Efficient Removal of Pb(Ⅱ) by Highly Porous Polymeric Sponges Self-Assembled from a Poly(Amic Acid)
Abstract
:1. Introduction
2. Results and Discussion
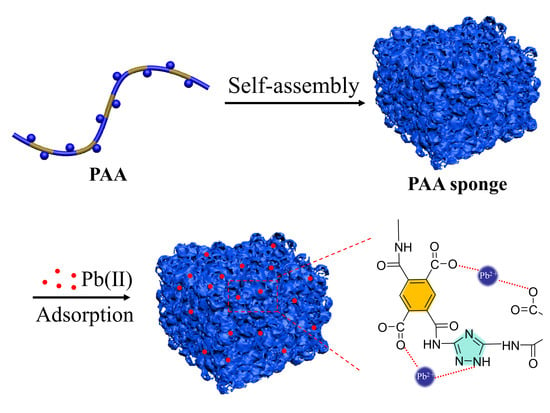
2.1. Synthesis and Self-Assembly of PAA
2.2. Effect of pH and PAA Sponge Dosage on Adsorption Performance
2.3. Adsorption Kinetics
| Heavy Metal Ion | Pseudo-First-Order | Pseudo-Second-Order | ||||
|---|---|---|---|---|---|---|
| qe1 (mg g−1) | k1 (min−1) | R2 | qe2 (mg g−1) | k2 (g (mg min)−1) | R2 | |
| Pb(II) | 379.3 | 1.892 | 0.9995 | 381.4 | 0.0413 | 0.9998 |
2.4. Adsorption Isotherms
2.5. Recyclability of the PAA Sponge
2.6. Mechanism for the Adsorption of Pb(II) by PAA Sponge
3. Materials and Methods
3.1. Materials
3.2. Experimental Section
3.2.1. Synthesis of Poly(amic acid) (PAA)
3.2.2. Self-Assembly of the PAA into a Sponge
3.2.3. Adsorption Experiments
3.3. Characterizations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Gao, Y.; Deng, S.-Q.; Jin, X.; Cai, S.-L.; Zheng, S.-R.; Zhang, W.-G. The construction of amorphous metal-organic cage-based solid for rapid dye adsorption and time-dependent dye separation from water. Chem. Eng. J. 2019, 357, 129–139. [Google Scholar] [CrossRef]
- Zhang, X.; Li, D.; Li, M.; Qin, P.; Zhu, S.; Gao, Y.; Mu, M.; Zhang, N.; Wang, Y.; Lu, M. Flower-like Co3O4/C3N5 composite as solid-phase microextraction coating for high-efficiency adsorption and preconcentration of polycyclic aromatic hydrocarbons and polychlorinated biphenyls in water. Chem. Eng. J. 2022, 443, 136293. [Google Scholar] [CrossRef]
- Cai, L.; Ying, D.; Liang, X.; Zhu, M.; Lin, X.; Xu, Q.; Cai, Z.; Xu, X.; Zhang, L. A novel cationic polyelectrolyte microsphere for ultrafast and ultra-efficient removal of heavy metal ions and dyes. Chem. Eng. J. 2021, 410, 128404. [Google Scholar] [CrossRef]
- Bao, S.; Yang, W.; Wang, Y.; Yu, Y.; Sun, Y. One-pot synthesis of magnetic graphene oxide composites as an efficient and recoverable adsorbent for Cd(II) and Pb(II) removal from aqueous solution. J. Hazard. Mater. 2020, 381, 120914. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Atieh, M.A.; Bakather, O.Y.; Tawabini, B.; Al-Tawbini, B.; Bukhari, A.A.; Abuilaiwi, F.A.; Fettouhi, M.B. Effect of carboxylic functional group functionalized on carbon nanotubes surface on the removal of lead from water. Bioinorg. Chem. Appl. 2010, 2010, 603978. [Google Scholar] [CrossRef] [Green Version]
- Singh, K.; Agarwal, M. Renu Heavy metal removal from wastewater using various adsorbents: A review. J. Water Reuse Desalin. 2017, 7, 387–419. [Google Scholar]
- Wan, S.; Wu, J.; Zhou, S.; Wang, R.; Gao, B.; He, F. Enhanced lead and cadmium removal using biochar-supported hydrated manganese oxide (HMO) nanoparticles: Behavior and mechanism. Sci. Total Environ. 2018, 616–617, 1298–1306. [Google Scholar] [CrossRef]
- Uddin, M.K. A review on the adsorption of heavy metals by clay minerals, with special focus on the past decade. Chem. Eng. J. 2017, 308, 438–462. [Google Scholar] [CrossRef]
- Wang, J.; Chen, B. Adsorption and coadsorption of organic pollutants and a heavy metal by graphene oxide and reduced graphene materials. Chem. Eng. J. 2015, 281, 379–388. [Google Scholar] [CrossRef]
