; Duin, E.C.; Liu, W. 2D/1D graphitic carbon nitride/titanate nanotubes heterostructure for efficient photocatalysis of sulfamethazine under solar light: Catalytic “hot spots” at the rutile–anatase–titanate interfaces. Appl. Catal. B-Environ. 2020, 263, 118357. [Google Scholar] [CrossRef]
- Mailhot, G.; Asif, A.; Bolte, M. Degradation of sodium 4-dodecylbenzenesulphonate photoinduced by Fe(III) in aqueous solution. Chemosphere 2000, 41, 363–370. [Google Scholar] [CrossRef]
- Ashokkumar, M.; Niblett, T.; Tantiongco, L.; Grieser, F. Sonochemical degradation of sodium dodecylbenzene sulfonate in aqueous solutions. Aust. J. Chem. 2003, 56, 1045. [Google Scholar] [CrossRef]
- Cai, Z.; Hao, X.; Sun, X.; Du, P.; Liu, W.; Fu, J. Highly active WO3@anatase-SiO2 aerogel for solar-light-driven phenanthrene degradation: Mechanism insight and toxicity assessment. Water Res. 2019, 162, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Lima, T.M.S.; Procópio, L.C.; Brandão, F.D.; Leão, B.A.; Tótola, M.R.; Borges, A.C. Evaluation of bacterial surfactant toxicity towards petroleum degrading microorganisms. Bioresour. Technol. 2011, 102, 2957–2964. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Ji, H.; Qi, J.; Huang, T.; Wang, C.; Liu, W. Degradation of acetaminophen by activated peroxymonosulfate using Co(OH)2 hollow microsphere supported titanate nanotubes: Insights into sulfate radical production pathway through CoOH+ activation. Chem. Eng. J. 2021, 406, 126877. [Google Scholar] [CrossRef]
- Li, M.; Qiang, Z.; Pulgarin, C.; Kiwi, J. Accelerated methylene blue (MB) degradation by Fenton reagent exposed to UV or VUV/UV light in an innovative micro photo-reactor. Appl. Catal. B-Environ. 2016, 187, 83–89. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Yang, Y.; Li, X.; Zhou, Z.; Feng, J.; Dai, Y.; Li, X.; Ren, J. Degradation of sulfamethazine by vacuum ultraviolet-activated sulfate radical-advanced oxidation: Efficacy, mechanism and influences of water constituents. Sep. Purif. Technol. 2022, 282, 120058. [Google Scholar] [CrossRef]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (⋅OH/⋅O− in aqueous solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef] [Green Version]
- Imoberdorf, G.; Mohseni, M. Modeling and experimental evaluation of vacuum-UV photoreactors for water treatment. Chem. Eng. Sci. 2011, 66, 1159–1167. [Google Scholar] [CrossRef]
- Bader, H.; Hoigné, J. Determination of ozone in water by the indigo method. Water Res. 1981, 15, 449–456. [Google Scholar] [CrossRef]
- Yin, R.; Chen, Y.; He, S.; Li, W.; Zeng, L.; Guo, W.; Zhu, M. In situ photoreduction of structural Fe(III) in a metal–organic framework for peroxydisulfate activation and efficient removal of antibiotics in real wastewater. J. Hazard. Mater. 2020, 388, 121996. [Google Scholar] [CrossRef]











Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Yang, Y.; Li, X.; Ullah, H. Remediation of Surfactants Used by VUV/O3 Techniques: Degradation Efficiency, Pathway and Toxicological Analysis. Molecules 2023, 28, 3312. https://doi.org/10.3390/molecules28083312
Li H, Yang Y, Li X, Ullah H. Remediation of Surfactants Used by VUV/O3 Techniques: Degradation Efficiency, Pathway and Toxicological Analysis. Molecules. 2023; 28(8):3312. https://doi.org/10.3390/molecules28083312
Chicago/Turabian StyleLi, Hang, Yanling Yang, Xing Li, and Habib Ullah. 2023. "Remediation of Surfactants Used by VUV/O3 Techniques: Degradation Efficiency, Pathway and Toxicological Analysis" Molecules 28, no. 8: 3312. https://doi.org/10.3390/molecules28083312