Echinophora tenuifolia subsp. sibthorpiana—Study of the Histochemical Localization of Essential Oil
Abstract
:1. Introduction
2. Results and Discussion
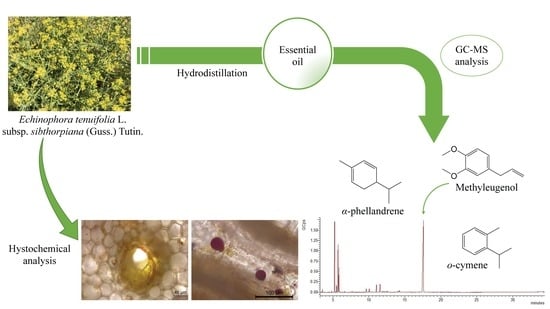
2.1. Microscopic Histochemical Analysis
2.2. Volatile Constituents of the E. tenuifolia ssp. sibthorpiana Essential Oil
3. Materials and Methods
3.1. Plant Material
3.2. Chemicals and Reagents
3.3. Microscopic Histochemical Analysis
3.4. Isolation of the Essential Oil
3.5. Chromatographic Conditions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Georgiou, C.; Koutsaviti, A.; Bazos, I.; Tzakou, O. Chemical Composition of Echinophora tenuifolia subsp. sibthorpiana Essential Oil from Greece. Rec. Nat. Prod. 2010, 4, 167. [Google Scholar]
- Stoyanov, K.; Raycheva, T.; Cheschmedzhiev, I. Key to the Native and Foreign Vascular Plants in Bulgaria; Agricultural University Plovdiv Academic Press: Plovdiv, Bulgaria, 2021. [Google Scholar]
- Flora Republicae Popularis Bulgaricae. In Serdicae; Jordanov, D. (Ed.) Aedibus Academiae Scientiarum Bulgaricae: Sofia, Bulgaria, 1982; Volume 8. [Google Scholar]
- Deghirmencioghlu, N.; Göçmen, D.; Daghdelen, A.; Daghdelen, F. Influence of Tarhana Herb (Echinophora sibthorpiana) on Fermentation of Tarhana, Turkish Traditional Fermented Food. Food Technol. Biotechnol. 2005, 43, 175–179. [Google Scholar]
- Georgala, A. The Nutritional Value of Two Fermented Milk/Cereal Foods Named ‘Greek Trahanas and Turkish Tarhana: A Review. J. Nutr. Disord. Ther. 2013, 3, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Ozdemir, S.; Gocmen, D.; Yildirim Kumral, A. A Traditional Turkish Fermented Cereal Food: Tarhana. Food Rev. Int. 2007, 23, 107–121. [Google Scholar] [CrossRef]
- Şanli, A.; Karadoğan, T.; Tosun, B.; Tonguç, M.; Erbaş, S. Growth Stage and Drying Methods Affect Essential Oil Content and Composition of Pickling Herb (Echinophora tenuifolia subsp. sibthorpiana Tutin). SDÜ Fen Bilim. Enstitüsü Dergisi. 2016, 20, 43–49. [Google Scholar] [CrossRef]
- Spano, M.; Di Matteo, G.; Ingallina, C.; Ambroselli, D.; Carradori, S.; Gallorini, M.; Giusti, A.M.; Salvo, A.; Grosso, M.; Mannina, L. Modulatory Properties of Food and Nutraceutical Components Targeting NLRP3 Inflammasome Activation. Nutrients 2022, 14, 490. [Google Scholar] [CrossRef]
- Hossain, M.B.; Ahmed, L.; Martin-Diana, A.B.; Brunton, N.P.; Barry-Ryan, C. Individual and Combined Antioxidant Activity of Spices and Spice Phenolics. Antioxidants 2023, 12, 308. [Google Scholar] [CrossRef]
- Avijgan, M.; Hafizi, M.; Saadat, M.; Nilforoushzadeh, M. Antifungal Effect of Echinophora Platyloba’s Extract against Candida Albicans. Iran. J. Pharm. Res. 2006, 5, 285–289. [Google Scholar] [CrossRef]
- Avijgan, M.; Mirzadeh, F.; Nia, E. The Comparative Study of Anti-Fungal Effect of Pharmaceutical Products Containing Hydroalcoholic Extract of Echinophora Platyloba DC and Flucona- Zole in Women with Chronic Recurrent Vaginitis Caused by Candida Albicans. Res. J. Med. Sci. 2012, 17, S103–S107. [Google Scholar]
- Delaram, M.; Kheiri, S.; Hodjati, M.R. Comparing the Effects of Echinophora-platyloba, Fennel and Placebo on Pre-Menstrual Syndrome. J. Reprod. Infertil. 2011, 12, 221–226. [Google Scholar] [PubMed]
