Sulphur- and Selenium-for-Oxygen Replacement as a Strategy to Obtain Dual Type I/Type II Photosensitizers for Photodynamic Therapy
Abstract
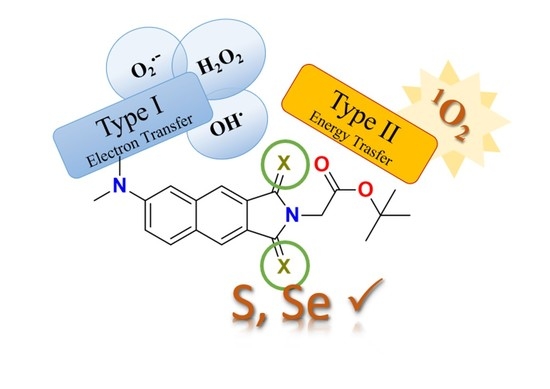
:1. Introduction
2. Results and Discussion
3. Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dąbrowski, J.M.; Pucelik, B.; Regiel-Futyra, A.; Brindell, M.; Mazuryk, O.; Kyzioł, A.; Stochel, G.; Macyk, W.; Arnaut, L.G. Engineering of Relevant Photodynamic Processes through Structural Modifications of Metallotetrapyrrolic Photosensitizers. Coord. Chem. Rev. 2016, 325, 67–101. [Google Scholar] [CrossRef]
- Yano, S.; Hirohara, S.; Obata, M.; Hagiya, Y.; Ogura, S.; Ikeda, A.; Kataoka, H.; Tanaka, M.; Joh, T. Current States and Future Views in Photodynamic Therapy. J. Photochem. Photobiol. C Photochem. Rev. 2011, 12, 46–67. [Google Scholar] [CrossRef]
- MacDonald, I.J.; Dougherty, T.J. Basic Principles of Photodynamic Therapy. J. Porphyr. Phthalocyanines 2001, 05, 105–129. [Google Scholar] [CrossRef]
- Baptista, M.S.; Cadet, J.; di Mascio, P.; Ghogare, A.A.; Greer, A.; Hamblin, M.R.; Lorente, C.; Nunez, S.C.; Ribeiro, M.S.; Thomas, A.H.; et al. Type I and Type II Photosensitized Oxidation Reactions: Guidelines and Mechanistic Pathways. Photochem. Photobiol. 2017, 93, 912–919. [Google Scholar] [CrossRef] [Green Version]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic Therapy of Cancer: An Update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef]
- Dabrowski, J.M.; Arnaut, L.G. Photodynamic Therapy (PDT) of Cancer: From Local to Systemic Treatment. Photochem. Photobiol. Sci. 2015, 14, 1765–1780. [Google Scholar] [CrossRef]
- dos Santos, A.F.; de Almeida, D.R.Q.; Terra, L.F.; Baptista, M.S.; Labriola, L. Photodynamic Therapy in Cancer Treatment—An Update Review. J. Cancer Metastasis Treat. 2019, 2019, 25. [Google Scholar] [CrossRef] [Green Version]
- Kossodo, S.; LaMuraglia, G.M. Clinical Potential of Photodynamic Therapy in Cardiovascular Disorders. Am. J. Cardiovasc. Drugs 2001, 1, 15–21. [Google Scholar] [CrossRef]
- DeRosa, M. Photosensitized Singlet Oxygen and Its Applications. Coord. Chem. Rev. 2002, 233–234, 351–371. [Google Scholar] [CrossRef]
- Romero, O.C.; Straub, A.P.; Kohn, T.; Nguyen, T.H. Role of Temperature and Suwannee River Natural Organic Matter on Inactivation Kinetics of Rotavirus and Bacteriophage MS2 by Solar Irradiation. Environ. Sci. Technol. 2011, 45, 10385–10393. [Google Scholar] [CrossRef] [Green Version]
- Aroso, R.T.; Schaberle, F.A.; Arnaut, L.G.; Pereira, M.M. Photodynamic Disinfection and Its Role in Controlling Infectious Diseases. Photochem. Photobiol. Sci. 2021, 20, 1497–1545. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Cao, W.; Cao, J. Treatment of Rheumatoid Arthritis by Phototherapy: Advances and Perspectives. Nanoscale 2021, 13, 14591–14608. [Google Scholar] [CrossRef] [PubMed]
- Monfrecola, G.; Megna, M.; Rovati, C.; Arisi, M.; Rossi, M.; Calzavara-Pinton, I.; Fabbrocini, G.; Calzavara-Pinton, P. A Critical Reappraisal of Off-Label Use of Photodynamic Therapy for the Treatment of Non-Neoplastic Skin Conditions. Dermatology 2021, 237, 262–276. [Google Scholar] [CrossRef] [PubMed]
- Stájer, A.; Kajári, S.; Gajdács, M.; Musah-Eroje, A.; Baráth, Z. Utility of Photodynamic Therapy in Dentistry: Current Concepts. Dent. J. 2020, 8, 43. [Google Scholar] [CrossRef]
