An Alternative Exploitation of Synechocystis sp. PCC6803: A Cascade Approach for the Recovery of High Added-Value Products
Abstract
:1. Introduction
2. Results
2.1. Synechocystis sp. PCC6803 Cultivation
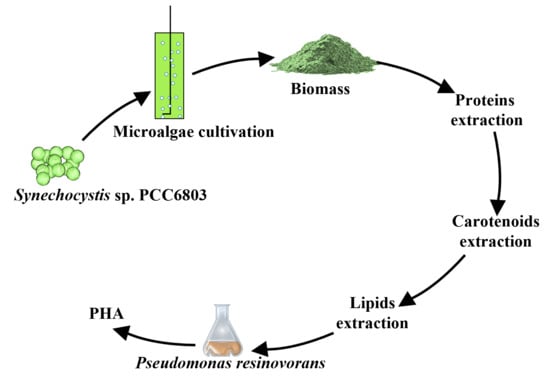
2.2. Biorefinery Strategy
2.2.1. Protein Extraction
2.2.2. Pigments Extraction and Identification
2.2.3. Lipidic Fraction Extraction and Quantification
2.3. Biotechnological Application of the Extracted Molecules
2.3.1. Protein Fraction
2.3.2. Carotenoids
2.3.3. Lipidic Fraction
3. Materials and Methods
3.1. Reagents
3.2. Synechocystis sp. PCC6803 Cultivation
3.3. Proteins Extraction and Quantification
3.4. Pigments Extraction and Characterization
3.5. Lipid Extraction
3.6. Cell Culture and Biocompatibility Assay
3.7. Carotenoids Antioxidant Activity
3.8. Microbial Production of Polyhydroxyalkanoates by Pseudomonas resinovorans
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Anderson, T.R.; Hawkins, E.; Jones, P.D. CO2, the Greenhouse Effect and Global Warming: From the Pioneering Work of Arrhenius and Callendar to Today’s Earth System Models. Endeavour 2016, 40, 178–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beattie, A.; Vermaas, W.; Darzins, A.; Holland, S.C.; Li, S.; McGowen, J.; Nielsen, D.; Quinn, J.C. A Probabilistic Economic and Environmental Impact Assessment of a Cyanobacteria-Based Biorefinery. Algal Res. 2021, 59, 102454. [Google Scholar] [CrossRef]
- Lopes, T.F.; Cabanas, C.; Silva, A.; Fonseca, D.; Santos, E.; Guerra, L.T.; Sheahan, C.; Reis, A.; Gírio, F. Process Simulation and Techno-Economic Assessment for Direct Production of Advanced Bioethanol Using a Genetically Modified Synechocystis Sp. Bioresour. Technol. Rep. 2019, 6, 113–122. [Google Scholar] [CrossRef]
- Koyande, A.K.; Chew, K.W.; Rambabu, K.; Tao, Y.; Chu, D.T.; Show, P.L. Microalgae: A Potential Alternative to Health Supplementation for Humans. Food Sci. Hum. Wellness 2019, 8, 16–24. [Google Scholar] [CrossRef]
- Chew, K.W.; Yap, J.Y.; Show, P.L.; Suan, N.H.; Juan, J.C.; Ling, T.C.; Lee, D.J.; Chang, J.S. Microalgae Biorefinery: High Value Products Perspectives. Bioresour. Technol. 2017, 229, 53–62. [Google Scholar] [CrossRef]
- Koller, M.; Salerno, A.; Strohmeier, K.; Schober, S.; Mittelbach, M.; Illieva, V.; Chiellini, E.; Braunegg, G. Novel precursors for production of 3-hydroxyvalerate-containing poly [(R)-hydroxyalkanoate]s. Biocatal. Biotransform. 2014, 32, 161–167. [Google Scholar] [CrossRef]
- Ferreira da Silva, A.; Brazinha, C.; Costa, L.; Caetano, N.S. Techno-Economic Assessment of a Synechocystis Based Biorefinery through Process Optimization. Energy Rep. 2020, 6, 509–514. [Google Scholar] [CrossRef]
- Tredici, M.R. Photobiology of Microalgae Mass Cultures: Understanding the Tools for the next Green Revolution. Biofuels 2014, 1, 143–162. [Google Scholar] [CrossRef]
- Chisti, Y. Biodiesel from Microalgae Beats Bioethanol. Trends Biotechnol. 2008, 26, 126–131. [Google Scholar] [CrossRef]
- Stanier, R.Y.; Kunisawa, R.; Mandel, M.; Cohen-Bazire, G. Purification and Properties of Unicellular Blue-Green Algae (Order Chroococcales). Bacteriol. Rev. 1971, 35, 171–205. [Google Scholar] [CrossRef]