- Heidarian, E.; Saffari, J.; Jafari-Dehkordi, E. Hepatoprotective Action of Echinophora Platyloba DC Leaves Against Acute Toxicity of Acetaminophen in Rats. J. Diet. Suppl. 2014, 11, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Shahneh, F.Z.; Baradaran, B.; Majidi, J.; Babaloo, Z. Echinophora Platyloba DC (Apiaceae) Crude Extract Induces Apoptosis in Human Prostate Adenocarcinoma Cells (PC 3). Biomed. J. 2014, 37, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Shahneh, F.Z.; Valiyari, S.; Azadmehr, A.; Hajiaghaee, R.; Yaripour, S.; Bandehagh, A.; Baradaran, B. Inhibition of Growth and Induction of Apoptosis in Fibrosarcoma Cell Lines by Echinophora Platyloba DC: In Vitro Analysis. Adv. Pharmacol. Sci. 2013, 2013, 512931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ezatpour, B.; Bahmani, M.; Azami, M.; Kheirandish, F.; Rafieian-Kopaei, M. The in Vitro Effects of Echinophora Cinerea on Cell Line, Giardia Lamblia Cyst, and Giardia Muris. Herb. Med. J. 2018, 3, 70–76. [Google Scholar] [CrossRef]
- Shokoohinia, Y.; Khajouei, S.; Ahmadi, F.; Ghiasvand, N.; Hosseinzadeh, L. Protective Effect of Bioactive Compounds from Echinophora Cinerea against Cisplatin-Induced Oxidative Stress and Apoptosis in the PC12 Cell Line. Iran. J. Basic Med. Sci. 2017, 20, 438–445. [Google Scholar] [CrossRef]
- Fraternale, D.; Genovese, S.; Ricci, D. Essential Oil Composition and Antimicrobial Activity of Aerial Parts and Ripe Fruits of Echinophora Spinosa (Apiaceae) from Italy. Nat. Prod. Commun. 2013, 8, 527–530. [Google Scholar] [CrossRef] [Green Version]
- Kaska, A.; Mammadov, R. Antioxidant Properties, Proximate Content and Cytotoxic Activity of Echinophora Tournefortii Jaub. & Spach. Food Sci. Technol. 2019, 39, 875–880. [Google Scholar] [CrossRef] [Green Version]
- Marrelli, M.; Statti, G.A.; Menichini, F.; Conforti, F. Echinophora Tenuifolia L. Inflorescences: Phytochemistry and in Vitro Antioxidant and Anti-Inflammatory Properties in LPS-Stimulated RAW 264.7 Macrophages. Plant Biosyst.—Int. J. Deal. All Asp. Plant Biol. 2017, 151, 1073–1081. [Google Scholar] [CrossRef]
- Marrelli, M.; Pisani, F.; Amodeo, V.; Duez, P.; Conforti, F. Echinophora Tenuifolia L. Branches Phytochemical Profile and Antiproliferative Activity on Human Cancer Cell Lines. Nat. Prod. Res. 2020, 34, 2664–2667. [Google Scholar] [CrossRef]
- Gokbulut, I.; Bilenler, T.; Karabulut, I. Determination of Chemical Composition, Total Phenolic, Antimicrobial, and Antioxidant Activities of Echinophora Tenuifolia Essential Oil. Int. J. Food Prop. 2013, 16, 1442–1451. [Google Scholar] [CrossRef]
- Najmi, Z.; Scalia, A.C.; De Giglio, E.; Cometa, S.; Cochis, A.; Colasanto, A.; Locatelli, M.; Coisson, J.D.; Iriti, M.; Vallone, L.; et al. Screening of Different Essential Oils Based on Their Physicochemical and Microbiological Properties to Preserve Red Fruits and Improve Their Shelf Life. Foods 2023, 12, 332. [Google Scholar] [CrossRef]
- Özcan, M.; Akgül, A. Essential Oil Composition of Turkish Pickling Herb (Echinophora Tenuifolia L. Subsp. sibthorpiana (Guss.) Tutin). Acta Bot. Hung. 2003, 45, 163–167. [Google Scholar] [CrossRef]
- Telci, I.; Hisil, Y. Essential Oil Composition of the Spice Plant Echinophora Tenuifolia L. Subsp. sibthorpiana Tutin from Turkey. Chem. Nat. Compd. 2008, 44, 534–536. [Google Scholar] [CrossRef]
- Mileski, K.; Dzamic, A.; Ciric, A.; Grujic, S.; Ristic, M.; Matevski, V.; Marin, P.D. Radical Scavenging and Antimicrobial Activity of Essential Oil and Extracts of Echinophora sibthorpiana Guss. from Macedonia. Arch. Biol. Sci. 2014, 66, 401–413. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, V.U.; Jassbi, A.R.; Pannahi, M.S.C. Analysis of the Essential Oil of Echinophora sibthorpiana Guss. by Means of GC, GC/MS and 13C-NMR Techniques. J. Essent. Oil Res. 1999, 11, 107–108. [Google Scholar] [CrossRef]