- Alberto, M.E.; Comuzzi, C.; Thandu, M.; Adamo, C.; Russo, N. 22π-Electrons [1.1.1.1.1] Pentaphyrin as a New Photosensitizing Agent for Water Disinfection: Experimental and Theoretical Characterization. Theor. Chem. Acc. 2016, 135, 29. [Google Scholar] [CrossRef] [Green Version]
- Bartolomeu, M.; Reis, S.; Fontes, M.; Neves, M.; Faustino, M.; Almeida, A. Photodynamic Action against Wastewater Microorganisms and Chemical Pollutants: An Effective Approach with Low Environmental Impact. Water 2017, 9, 630. [Google Scholar] [CrossRef] [Green Version]
- Semenova, O.; Kobzev, D.; Hovor, I.; Atrash, M.; Nakonechny, F.; Kulyk, O.; Bazylevich, A.; Gellerman, G.; Patsenker, L. Effect of Solubilizing Group on the Antibacterial Activity of Heptamethine Cyanine Photosensitizers. Pharmaceutics 2023, 15, 247. [Google Scholar] [CrossRef]
- Henderson, B.W.; Gollnick, S.O.; Snyder, J.W.; Busch, T.M.; Kousis, P.C.; Cheney, R.T.; Morgan, J. Choice of Oxygen-Conserving Treatment Regimen Determines the Inflammatory Response and Outcome of Photodynamic Therapy of Tumors. Cancer Res. 2004, 64, 2120–2126. [Google Scholar] [CrossRef] [Green Version]
- Falk-Mahapatra, R.; Gollnick, S.O. Photodynamic Therapy and Immunity: An Update. Photochem. Photobiol. 2020, 96, 550–559. [Google Scholar] [CrossRef] [Green Version]
- Castano, A.P.; Mroz, P.; Hamblin, M.R. Photodynamic Therapy and Anti-Tumour Immunity. Nat. Rev. Cancer 2006, 6, 535–545. [Google Scholar] [CrossRef] [Green Version]
- Gourdon, L.; Cariou, K.; Gasser, G. Phototherapeutic Anticancer Strategies with First-Row Transition Metal Complexes: A Critical Review. Chem. Soc. Rev. 2022, 51, 1167–1195. [Google Scholar] [CrossRef] [PubMed]
- Algorri, J.F.; Ochoa, M.; Roldán-Varona, P.; Rodríguez-Cobo, L.; López-Higuera, J.M. Photodynamic Therapy: A Compendium of Latest Reviews. Cancers 2021, 13, 4447. [Google Scholar] [CrossRef] [PubMed]
- Alberto, M.; Pirillo, J.; Russo, N.; Adamo, C. Theoretical Exploration of Type I/Type II Dual Photoreactivity of Promising Ru(II) Dyads for PDT Approach. Inorg. Chem. 2016, 55, 11185–11192. [Google Scholar] [CrossRef] [Green Version]
- Alberto, M.E.; Francés-Monerris, A. A Multiscale Free Energy Method Reveals an Unprecedented Photoactivation of a Bimetallic Os(II)–Pt(II) Dual Anticancer Agent. Phys. Chem. Chem. Phys. 2022, 24, 19584–19594. [Google Scholar] [CrossRef] [PubMed]
- Ponte, F.; Alberto, M.E.; De Simone, B.C.; Russo, N.; Sicilia, E. Photophysical Exploration of Dual-Approach PtII –BODIPY Conjugates: Theoretical Insights. Inorg. Chem. 2019, 58, 9882–9889. [Google Scholar] [CrossRef]
- Monro, S.; Colón, K.L.; Yin, H.; Roque, J.; Konda, P.; Gujar, S.; Thummel, R.P.; Lilge, L.; Cameron, C.G.; McFarland, S.A. Transition Metal Complexes and Photodynamic Therapy from a Tumor-Centered Approach: Challenges, Opportunities, and Highlights from the Development of TLD1433. Chem. Rev. 2019, 119, 797–828. [Google Scholar] [CrossRef]
- Roque, J.A.; Barrett, P.C.; Cole, H.D.; Lifshits, L.M.; Shi, G.; Monro, S.; von Dohlen, D.; Kim, S.; Russo, N.; Deep, G.; et al. Breaking the Barrier: An Osmium Photosensitizer with Unprecedented Hypoxic Phototoxicity for Real World Photodynamic Therapy. Chem. Sci. 2020, 11, 9784–9806. [Google Scholar] [CrossRef]
- Lameijer, L.N.; Ernst, D.; Hopkins, S.L.; Meijer, M.S.; Askes, S.H.C.; Le Dévédec, S.E.; Bonnet, S. A Red-Light-Activated Ruthenium-Caged NAMPT Inhibitor Remains Phototoxic in Hypoxic Cancer Cells. Angew. Chem. Int. Ed. 2017, 56, 11549–11553. [Google Scholar] [CrossRef] [Green Version]