- Barten, R.; Lill, H. DNA-Uptake in the Naturally Competent Cyanobacterium, Synechocystis Sp. PCC 6803. FEMS Microbiol. Lett. 1995, 129, 83–87. [Google Scholar] [CrossRef]
- Khetkorn, W.; Incharoensakdi, A.; Lindblad, P.; Jantaro, S. Enhancement of Poly-3-Hydroxybutyrate Production in Synechocystis Sp. PCC 6803 by Overexpression of Its Native Biosynthetic Genes. Bioresour. Technol. 2016, 214, 761–768. [Google Scholar] [CrossRef]
- Koch, M.; Doello, S.; Gutekunst, K.; Forchhammer, K. PHB Is Produced from Glycogen Turn-over during Nitrogen Starvation in Synechocystis sp. PCC 6803. Int. J. Mol. Sci. 2019, 20, 1942. [Google Scholar] [CrossRef] [Green Version]
- Durall, C.; Lindberg, P.; Yu, J.; Lindblad, P. Increased Ethylene Production by Overexpressing Phosphoenolpyruvate Carboxylase in the Cyanobacterium Synechocystis PCC 6803. Biotechnol. Biofuels 2020, 13, 16. [Google Scholar] [CrossRef]
- Veetil, V.P.; Angermayr, S.A.; Hellingwerf, K.J. Ethylene Production with Engineered Synechocystis sp PCC 6803 Strains. Microb. Cell Fact. 2017, 16, 34. [Google Scholar] [CrossRef] [Green Version]
- Trentin, G.; Bertucco, A.; Sforza, E. Mixotrophy in Synechocystis Sp. for the Treatment of Wastewater with High Nutrient Content: Effect of CO2 and Light. Bioprocess Biosyst. Eng. 2019, 42, 1661–1669. [Google Scholar] [CrossRef]
- Srimongkol, P.; Sangtanoo, P.; Songserm, P.; Watsuntorn, W.; Karnchanatat, A. Microalgae-Based Wastewater Treatment for Developing Economic and Environmental Sustainability: Current Status and Future Prospects. Front. Bioeng. Biotechnol. 2022, 10, 1–18. [Google Scholar] [CrossRef]
- Touloupakis, E.; Cicchi, B.; Benavides, A.M.S.; Torzillo, G. Effect of High PH on Growth of Synechocystis Sp. PCC 6803 Cultures and Their Contamination by Golden Algae (Poterioochromonas sp.). Appl. Microbiol. Biotechnol. 2016, 100, 1333–1341. [Google Scholar] [CrossRef] [Green Version]
- Straka, L.; Rittmann, B.E. Effect of Culture Density on Biomass Production and Light Utilization Efficiency of Synechocystis Sp. PCC 6803. Biotechnol. Bioeng. 2018, 115, 507–511. [Google Scholar] [CrossRef]
- Imbimbo, P.; Romanucci, V.; Pollio, A.; Fontanarosa, C.; Amoresano, A.; Zarrelli, A.; Olivieri, G.; Monti, D.M. A Cascade Extraction of Active Phycocyanin and Fatty Acids from Galdieria Phlegrea. Appl. Microbiol. Biotechnol. 2019, 103, 9455–9464. [Google Scholar] [CrossRef]
- Hsieh-Lo, M.; Castillo, G.; Ochoa-Becerra, M.A.; Mojica, L. Phycocyanin and Phycoerythrin: Strategies to Improve Production Yield and Chemical Stability. Algal Res. 2019, 42, 101600. [Google Scholar] [CrossRef]
- Gorgich, M.; Passos, M.L.C.; Mata, T.M.; Martins, A.A.; Saraiva, M.L.M.F.S.; Caetano, N.S. Enhancing Extraction and Purification of Phycocyanin from Arthrospira sp. with Lower Energy Consumption. Energy Rep. 2020, 6, 312–318. [Google Scholar] [CrossRef]
- Archanaa, S.; Moise, S.; Suraishkumar, G.K. Chlorophyll Interference in Microalgal Lipid Quantification through the Bligh and Dyer Method. Biomass Bioenergy 2012, 46, 805–808. [Google Scholar] [CrossRef]