- Akgül, A.; Chialva, F. Constituents of the Essential Oil of Echinophora Tenuifolia L. Subsp. sibthorpiana (Guss.) Tutin from Turkey. Flavour Fragr. J. 1989, 4, 67–68. [Google Scholar] [CrossRef]
- Chalchat, J.C.; Ozcan, M.M.; Dagdelen, A.; Akgul, A. Variability of Essential Oil Composition of Echinophora Tenuifolia Subsp. sibthorpiana Tutin by Harvest Location and Year and Oil Storage. Chem. Nat. Compd. 2007, 43, 225–227. [Google Scholar] [CrossRef]
- Chalchat, J.; Özcan, M.; Figueredo, G.; Chalard, P. The Effect of Harvest Years on Chemical Composition of Essential Oil of Pickling Herb (Echinophora Tenuifolia Subsp. sibthorpiana) Leaves Used as Medicinal Plant. Acta Bot. Hung. 2011, 53, 73–77. [Google Scholar] [CrossRef]
- Sefidkon, F. Extraction and Identification of Volatile Components of Echinophora sibthorpiana Guss. Iran. J. Med. Aromat. Plants Res. 2004, 20, 149–158. [Google Scholar]
- Baser, K.H.C.; Erdemgil, F.Z.; Özek, T. Essential Oil of Echinophora Tenuifolia L. Subsp. sibthorpiana (Guss.) Tutin. J. Essent. Oil Res. 1994, 6, 399–400. [Google Scholar] [CrossRef]
- Sanli, A.; Ok, F.Z. Chemical Composition and Antimicrobial Activity against Phytopathogenic Fungi of Essential Oils Obtained from Echinophora tenuifolia subsp. sibthorpiana Grown in Wild and Cultivated Conditions in Turkey. Molecules 2023, 28, 585. [Google Scholar] [CrossRef]
- Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Scheffer, J.J.C. Factors Affecting Secondary Metabolite Production in Plants: Volatile Components and Essential Oils. Flavour Fragr. J. 2008, 23, 213–226. [Google Scholar] [CrossRef]
- Glamoclija, J.M.; Sokovic, M.D.; Siljegovic, J.D.; Ristic, M.S.; Ciric, A.; Grubisic, D.V. Chemical Composition and Anti-Microbial Activity of Echinophora Spinosa L. (Apiaceae) Essential Oil. Rec. Nat. Prod. 2011, 5, 319. [Google Scholar]
- Pavela, R.; Maggi, F.; Cianfaglione, K.; Canale, A.; Benelli, G. Promising Insecticidal Efficacy of the Essential Oils from the Halophyte Echinophora Spinosa (Apiaceae) Growing in Corsica Island, France. Environ. Sci. Pollut. Res. 2020, 27, 14454–14464. [Google Scholar] [CrossRef]
- Sodeifian, G.; Sajadian, S.A. Investigation of Essential Oil Extraction and Antioxidant Activity of Echinophora Platyloba DC. Using Supercritical Carbon Dioxide. J. Supercrit. Fluids 2017, 121, 52–62. [Google Scholar] [CrossRef]
- Moghaddam, M.; Taheri, P.; Pirbalouti, A.G.; Mehdizadeh, L. Chemical Composition and Antifungal Activity of Essential Oil from the Seed of Echinophora Platyloba DC. against Phytopathogens Fungi by Two Different Screening Methods. LWT Food Sci. Technol. 2015, 61, 536–542. [Google Scholar] [CrossRef]
- Asghari, G.; Abedi, D.; Jalali, M.; Farsi, S. Antimicrobial Activities and Phytochemical Composition of Echinophora Platyloba DC. Essential Oils from Isfahan. J. Essent. Oil Bear. Plants 2007, 10, 76–82. [Google Scholar] [CrossRef]
- Baser, K.H.C.; Biçakçi, A.; Malyer, H. Composition of the Essential Oil of Echinophora Lamondiana B.Yildiz et Z.Bahçecioglu. J. Essent. Oil Res. 2000, 12, 147–148. [Google Scholar] [CrossRef]
- Ali, A.; Tabanca, N.; Ozek, G.; Ozek, T.; Aytac, Z.; Bernier, U.R.; Agramonte, N.M.; Baser, K.H.C.; Khan, I.A. Essential Oils of Echinophora Lamondiana (Apiales: Umbelliferae): A Relationship Between Chemical Profile and Biting Deterrence and Larvicidal Activity Against Mosquitoes (Diptera: Culicidae). J. Med. Entomol. 2015, 52, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Baser, K.H.C.; Kürkcüoglu, M.; Malyer, H.; Bicakci, A. Essential Oils of Six Echinophora Species from Turkey. J. Essent. Oil Res. 1998, 10, 345–351. [Google Scholar] [CrossRef]