- Loftus, L.M.; Al-Afyouni, K.F.; Turro, C. New Ru II Scaffold for Photoinduced Ligand Release with Red Light in the Photodynamic Therapy (PDT) Window. Chem. Eur. J. 2018, 24, 11550–11553. [Google Scholar] [CrossRef]
- Roque, J.A., III; Cole, H.D.; Barrett, P.C.; Lifshits, L.M.; Hodges, R.O.; Kim, S.; Deep, G.; Francés-Monerris, A.; Alberto, M.E.; Cameron, C.G.; et al. Intraligand Excited States Turn a Ruthenium Oligothiophene Complex into a Light-Triggered Ubertoxin with Anticancer Effects in Extreme Hypoxia. J. Am. Chem. Soc. 2022, 144, 8317–8336. [Google Scholar] [CrossRef]
- El-Sayed, M.A. Triplet State. Its Radiative and Nonradiative Properties. Acc. Chem. Res. 1968, 1, 8–16. [Google Scholar] [CrossRef]
- Banfi, S.; Caruso, E.; Caprioli, S.; Mazzagatti, L.; Canti, G.; Ravizza, R.; Gariboldi, M.; Monti, E. Photodynamic Effects of Porphyrin and Chlorin Photosensitizers in Human Colon Adenocarcinoma Cells. Bioorg. Med. Chem. 2004, 12, 4853–4860. [Google Scholar] [CrossRef] [PubMed]
- Alberto, M.E.; Marino, T.; Quartarolo, A.D.; Russo, N. Photophysical Origin of the Reduced Photodynamic Therapy Activity of Temocene Compared to Foscan®: Insights from Theory. Phys. Chem. Chem. Phys. 2013, 15, 16167. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Xu, Q.; Wang, W.; Shao, J.; Huang, W.; Dong, X. Type I Photosensitizers Revitalizing Photodynamic Oncotherapy. Small 2021, 17, 2006742. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhuang, Z.; Zhao, Z.; Tang, B.Z. Type I AIE Photosensitizers: Mechanism and Application. VIEW 2022, 3, 20200121. [Google Scholar] [CrossRef]
- Ignarro, L.J.; Freeman, B.A. Nitric Oxide Biology and Pathobiology; Ignarro, L., Ed.; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Herzberg, G. Spectra of Diatomic Molecules, 2nd ed.; Van Nostrand Reinhold: New York, NY, USA, 1950. [Google Scholar]
- Alberto, M.E.; de Simone, B.C.; Liuzzi, S.; Marino, T.; Russo, N.; Toscano, M. Iodine Substituted Phosphorus Corrole Complexes as Possible Photosensitizers in Photodynamic Therapy: Insights from Theory. J. Comput. Chem. 2020, 41, 1395–1401. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-H.; Tang, X.-F.; Chang, X.-P.; Zhang, T.-S.; Xie, B.-B.; Cui, G. Mechanistic Photophysics of Tellurium-Substituted Uracils: Insights from Multistate Complete-Active-Space Second-Order Perturbation Calculations. J. Phys. Chem. A 2021, 125, 8816–8826. [Google Scholar] [CrossRef]
- Jena, S.; Tulsiyan, K.D.; Kumari, A.; Das, R.; Biswal, H.S. Thiolumazines as Heavy-Atom-Free Photosensitizers for Applications in Daylight Photodynamic Therapy: Insights from Ultrafast Excited-State Dynamics. J. Phys. Chem. B 2022, 126, 6083–6094. [Google Scholar] [CrossRef]
- Farrell, K.M.; Brister, M.M.; Pittelkow, M.; Sølling, T.I.; Crespo-Hernández, C.E. Heavy-Atom-Substituted Nucleobases in Photodynamic Applications: Substitution of Sulfur with Selenium in 6-Thioguanine Induces a Remarkable Increase in the Rate of Triplet Decay in 6-Selenoguanine. J. Am. Chem. Soc. 2018, 140, 11214–11218. [Google Scholar] [CrossRef]
- Fang, Y.-G.; Valverde, D.; Mai, S.; Canuto, S.; Borin, A.C.; Cui, G.; González, L. Excited-State Properties and Relaxation Pathways of Selenium-Substituted Guanine Nucleobase in Aqueous Solution and DNA Duplex. J. Phys. Chem. B 2021, 125, 1778–1789. [Google Scholar] [CrossRef]
