Blue, Yellow, and Red Carbon Dots from Aromatic Precursors for Light-Emitting Diodes
Abstract
:1. Introduction
2. Results and Discussion
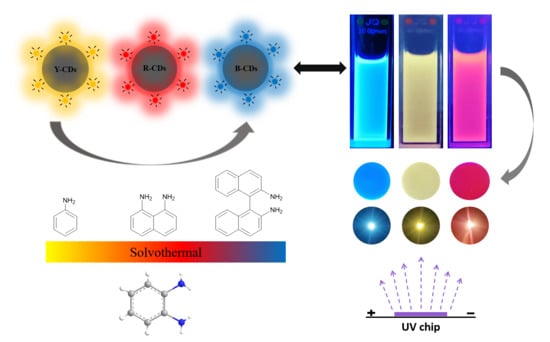
2.1. Preparation and Characterization of the Prepared CDs
2.2. Spectral Properties of the Prepared CDs
2.3. The Applications of MCDs
3. Experiments
3.1. Materials
3.2. Characterizations
3.3. Synthesis of CDs
3.4. The Determination of the Relative Fluorescence Quantum Yield and Fluorescence Lifetime
3.5. Preparation of CDs/PVA Films
3.6. The Fabrication of CDs/PVA Composites-Based LEDs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Hu, T.; Wen, Z.; Wang, C.; Thomas, T.; Wang, C.; Song, Q.; Yang, M. Temperature-controlled spectral tuning of full-color carbon dots and their strongly fluorescent solid-state polymer composites for light-emitting diodes. Nanoscale Adv. 2019, 1, 1413–1420. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Kang, Z.; Liu, Y.; Lee, S.-T. Carbon nanodots: Synthesis, properties and applications. J. Mater. Chem. 2012, 22. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, J.; Yang, Y.; Liu, X.; Qiu, J.; Tian, Y. Tunable full-color solid-state fluorescent carbon dots for light emitting diodes. Carbon 2022, 190, 22–31. [Google Scholar] [CrossRef]
- Chang, Q.; Zhou, X.; Xiang, G.; Jiang, S.; Li, L.; Wang, Y.; Li, Y.; Cao, Z.; Tang, X.; Ling, F.; et al. Full color fluorescent carbon quantum dots synthesized from triammonium citrate for cell imaging and white LEDs. Dyes Pigments 2021, 193, 109478. [Google Scholar] [CrossRef]
- Hola, K.; Zhang, Y.; Wang, Y.; Giannelis, E.P.; Zboril, R.; Rogach, A.L. Carbon dots—Emerging light emitters for bioimaging, cancer therapy and optoelectronics. Nano Today 2014, 9, 590–603. [Google Scholar] [CrossRef]
- Dong, H.; Wang, M.; Zhao, L.; Yan, M.; Zhang, H.; Qiu, S.; Shan, M.; Song, Y.; Dong, X.; Zhou, Y.; et al. Red-emitting carbon dots aggregates-based fluorescent probe for monitoring Cu(2). Mikrochim. Acta 2022, 190, 12. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wu, J.; Xing, Y.; Wang, Z. Solvent-dependent red emissive carbon dots and their applications in sensing and solid-state luminescence. Sens. Actuators B Chem. 2022, 360, 131645. [Google Scholar] [CrossRef]
- Barhum, H.; Alon, T.; Attrash, M.; Machnev, A.; Shishkin, I.; Ginzburg, P. Multicolor Phenylenediamine Carbon Dots for Metal-Ion Detection with Picomolar Sensitivity. ACS Appl. Nano Mater. 2021, 4, 9919–9931. [Google Scholar] [CrossRef]
- Jiang, K.; Sun, S.; Zhang, L.; Lu, Y.; Wu, A.; Cai, C.; Lin, H. Red, green, and blue luminescence by carbon dots: Full-color emission tuning and multicolor cellular imaging. Angew. Chem. Int. Ed. Engl. 2015, 54, 5360–5363. [Google Scholar] [CrossRef]