- Inyang, M.; Gao, B.; Yao, Y.; Xue, Y.; Zimmerman, A.R.; Pullammanappallil, P.; Cao, X. Removal of heavy metals from aqueous solution by biochars derived from anaerobically digested biomass. Bioresour. Technol. 2012, 110, 50–56. [Google Scholar] [CrossRef]
- Demirbas, A. Heavy metal adsorption onto agro-based waste materials: A review. J. Hazard. Mater. 2008, 157, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Ahluwalia, S.S.; Goyal, D. Microbial and plant derived biomass for removal of heavy metals from wastewater. Bioresour. Technol. 2007, 98, 2243–2257. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Pan, B.; Chen, X.; Zhang, W.; Zhang, X.; Zhang, Q.; Zhang, Q.; Chen, J. Preparation and preliminary assessment of polymer-supported zirconium phosphate for selective lead removal from contaminated water. Water Res. 2006, 40, 2938–2946. [Google Scholar] [CrossRef]
- Tan, Y.; Chen, M.; Hao, Y. High efficient removal of Pb (II) by amino-functionalized Fe3O4 magnetic nano-particles. Chem. Eng. J. 2012, 191, 104–111. [Google Scholar] [CrossRef]
- Ren, H.; Gao, Z.; Wu, D.; Jiang, J.; Sun, Y.; Luo, C. Efficient Pb(II) removal using sodium alginate-carboxymethyl cellulose gel beads: Preparation, characterization, and adsorption mechanism. Carbohydr. Polym. 2016, 137, 402–409. [Google Scholar] [CrossRef]
- Mei, D.; Liu, L.; Yan, B. Adsorption of uranium (VI) by metal-organic frameworks and covalent-organic frameworks from water. Coord. Chem. Rev. 2023, 475, 214917. [Google Scholar] [CrossRef]
- Liu, Z.; Xiong, B.; Dong, Y.; Ning, Y.; Li, D. Metal–Organic Frameworks@Calcite Composite Crystals. Inorg. Chem. 2022, 61, 16203–16210. [Google Scholar] [CrossRef]
- Yin, N.; Wang, K.; Xia, Y.A.; Li, Z. Novel melamine modified metal-organic frameworks for remarkably high removal of heavy metal Pb (II). Desalination 2018, 430, 120–127. [Google Scholar] [CrossRef]
- Kobielska, P.A.; Howarth, A.J.; Farha, O.K.; Nayak, S. Metal–organic frameworks for heavy metal removal from water. Coord. Chem. Rev. 2018, 358, 92–107. [Google Scholar] [CrossRef]
- Ullah, I.; Shah, A.; Khan, M.; Khan, S.Z.; ur-Rehman, Z.; Badshah, A. Synthesis and Spectrophotometric Study of Toxic Metals Extraction by Novel Thio-Based Non-Ionic Surfactant. Tenside, Surfactants, Deterg. 2015, 52, 406–413. [Google Scholar] [CrossRef]
- Rauf, A.; Shah, A.; Abbas, S.; Rana, U.A.; Khan, S.U.; Ali, S.; Zia Ur, R.; Qureshi, R.; Kraatz, H.B.; Belanger-Gariepy, F. Synthesis, spectroscopic characterization and pH dependent photometric and electrochemical fate of Schiff bases. Spectrochim. Acta, Part A 2015, 138, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Fan, L.; Yang, B.; Du, J. Multifunctional Homopolymer Vesicles for Facile Immobilization of Gold Nanoparticles and Effective Water Remediation. ACS Nano 2014, 8, 5022–5031. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Y.; Bi, G.; Liu, J.; Wang, Z.; Xu, Q.; Xu, H.; Li, X. Mussel-inspired polydopamine biopolymer decorated with magnetic nanoparticles for multiple pollutants removal. J. Hazard. Mater. 2014, 270, 27–34. [Google Scholar] [CrossRef]