- Humans, I.W.G. On the E. of C.R. to Methyleugenol; International Agency for Research on Cancer: Lyon, France, 2013. [Google Scholar]
- Public Statement on the Use of Herbal Medicinal Products Containing Methyleugenol; European Medicines Agency: Amsterdam, The Netherlands, 2005; Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/public-statement-use-herbal-medicinal-products-containing-methyleugenol_en.pdf (accessed on 25 February 2023).
- Tang, F.; Chen, F.; Ling, X.; Huang, Y.; Zheng, X.; Tang, Q.; Tan, X. Inhibitory Effect of Methyleugenol on IgE-Mediated Allergic Inflammation in RBL-2H3 Cells. Mediat. Inflamm. 2015, 2015, 463530. [Google Scholar] [CrossRef] [Green Version]
- Shin, B.-K.; Lee, E.-H.; Kim, H.-M. Suppression Ofl-Histidine Decarboxylase MRNA Expression by Methyleugenol. Biochem. Biophys. Res. Commun. 1997, 232, 188–191. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.K.; Cho, G.-S.; Hwang, S.; Kim, B.W.; Lim, J.H.; Lee, J.-C.; Kim, H.C.; Kim, W.-K.; Kim, Y.S. Methyleugenol Reduces Cerebral Ischemic Injury by Suppression of Oxidative Injury and Inflammation. Free Radic. Res. 2010, 44, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Norte, M.C.B.; Cosentino, R.M.; Lazarini, C.A. Effects of Methyl-Eugenol Administration on Behavioral Models Related to Depression and Anxiety, in Rats. Phytomedicine 2005, 12, 294–298. [Google Scholar] [CrossRef]
- Liu, Y.-M.; Fan, H.-R.; Deng, S.; Zhu, T.; Yan, Y.; Ge, W.-H.; Li, W.-G.; Li, F. Methyleugenol Potentiates Central Amygdala GABAergic Inhibition and Reduces Anxiety. J. Pharmacol. Exp. Ther. 2019, 368, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, T.; Yan, Y.; Deng, S.; Liu, Y.-M.; Fan, H.-R.; Ma, B.; Meng, B.; Mei, B.; Li, W.-G.; Li, F. Methyleugenol Counteracts Anorexigenic Signals in Association with GABAergic Inhibition in the Central Amygdala. Neuropharmacology 2018, 141, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Huang, C.; Peng, Z.; Xie, Y.; Deng, S.; Nie, Y.-Z.; Xu, T.-L.; Ge, W.-H.; Li, W.-G.; Li, F. Electrophysiological Characterization of Methyleugenol: A Novel Agonist of GABA(A) Receptors. ACS Chem. Neurosci. 2014, 5, 803–811. [Google Scholar] [CrossRef]
- Lima, C.C.; Criddle, D.N.; Coelho-de-Souza, A.N.; Monte, F.J.Q.; Jaffar, M.; Leal-Cardoso, J.H. Relaxant and Antispasmodic Actions of Methyleugenol on Guinea-Pig Isolated Ileum. Planta Med. 2000, 66, 408–411. [Google Scholar] [CrossRef]
- Lahlou, S.; Figueiredo, A.F.; Magalhães, P.J.C.; Leal-Cardoso, J.H.; Gloria, P.D. Cardiovascular Effects of Methyleugenol, a Natural Constituent of Many Plant Essential Oils, in Normotensive Rats. Life Sci. 2004, 74, 2401–2412. [Google Scholar] [CrossRef]
- Xie, Y.; Huang, Q.; Wang, Z.; Cao, H.; Zhang, D. Structure-Activity Relationships of Cinnamaldehyde and Eugenol Derivatives against Plant Pathogenic Fungi. Ind. Crops Prod. 2017, 97, 388–394. [Google Scholar] [CrossRef]
- Ahmad, A.; Khan, A.; Khan, L.A.; Manzoor, N. In Vitro Synergy of Eugenol and Methyleugenol with Fluconazole against Clinical Candida Isolates. J. Med. Microbiol. 2010, 59, 1178–1184. [Google Scholar] [CrossRef]
- Götz, M.E.; Sachse, B.; Schäfer, B.; Eisenreich, A. Myristicin and Elemicin: Potentially Toxic Alkenylbenzenes in Food. Foods 2022, 11, 1988. [Google Scholar] [CrossRef]