- Valverde, D.; Mai, S.; Canuto, S.; Borin, A.C.; González, L. Ultrafast Intersystem Crossing Dynamics of 6-Selenoguanine in Water. JACS Au 2022, 2, 1699–1711. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Rodríguez, L.A.; Crespo-Hernández, C.E. Thionated Organic Compounds as Emerging Heavy-Atom-Free Photodynamic Therapy Agents. Chem. Sci. 2020, 11, 11113–11123. [Google Scholar] [CrossRef] [PubMed]
- Salon, J.; Gan, J.; Abdur, R.; Liu, H.; Huang, Z. Synthesis of 6-Se-Guanosine RNAs for Structural Study. Org. Lett. 2013, 15, 3934–3937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassan, A.E.A.; Sheng, J.; Zhang, W.; Huang, Z. High Fidelity of Base Pairing by 2-Selenothymidine in DNA. J. Am. Chem. Soc. 2010, 132, 2120–2121. [Google Scholar] [CrossRef]
- Faustino, I.; Curutchet, C.; Luque, F.J.; Orozco, M. The DNA-Forming Properties of 6-Selenoguanine. Phys. Chem. Chem. Phys. 2014, 16, 1101–1110. [Google Scholar] [CrossRef]
- Nguyen, V.-N.; Qi, S.; Kim, S.; Kwon, N.; Kim, G.; Yim, Y.; Park, S.; Yoon, J. An Emerging Molecular Design Approach to Heavy-Atom-Free Photosensitizers for Enhanced Photodynamic Therapy under Hypoxia. J. Am. Chem. Soc. 2019, 141, 16243–16248. [Google Scholar] [CrossRef]
- Tang, J.; Wang, L.; Loredo, A.; Cole, C.; Xiao, H. Single-Atom Replacement as a General Approach towards Visible-Light/near-Infrared Heavy-Atom-Free Photosensitizers for Photodynamic Therapy. Chem. Sci. 2020, 11, 6701–6708. [Google Scholar] [CrossRef]
- Ortiz-Rodríguez, L.A.; Hoehn, S.J.; Loredo, A.; Wang, L.; Xiao, H.; Crespo-Hernández, C.E. Electronic Relaxation Pathways in Heavy-Atom-Free Photosensitizers Absorbing Near-Infrared Radiation and Exhibiting High Yields of Singlet Oxygen Generation. J. Am. Chem. Soc. 2021, 143, 2676–2681. [Google Scholar] [CrossRef]
- Alberto, M.E.; de Simone, B.C.; Marino, T.; Toscano, M.; Russo, N. Chalcogen Effects in the Photophysical Properties of Dimethylamino-1,8-Naphthalimide Dyes Revealed by DFT Investigation. J. Phys. Chem. A 2022, 126, 5167–5172. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron Density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [Green Version]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian, 16, Revision C.1; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005, 105, 2999–3094. [Google Scholar] [CrossRef] [PubMed]
- Hirata, S.; Head-Gordon, M. Time-Dependent Density Functional Theory within the Tamm–Dancoff Approximation. Chem. Phys. Lett. 1999, 314, 291–299. [Google Scholar] [CrossRef]
- Peach, M.J.G.; Williamson, M.J.; Tozer, D.J. Influence of Triplet Instabilities in TDDFT. J. Chem. Theory. Comput. 2011, 7, 3578–3585. [Google Scholar] [CrossRef] [PubMed]
- Alberto, M.E.; de Simone, B.C.; Mazzone, G.; Sicilia, E.; Russo, N. The Heavy Atom Effect on Zn(II) Phthalocyanine Derivatives: A Theoretical Exploration of the Photophysical Properties. Phys. Chem. Chem. Phys. 2015, 17, 23595–23601. [Google Scholar] [CrossRef] [PubMed]
- Ruud, K.; Schimmelpfennig, B.; Ågren, H. Internal and External Heavy-Atom Effects on Phosphorescence Radiative Lifetimes Calculated Using a Mean-Field Spin–Orbit Hamiltonian. Chem. Phys. Lett. 1999, 310, 215–221. [Google Scholar] [CrossRef]
- Dalton, a Molecular Electronic Structure Program, Release 2011-07-20 (2011). Available online: http://daltonprogram.org (accessed on 9 February 2023).