- Li, H.; He, X.; Kang, Z.; Huang, H.; Liu, Y.; Liu, J.; Lian, S.; Tsang, C.H.; Yang, X.; Lee, S.T. Water-soluble fluorescent carbon quantum dots and photocatalyst design. Angew. Chem. Int. Ed. Engl. 2010, 49, 4430–4434. [Google Scholar] [CrossRef]
- Li, J.; Gong, X. The Emerging Development of Multicolor Carbon Dots. Small 2022, 18, e2205099. [Google Scholar] [CrossRef]
- Yu, J.; Yong, X.; Tang, Z.; Yang, B.; Lu, S. Theoretical Understanding of Structure-Property Relationships in Luminescence of Carbon Dots. J. Phys. Chem. Lett. 2021, 12, 7671–7687. [Google Scholar] [CrossRef]
- Yuan, F.; Wang, Z.; Li, X.; Li, Y.; Tan, Z.; Fan, L.; Yang, S. Bright Multicolor Bandgap Fluorescent Carbon Quantum Dots for Electroluminescent Light-Emitting Diodes. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef]
- Bao, L.; Liu, C.; Zhang, Z.L.; Pang, D.W. Photoluminescence-tunable carbon nanodots: Surface-state energy-gap tuning. Adv. Mater. 2015, 27, 1663–1667. [Google Scholar] [CrossRef]
- Zhan, J.; Geng, B.; Wu, K.; Xu, G.; Wang, L.; Guo, R.; Lei, B.; Zheng, F.; Pan, D.; Wu, M. A solvent-engineered molecule fusion strategy for rational synthesis of carbon quantum dots with multicolor bandgap fluorescence. Carbon 2018, 130, 153–163. [Google Scholar] [CrossRef]
- Tian, Z.; Zhang, X.; Li, D.; Zhou, D.; Jing, P.; Shen, D.; Qu, S.; Zboril, R.; Rogach, A.L. Full-Color Inorganic Carbon Dot Phosphors for White-Light-Emitting Diodes. Adv. Opt. Mater. 2017, 5. [Google Scholar] [CrossRef]
- Dai, R.; Chen, X.; Ouyang, N.; Hu, Y. A pH-controlled synthetic route to violet, green, and orange fluorescent carbon dots for multicolor light-emitting diodes. Chem. Eng. J. 2022, 431, 134172. [Google Scholar] [CrossRef]
- Chen, J.; Luo, J.B.; Hu, M.Y.; Zhou, J.; Huang, C.Z.; Liu, H. Controlled Synthesis of Multicolor Carbon Dots Assisted by Machine Learning. Adv. Funct. Mater. 2022, 33, 2210095. [Google Scholar] [CrossRef]
- Lei, X.; Li, D.; Chen, Y.; Liu, Q.; Yan, Q.; Wang, J.; Han, B.; He, G.; An, B. RGB-multicolor fluorescent carbon dots by changing the reaction solvent type for white light-emitting diodes. New J. Chem. 2022, 46, 4979–4982. [Google Scholar] [CrossRef]
- Huo, F.; Liang, W.; Tang, Y.; Zhang, W.; Liu, X.; Pei, D.; Wang, H.; Jia, W.; Jia, P.; Yang, F. Full-color carbon dots with multiple red-emission tuning: On/off sensors, in vitro and in vivo multicolor bioimaging. J. Mater. Sci. 2019, 54, 6815–6825. [Google Scholar] [CrossRef]
- Li, P.; Sun, L.; Xue, S.; Qu, D.; An, L.; Wang, X.; Sun, Z. Recent advances of carbon dots as new antimicrobial agents. SmartMat 2022, 3, 226–248. [Google Scholar] [CrossRef]