- Quan, N.V.; Dang Xuan, T.; Teschke, R. Potential Hepatotoxins Found in Herbal Medicinal Products: A Systematic Review. Int. J. Mol. Sci. 2020, 21, 5011. [Google Scholar] [CrossRef] [PubMed]
- Eisenreich, A.; Götz, M.E.; Sachse, B.; Monien, B.H.; Herrmann, K.; Schäfer, B. Alkenylbenzenes in Foods: Aspects Impeding the Evaluation of Adverse Health Effects. Foods 2021, 10, 2139. [Google Scholar] [CrossRef] [PubMed]
- Smith, F. Therapeutics within a Naturopathic Approach. In Naturopathic Medicine; Springer International Publishing: Cham, Switzerland, 2022; pp. 129–202. ISBN 978-3-031-13387-9. [Google Scholar]
- Radice, M.; Durofil, A.; Buzzi, R.; Baldini, E.; Martínez, A.P.; Scalvenzi, L.; Manfredini, S. Alpha-Phellandrene and Alpha-Phellandrene-Rich Essential Oils: A Systematic Review of Biological Activities, Pharmaceutical and Food Applications. Life 2022, 12, 1602. [Google Scholar] [CrossRef] [PubMed]
- Thangaleela, S.; Sivamaruthi, B.S.; Kesika, P.; Tiyajamorn, T.; Bharathi, M.; Chaiyasut, C. A Narrative Review on the Bioactivity and Health Benefits of Alpha-Phellandrene. Sci. Pharm. 2022, 90, 57. [Google Scholar] [CrossRef]
- Evergetis, E.; Michaelakis, A.; Haroutounian, S.A. Exploitation of Apiaceae Family Essential Oils as Potent Biopesticides and Rich Source of Phellandrenes. Ind. Crops Prod. 2013, 41, 365–370. [Google Scholar] [CrossRef]
- Dalli, M.; Azizi, S.; Benouda, H.; Azghar, A.; Tahri, M.; Bouammali, B.; Maleb, A.; Gseyra, N. Molecular Composition and Antibacterial Effect of Five Essential Oils Extracted from Nigella Sativa L. Seeds against Multidrug-Resistant Bacteria: A Comparative Study. Evid.-Based Complement. Altern. Med. 2021, 2021, 6643765. [Google Scholar] [CrossRef]
- Salas-Oropeza, J.; Jimenez-Estrada, M.; Perez-Torres, A.; Castell-Rodriguez, A.E.; Becerril-Millan, R.; Rodriguez-Monroy, M.A.; Jarquin-Yañez, K.; Canales-Martinez, M.M. Wound Healing Activity of α-Pinene and α-Phellandrene. Molecules 2021, 26, 2488. [Google Scholar] [CrossRef]
- de Christo Scherer, M.M.; Marques, F.M.; Figueira, M.M.; Peisino, M.C.O.; Schmitt, E.F.P.; Kondratyuk, T.P.; Endringer, D.C.; Scherer, R.; Fronza, M. Wound Healing Activity of Terpinolene and α-Phellandrene by Attenuating Inflammation and Oxidative Stress in Vitro. J. Tissue Viability 2019, 28, 94–99. [Google Scholar] [CrossRef]
- Chaaban, A.; Richardi, V.S.; Carrer, A.R.; Brum, J.S.; Cipriano, R.R.; Martins, C.E.N.; Silva, M.A.N.; Deschamps, C.; Molento, M.B. Insecticide Activity of Curcuma Longa (Leaves) Essential Oil and Its Major Compound α-Phellandrene against Lucilia Cuprina Larvae (Diptera: Calliphoridae): Histological and Ultrastructural Biomarkers Assessment. Pestic. Biochem. Physiol. 2019, 153, 17–27. [Google Scholar] [CrossRef]
- Kang, W.; Park, S.; Choi, D.; Son, B.; Park, T. Activation of CAMP Signaling in Response to α-Phellandrene Promotes Vascular Endothelial Growth Factor Levels and Proliferation in Human Dermal Papilla Cells. Int. J. Mol. Sci. 2022, 23, 8959. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Lin, C.L.; Huang, C.Y.; Hsieh, S.; Liu, C.H.; Hsieh, S.L. α-Phellandrene Enhances the Immune Response and Resistance against Vibrio Alginolyticus in White Shrimp (Litopenaeus Vannamei). Fish Shellfish Immunol. 2019, 84, 1108–1114. [Google Scholar] [CrossRef] [PubMed]