- Cai, T.; Ge, X.; Park, S.Y.; Li, Y. Comparison of Synechocystis Sp. PCC6803 and Nannochloropsis Salina for Lipid Production Using Artificial Seawater and Nutrients from Anaerobic Digestion Effluent. Bioresour. Technol. 2013, 144, 255–260. [Google Scholar] [CrossRef] [PubMed]
- de Farias Silva, C.E.; Bertucco, A. Bioethanol from Microalgae and Cyanobacteria: A Review and Technological Outlook. Process Biochem. 2016, 51, 1833–1842. [Google Scholar] [CrossRef]
- Neag, E.; Török, A.I.; Cadar, O.; Băbălău—Fuss, V.; Roman, C. Enhancing Lipid Production of Synechocystis PCC 6803 for Biofuels Production, through Environmental Stress Exposure. Renew. Energy 2019, 143, 243–251. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A Rapid Method of Total Lipid Extraction and Purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Ferraro, G.; Imbimbo, P.; Marseglia, A.; Illiano, A.; Fontanarosa, C.; Amoresano, A.; Olivieri, G.; Pollio, A.; Monti, D.M.; Merlino, A. A Thermophilic C-Phycocyanin with Unprecedented Biophysical and Biochemical Properties. Int. J. Biol. Macromol. 2020, 150, 38–51. [Google Scholar] [CrossRef]
- D’Elia, L.; Imbimbo, P.; Liberti, D.; Bolinesi, F.; Pollio, A.; Mangoni, O.; Brilman, W.; Olivieri, G.; Monti, D.M. Switchable Solvent Selective Extraction of Hydrophobic Antioxidants from Synechococcus Bigranulatus. ACS Sustain. Chem. Eng. 2021, 9, 13798–13806. [Google Scholar] [CrossRef]
- Turco, R.; Corrado, I.; Zannini, D.; Gargiulo, L.; Di Serio, M.; Pezzella, C.; Santagata, G. Upgrading Cardoon Biomass into Polyhydroxybutyrate Based Blends: A Holistic Approach for the Synthesis of Biopolymers and Additives. Bioresour. Technol. 2022, 363, 127954. [Google Scholar] [CrossRef]
- Kumar, J.; Singh, D.; Tyagi, M.B.; Kumar, A. Cyanobacteria: Applications in Biotechnology; Elsevier Inc.: Amsterdam, The Netherlands, 2018; Volume 7421, ISBN 9780128146682. [Google Scholar]
- Arguelles, E.D.L.R. Total Phenolic Content and in Vitro Analysis of Antioxidant, Antibacterial, and Alpha-Glucosidase Inhibition Properties of Chroococcus Minutus (Kützing) Nägeli (Chroococcales, Cyanobacteria). Ankara Univ. Eczac. Fak. Derg. 2022, 46, 170–181. [Google Scholar] [CrossRef]
- Tan, F.H.P.; Nadir, N.; Sudesh, K. Microalgal Biomass as Feedstock for Bacterial Production of PHA: Advances and Future Prospects. Front. Bioeng. Biotechnol. 2022, 10, 879476. [Google Scholar] [CrossRef]
- Utharn, S.; Yodsang, P.; Incharoensakdi, A.; Jantaro, S. Cyanobacterium Synechocystis Sp. PCC 6803 Lacking Adc1 Gene Produces Higher Polyhydroxybutyrate Accumulation under Modified Nutrients of Acetate Supplementation and Nitrogen-Phosphorus Starvation. Biotechnol. Rep. 2021, 31, e00661. [Google Scholar] [CrossRef]
- Andersen, R. Algal Culturing Techniques. J. Phycol. 2005, 41, 906–908. [Google Scholar] [CrossRef]
- Bennett, A.; Bogobad, L. Complementary Chromatic Adaptation in a Filamentous Blue-Green Alga. J. Cell Biol. 1973, 58, 419–435. [Google Scholar] [CrossRef]
- Aremu, A.O.; Masondo, N.A.; Molnár, Z.; Stirk, W.A.; Ördög, V.; Van Staden, J. Changes in Phytochemical Content and Pharmacological Activities of Three Chlorella Strains Grown in Different Nitrogen Conditions. J. Appl. Phycol. 2016, 28, 149–159. [Google Scholar] [CrossRef]