- Ji, S.; Ge, J.; Escudero, D.; Wang, Z.; Zhao, J.; Jacquemin, D. Molecular Structure–Intersystem Crossing Relationship of Heavy-Atom-Free BODIPY Triplet Photosensitizers. J. Org. Chem. 2015, 80, 5958–5963. [Google Scholar] [CrossRef]
- Brémond, É.; Alberto, M.E.; Russo, N.; Ricci, G.; Ciofini, I.; Adamo, C. Photophysical Properties of NIR-Emitting Fluorescence Probes: Insights from TD-DFT. Phys. Chem. Chem. Phys. 2013, 15, 10019. [Google Scholar] [CrossRef]
- Alberto, M.E.; Marino, T.; Russo, N.; Sicilia, E.; Toscano, M. The Performance of Density Functional Based Methods in the Description of Selected Biological Systems and Processes. Phys. Chem. Chem. Phys. 2012, 14, 14943. [Google Scholar] [CrossRef]
- de Simone, B.C.; Marino, T.; Prejanò, M.; Russo, N. Can Fused Thiophene–Pyrrole-Containing Rings Act as Possible New Electrochromic Dyes? A Computational Prediction. Theor. Chem. Acc. 2016, 135, 238. [Google Scholar] [CrossRef]
- Li, Y.; Prejanò, M.; Toscano, M.; Russo, N. Oenin/Syringic Acid Copigmentation: Insights From a Theoretical Study. Front. Chem. 2019, 7, 579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marian, C.M. Spin-Orbit Coupling and Intersystem Crossing in Molecules. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 187–203. [Google Scholar] [CrossRef]






| Cpds | VEA (S0) | VIP (S0) | VEA (T1) | VIP (T1) |
|---|---|---|---|---|
| DMNP | −2.48 | 5.42 | −4.66 | 3.56 |
| SDMNP | −3.51 | 5.43 | −4.83 | 4.23 |
| SeDMNP | −3.71 | 5.42 | −4.84 | 4.39 |
| O2 | −3.66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prejanò, M.; Alberto, M.E.; De Simone, B.C.; Marino, T.; Toscano, M.; Russo, N. Sulphur- and Selenium-for-Oxygen Replacement as a Strategy to Obtain Dual Type I/Type II Photosensitizers for Photodynamic Therapy. Molecules 2023, 28, 3153. https://doi.org/10.3390/molecules28073153
Prejanò M, Alberto ME, De Simone BC, Marino T, Toscano M, Russo N. Sulphur- and Selenium-for-Oxygen Replacement as a Strategy to Obtain Dual Type I/Type II Photosensitizers for Photodynamic Therapy. Molecules. 2023; 28(7):3153. https://doi.org/10.3390/molecules28073153
Chicago/Turabian StylePrejanò, Mario, Marta Erminia Alberto, Bruna Clara De Simone, Tiziana Marino, Marirosa Toscano, and Nino Russo. 2023. "Sulphur- and Selenium-for-Oxygen Replacement as a Strategy to Obtain Dual Type I/Type II Photosensitizers for Photodynamic Therapy" Molecules 28, no. 7: 3153. https://doi.org/10.3390/molecules28073153