- Hayouni, E.A.; Chraief, I.; Abedrabba, M.; Bouix, M.; Leveau, J.-Y.; Mohammed, H.; Hamdi, M. Tunisian Salvia Officinalis L. and Schinus Molle L. Essential Oils: Their Chemical Compositions and Their Preservative Effects against Salmonella Inoculated in Minced Beef Meat. Int. J. Food Microbiol. 2008, 125, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Ahluwalia, V.; Singh, P.; Kumar, N.; Prakash Sati, O.; Sati, N. Antifungal and Phytotoxic Activity of Essential Oil from Root of Senecio Amplexicaulis Kunth. (Asteraceae) Growing Wild in High Altitude-Himalayan Region. Nat. Prod. Res. 2016, 30, 1875–1879. [Google Scholar] [CrossRef] [PubMed]
- Magwa, M.L.; Gundidza, M.; Gweru, N.; Humphrey, G. Chemical Composition and Biological Activities of Essential Oil from the Leaves of Sesuvium portulacastrum. J. Ethnopharmacol. 2006, 103, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Al-Burtamani, S.K.S.; Fatope, M.O.; Marwah, R.G.; Onifade, A.K.; Al-Saidi, S.H. Chemical Composition, Antibacterial and Antifungal Activities of the Essential Oil of Haplophyllum tuberculatum from Oman. J. Ethnopharmacol. 2005, 96, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Tchoumbougnang, F.; Zollo, P.H.; Dagne, E.; Mekonnen, Y. In Vivo Antimalarial Activity of Essential Oils from Cymbopogon citratus and Ocimum gratissimum on Mice Infected with Plasmodium berghei. Planta Med. 2005, 71, 20–23. [Google Scholar] [CrossRef]
- Bonesi, M.; Menichini, F.; Tundis, R.; Loizzo, M.R.; Conforti, F.; Passalacqua, N.G.; Statti, G.A.; Menichini, F. Acetylcholinesterase and Butyrylcholinesterase Inhibitory Activity of Pinus Species Essential Oils and Their Constituents. J. Enzym. Inhib. Med. Chem. 2010, 25, 622–628. [Google Scholar] [CrossRef] [Green Version]
- Caballero-Gallardo, K.; Fuentes-Lopez, K.; Stashenko, E.E.; Olivero-Verbel, J. Chemical Composition, Repellent Action, and Toxicity of Essential Oils from Lippia Origanoide, Lippia. alba Chemotypes, and Pogostemon cablin on Adults of Ulomoides dermestoides (Coleoptera: Tenebrionidae). Insects 2022, 14, 41. [Google Scholar] [CrossRef]
- Alcala-Orozco, M.; Caballero-Gallardo, K.; Stashenko, E.E.; Olivero-Verbel, J. Repellent and Fumigant Actions of the Essential Oils from Elettaria Cardamomum (L.) Maton, Salvia Officinalis (L.) Linnaeus, and Lippia Origanoides (V.) Kunth Against Tribolium Castaneum and Ulomoides dermestoides. J. Essent. Oil Bear. Plants 2019, 22, 18–30. [Google Scholar] [CrossRef]
- Pellicciari, C.; Biggiogera, M. (Eds.) Histochemistry of Single Molecules: Methods and Protocols; Springer New York: New York, NY, USA, 2017; Volume 1560. [Google Scholar]




| Study Objectives | Study Design | Main Results | Ref. |
|---|---|---|---|
| Echinophora platyloba (E. platyloba) (ethanolic extract) | In vitro testing for anti-Candida albicans activity | E. platyloba extract, in a concentration ≥ 2 mg/mL, effectively inhibited Candida albicans growth. | [10] |
| Echinophora platyloba | Double-blind randomized clinical trial. The study included 60 women diagnosed with candida albicans vaginitis. The authors randomized patients into two groups: an Echinophora cream plus fluconazole group and a fluconazole group. | The cream containing E. platyloba extract was effective at reducing candida vaginitis in women with the disease. | [11] |
| Echinophora platyloba (extract) | Single-blind randomized clinical trial. The study included 90 women diagnosed with moderate-to-severe premenstrual syndrome (PMS). The authors randomized participants into three groups: an Echinophora-platyloba extract group, a fennel extract group, and a placebo group. | The E. platyloba and fennel extracts reduced the severity of PMS. | [12] |