- Imbimbo, P.; Bueno, M.; D’Elia, L.; Pollio, A.; Ibañez, E.; Olivieri, G.; Monti, D.M. Green Compressed Fluid Technologies to Extract Antioxidants and Lipids from Galdieria Phlegrea in a Biorefinery Approach. ACS Sustain. Chem. Eng. 2020, 8, 2939–2947. [Google Scholar] [CrossRef]
- Gallucci, N.; Vitiello, G.; Di Girolamo, R.; Imbimbo, P.; Monti, D.M.; Tarallo, O.; Vergara, A.; Krauss, I.R.; Paduano, L. Towards the Development of Antioxidant Cerium Oxide Nanoparticles for Biomedical Applications: Controlling the Properties by Tuning Synthesis Conditions. Nanomaterials 2021, 11, 542. [Google Scholar] [CrossRef]
- Vastano, M.; Corrado, I.; Sannia, G.; Solaiman, D.K.Y.; Pezzella, C. Conversion of No/Low Value Waste Frying Oils into Biodiesel and Polyhydroxyalkanoates. Sci. Rep. 2019, 9, 13751. [Google Scholar] [CrossRef] [Green Version]
- GlobalNewswire. FiorMarkets Global Carotenoids Market Is Expected to Reach USD 3.59 Billion by 2025: Fior Markets. 2019. Available online: https://www.globenewswire.com/news-release/2019/10/15/1929461/0/en/Global-Carotenoids-Market-is-Expected-to-Reach-USD-3-59-Billion-by-2025-Fior-Markets.html (accessed on 15 October 2019).
- Manirafasha, E.; Murwanashyaka, T.; Ndikubwimana, T.; Yue, Q.; Zeng, X.; Lu, Y.; Jing, K. Ammonium Chloride: A Novel Effective and Inexpensive Salt Solution for Phycocyanin Extraction from Arthrospira (Spirulina) Platensis. J. Appl. Phycol. 2017, 29, 1261–1270. [Google Scholar] [CrossRef]
- Olaizola, M.; Grewe, C. Commercial Microalgal Cultivation Systems. In Grand Challenges in Algae Biotechnology; Springer: Cham, Switzerland, 2019; ISBN 9783030252328. [Google Scholar]
- Aljuraifani, A.A.; Berekaa, M.M.; Ghazwani, A.A. Bacterial Biopolymer (Polyhydroxyalkanoate) Production from Low-Cost Sustainable Sources. Microbiologyopen 2019, 8, e00755. [Google Scholar] [CrossRef] [PubMed]








| Retention Time (min) | Compound | Raw Biomass Extract (µg/gextract) | Residual Biomass Extract (µg/gextract) |
|---|---|---|---|
| 8.057 | Zeaxanthin | 17,551 | 10,441 |
| 18.651 | β-Carotene | 216 | 187 |
| 19.800 | β-Carotene isomer | 64 | 33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imbimbo, P.; D’Elia, L.; Corrado, I.; Alvarez-Rivera, G.; Marzocchella, A.; Ibáñez, E.; Pezzella, C.; Branco dos Santos, F.; Monti, D.M. An Alternative Exploitation of Synechocystis sp. PCC6803: A Cascade Approach for the Recovery of High Added-Value Products. Molecules 2023, 28, 3144. https://doi.org/10.3390/molecules28073144
Imbimbo P, D’Elia L, Corrado I, Alvarez-Rivera G, Marzocchella A, Ibáñez E, Pezzella C, Branco dos Santos F, Monti DM. An Alternative Exploitation of Synechocystis sp. PCC6803: A Cascade Approach for the Recovery of High Added-Value Products. Molecules. 2023; 28(7):3144. https://doi.org/10.3390/molecules28073144
Chicago/Turabian StyleImbimbo, Paola, Luigi D’Elia, Iolanda Corrado, Gerardo Alvarez-Rivera, Antonio Marzocchella, Elena Ibáñez, Cinzia Pezzella, Filipe Branco dos Santos, and Daria Maria Monti. 2023. "An Alternative Exploitation of Synechocystis sp. PCC6803: A Cascade Approach for the Recovery of High Added-Value Products" Molecules 28, no. 7: 3144. https://doi.org/10.3390/molecules28073144