- Chen, Y.; Zheng, M.; Xiao, Y.; Dong, H.; Zhang, H.; Zhuang, J.; Hu, H.; Lei, B.; Liu, Y. A Self-Quenching-Resistant Carbon-Dot Powder with Tunable Solid-State Fluorescence and Construction of Dual-Fluorescence Morphologies for White Light-Emission. Adv. Mater. 2016, 28, 312–318. [Google Scholar] [CrossRef]
- Bhunia, S.K.; Nandi, S.; Manna, J.; Shikler, R.; Jelinek, R. Tuneable light-emitting carbon-dot/polymer flexible films prepared through one-pot synthesis. Nanoscale 2016, 8, 3400–3406. [Google Scholar] [CrossRef]
- Lin, S.; Lin, C.; He, M.; Yuan, R.; Zhang, Y.; Zhou, Y.; Xiang, W.; Liang, X. Solvatochromism of bright carbon dots with tunable long-wavelength emission from green to red and their application as solid-state materials for warm WLEDs. RSC Adv. 2017, 7, 41552–41560. [Google Scholar] [CrossRef] [Green Version]
- An, Y.; Lin, X.; Guo, Z.; Yin, Q.; Li, Y.; Zheng, Y.; Shi, Z.; Zhang, W.; Liu, C. Red Emission Carbon Dots Prepared by 1,4-Diaminonaphthalene for Light-Emitting Diode Application and Metal Ion Detection. Materials 2021, 14, 4716. [Google Scholar] [CrossRef]
- Han, Q.; Xu, W.; Ji, C.; Xiong, G.; Shi, C.; Zhang, D.; Shi, W.; Jiang, Y.; Peng, Z. Multicolor and Single-Component White Light-Emitting Carbon Dots from a Single Precursor for Light-Emitting Diodes. ACS Appl. Nano Mater. 2022, 5, 15914–15924. [Google Scholar] [CrossRef]
- Ding, H.; Yu, S.B.; Wei, J.S.; Xiong, H.M. Full-Color Light-Emitting Carbon Dots with a Surface-State-Controlled Luminescence Mechanism. ACS Nano 2016, 10, 484–491. [Google Scholar] [CrossRef]
- Da, X.; Han, Z.; Yang, Z.; Zhang, D.; Hong, R.; Tao, C.; Lin, H.; Huang, Y. Preparation of multicolor carbon dots with high fluorescence quantum yield and application in white LED. Chem. Phys. Lett. 2022, 794. [Google Scholar] [CrossRef]
- Wang, Z.; Yuan, F.; Li, X.; Li, Y.; Zhong, H.; Fan, L.; Yang, S. 53% Efficient Red Emissive Carbon Quantum Dots for High Color Rendering and Stable Warm White-Light-Emitting Diodes. Adv. Mater. 2017, 29, 466. [Google Scholar] [CrossRef]
- Miao, X.; Qu, D.; Yang, D.; Nie, B.; Zhao, Y.; Fan, H.; Sun, Z. Synthesis of Carbon Dots with Multiple Color Emission by Controlled Graphitization and Surface Functionalization. Adv. Mater. 2018, 30, 1704548. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Lu, X.; Liu, M.; Wang, W. Blue, Yellow, and Red Carbon Dots from Aromatic Precursors for Light-Emitting Diodes. Molecules 2023, 28, 2957. https://doi.org/10.3390/molecules28072957
Liu Z, Lu X, Liu M, Wang W. Blue, Yellow, and Red Carbon Dots from Aromatic Precursors for Light-Emitting Diodes. Molecules. 2023; 28(7):2957. https://doi.org/10.3390/molecules28072957
Chicago/Turabian StyleLiu, Zhenzhen, Xiaofei Lu, Menglin Liu, and Wenjing Wang. 2023. "Blue, Yellow, and Red Carbon Dots from Aromatic Precursors for Light-Emitting Diodes" Molecules 28, no. 7: 2957. https://doi.org/10.3390/molecules28072957