| Echinophora platyloba (ethanolic extract) | In vivo evaluation of hepatoprotective activity against acetaminophen-induced hepatotoxicity in rats. | E. platyloba extract pretreatment showed potent hepatoprotective effects. | [13] |
| Echinophora platyloba (methanolic extract) | In vitro evaluation of cytotoxicity and the mechanism of cell death against the human prostate adenocarcinoma PC 3 and human umbilical vein endothelial cells (HUVEC) cell line. | E. platyloba extract inhibited the human prostate adenocarcinoma cell proliferation possibly via apoptosis and might be valuable for human prostate adenocarcinoma treatment. | [14] |
| Echinophora platyloba (methanolic extract) | In vitro examination of the cytotoxic activity and mechanism of the cell death of E.platyloba methanolic extracts on mouse fibrosarcoma cells (WEHI-164). | The extract time- and dose-dependently inhibited the proliferation of fibrosarcoma cells possibly via an apoptosis-dependent pathway. | [15] |
| Echinophora cinerea (E. cinerea) (extract) | In vitro investigation of antiprotozoal activity of E. cinerea against Giardia lamblia and Giardia muris, as well as the cytotoxicity of this plant extract. | E. cinerea showed considerable antigiardial activity against G. lamblia and exerted low cytotoxicity on the studied cell lines. | [16] |
| Echinophora cinerea | In vitro assessment of the protective effect of six compounds isolated from E. cinerea (quercetrin-3-O-β-d-glucopyranoside, osthol, verbenone-5-O-β-d-glycopyranoside, Isoimperatorin, kaempferol-3-O-β-d-glucopyranoside, and echinophorin B) against oxidative stress and apoptosis induced by cisplatin in PC12 cells. | Quercetrin-3-O-β-d-glucopyranoside and osthol showed apoptosis inhibition and effectively blocked the cisplatin-induced neurotoxicity. | [17] |
| Echinophora spinosa L. (E. spinosa) aerial parts and ripe fruits (essential oil) | In vitro determination of the antimicrobial activity of the two essential oils against some bacteria responsible for intestinal dysbiosis. | The E. spinosa essential oils showed selective antibacterial activity against potentially pathogenic intestinal bacteria such as Clostridium difficile, Clostridium perfringens, Enterococcus faecalis, Eubacterium limosum, Peptostreptococcus anaerobius, and the fungus Candida albicans, and they were less active against bifidobacteria and lactobacilli. | [18] |
| Echinophora tournefortii Jaub. and Spach (E. tournefortii) (methanol, acetone, and water extracts) | In vitro examination of the antioxidant capacities of the extracts using six complementary methods and cytotoxic activity. | Generally, all the extracts showed strong antioxidant activity and exhibited cytotoxic activities. | [19] |
| Echinophora tenuifolia L. (E. tenifolia) inflorescence (methanolic extract) | In vitro investigation of antioxidant and anti-inflammatory activities. | The n-hexane fraction and the dichloromethane fraction of the extract showed considerable anti-inflammatory activity | [20] |
| Echinophora tenuifolia L. branches (methanolic extract) | In vitro investigation of the cell growth inhibitory activity on different human cancer cell lines and normal BJ fibroblasts | All the samples showed effectivity against the melanoma cell line C32, with IC50 values from 22.8 ± 0.8 to 78.7 ± 1.2 µg/mL, with the dichloromethane fraction activity being the highest. | [21] |
| Echinophora tenuifolia L. subsp. sibthorpiana (essential oil) | In vitro investigation of the antioxidant and antimicrobial activities | The essential oil showed antioxidant as well as antimicrobial effects, especially against Bacillus cereus and Staphylocoocus spp. | [22] |
| No. | Compound | RI | Formula | Class of Compound | % of Total EO |
|---|---|---|---|---|---|
| 1 | α-thujene | 947 | C10H16 | MH | tr |
| 2 | α-pinene | 952 | C10H16 | MH | 0.56 |
| 3 | β-pinene | 982 | C10H16 | MH | tr |
| 4 | β-myrcene | 993 | C10H16 | MH | 0.24 |
| 5 | α-phellandrene | 1007 | C10H16 | MH | 20.51 |
| 6 | α-terpinene | 1014 | C10H16 | MH | 1.66 |
| 7 | o-cymene | 1022 | C10H14 | MH | 12.30 |
| 8 | β-phellandrene | 1026 | C10H16 | MH | 6.0 |
| 9 | γ-terpinene | 1051 | C10H16 | MH | 0.20 |
| 10 | α-terpinolene | 1085 | C10H16 | MH | 0.21 |
| 11 | β-terpineol | 1149 | C10H18O | MO | 0.96 |
| 12 | cryptone | 1175 | C9H14O | MO | 0.12 |
| 13 | p-ment-1(7)-en-2-one | 1240 | C10H16O | MO | 0.08 |
| 14 | isocarveol | 1290 | C10H16O | MO | 0.35 |
| 15 | carvacrol | 1298 | C10H14O | MO | 0.71 |
| 16 | methyleugenol | 1405 | C11H14O | PP | 48.13 |
| Terpene classes | |||||
| MH—monoterpene hydrocarbons | 41.68 | ||||
| MO—oxygenated monoterpenes | 2.22 | ||||
| PP—phenylpropanoids | 48.13 | ||||
| Total identified | 92.03 |
| Plants Collecting Region | Main Volatile Compounds % | Other Volatile Compounds | Ref. |
|---|---|---|---|
| Bulgaria | methyleugenol (48.13%) | α-phellandrene (20.51%) o-cymene (12.30%) | Present study |
| Greece | α-phellandrene (43.80%) | methyleugenol (28.60%) | [1] |
| Iran | methyleugenol (50.40%) | α-phellandrene (16.30%) δ-3-carene (17.40%) | [27] |
| Iran | δ-3-carene (31.80%) α-phellandrene (31.00%) | methyleugenol (16.90%) | [31] |
| Republic of North Macedonia | methyleugenol (60.40%) | p-cymene (11.18%) α-phellandrene (10.23%) | [26] |
| Turkey | α-phellandrene (47.43–66.39%) methyl eugenol (21.29–38.72%) | [7] | |
| Turkey | δ-3-carene (17.93%) methyleugenol (16.41%) | p-cymene (8.99%) α-phellandrene (9.33%) | [22] |
| Turkey | δ-3-carene (30.01–38.80%) | methyleugenol (22.10–25.96%) α-phellandrene (14.50–29.26%) | [24] |
| Turkey | methyleugenol (41.80–62.90%) | α-phellandrene (30.40%) p-cymene (7.80–9.10%) | [25] |
| Turkey | methyleugenol (36.60%) δ-3-carene (36.60%) | p-cymene (7.60%) α-phellandrene (6.10%) | [28] |
| Turkey | methyleugenol (9.61–80.65%) δ-3-carene (2.27–61.64%) | [29] | |
| Turkey | methyleugenol (24.99–90.16%) δ-3-carene (2.57–34.80%) | p-cymene (1.23–9.81%) | [30] |
| Turkey | α-phellandrene (51.52%) | methyleugenol (17.46%) p-cymene (14.66%) | [32] |
| Turkey | α-phellandrene (13.22–55.27%) δ-3-carene (49.29–4.03%) | methyleugenol (22.59–25.69%) | [33] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanova, S.; Dyankov, S.; Karcheva-Bahchevanska, D.; Todorova, V.; Georgieva, Y.; Benbassat, N.; Ivanov, K. Echinophora tenuifolia subsp. sibthorpiana—Study of the Histochemical Localization of Essential Oil. Molecules 2023, 28, 2918. https://doi.org/10.3390/molecules28072918
Ivanova S, Dyankov S, Karcheva-Bahchevanska D, Todorova V, Georgieva Y, Benbassat N, Ivanov K. Echinophora tenuifolia subsp. sibthorpiana—Study of the Histochemical Localization of Essential Oil. Molecules. 2023; 28(7):2918. https://doi.org/10.3390/molecules28072918
Chicago/Turabian StyleIvanova, Stanislava, Stanislav Dyankov, Diana Karcheva-Bahchevanska, Velislava Todorova, Yoana Georgieva, Niko Benbassat, and Kalin Ivanov. 2023. "Echinophora tenuifolia subsp. sibthorpiana—Study of the Histochemical Localization of Essential Oil" Molecules 28, no. 7: 2918. https://doi.org/10.3390/molecules28072918