- Dupont, D.; Brullot, W.; Bloemen, M.; Verbiest, T.; Binnemans, K. Selective uptake of rare earths from aqueous solutions by EDTA-functionalized magnetic and nonmagnetic nanoparticles. ACS Appl. Mater. Interfaces 2014, 6, 4980–4988. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Su, G.; Zhang, B.; Jiang, G.; Yan, B. Nanoparticle-based strategies for detection and remediation of environmental pollutants. Analyst 2011, 136, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Jabeen, H.; Chandra, V.; Jung, S.; Lee, J.W.; Kim, K.S.; Kim, S.B. Enhanced Cr(vi) removal using iron nanoparticle decorated graphene. Nanoscale 2011, 3, 3583–3585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alotaibi, K.M.; Shiels, L.; Lacaze, L.; Peshkur, T.A.; Anderson, P.; Machala, L.; Critchley, K.; Patwardhan, S.V.; Gibson, L.T. Iron supported on bioinspired green silica for water remediation. Chem. Sci. 2017, 8, 567–576. [Google Scholar] [CrossRef] [Green Version]
- Manos, M.J.; Kanatzidis, M.G. Metal sulfide ion exchangers: Superior sorbents for the capture of toxic and nuclear waste-related metal ions. Chem. Sci. 2016, 7, 4804–4824. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Chen, S.; Li, X.; Leng, Y.; Zhou, X.; Du, J. Lateral growth of cylinders. Nat. Commun. 2022, 13, 2170. [Google Scholar] [CrossRef]
- Sun, H.; Zhou, X.; Leng, Y.; Li, X.; Du, J. Transformation of Amorphous Nanobowls to Crystalline Ellipsoids Induced by Trans-Cis Isomerization of Azobenzene. Macromol. Rapid Commun. 2022, 43, 2200131. [Google Scholar] [CrossRef]
- Sun, H.; Leng, Y.; Zhou, X.; Li, X.; Wang, T. Regulation of the nanostructures self-assembled from an amphiphilic azobenzene homopolymer: Influence of initial concentration and solvent solubility parameter. Soft Matter 2023, 19, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Jiang, J.; Xiao, Y.; Du, J. Efficient Removal of Polycyclic Aromatic Hydrocarbons, Dyes, and Heavy Metal Ions by a Homopolymer Vesicle. ACS Appl. Mater. Interfaces 2018, 10, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Leng, Y.; Jin, K.; Wang, T.; Sun, H. Facile Preparation of Cobalt Nanoparticles Encapsulated Nitrogen-Doped Carbon Sponge for Efficient Oxygen Reduction Reaction. Polymers 2023, 15, 521. [Google Scholar] [CrossRef]
- Sun, H.; Jin, K.; Li, X.; Wang, T.; Lai, X. Nitrogen-doped carbon sponge derived from the self-assembly of poly(amic acid) for high performance oxygen reduction reaction. J. Chem. 2023, 47, 3297–3305. [Google Scholar] [CrossRef]
- Yang, W.; Cao, M. Synthesis of ZIF-8@GO-COOH and its adsorption for Cu(II) and Pb(II) from water: Capability and mechanism. Sep. Purif. Technol. 2023, 309, 122957. [Google Scholar] [CrossRef]
- Xu, X.; Ouyang, X.-k.; Yang, L.-Y. Adsorption of Pb(II) from aqueous solutions using crosslinked carboxylated chitosan/carboxylated nanocellulose hydrogel beads. J. Mol. Liq. 2021, 322, 114523. [Google Scholar] [CrossRef]
- Wang, C.; Xiong, C.; He, Y.; Yang, C.; Li, X.; Zheng, J.; Wang, S. Facile preparation of magnetic Zr-MOF for adsorption of Pb(II) and Cr(VI) from water: Adsorption characteristics and mechanisms. Chem. Eng. J. 2021, 415, 128923. [Google Scholar] [CrossRef]
- Liu, X.; Guan, J.; Lai, G.; Xu, Q.; Bai, X.; Wang, Z.; Cui, S. Stimuli-responsive adsorption behavior toward heavy metal ions based on comb polymer functionalized magnetic nanoparticles. J. Clean. Prod. 2020, 253, 119915. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption kinetic models: Physical meanings, applications, and solving methods. J. Hazard. Mater. 2020, 390, 122156. [Google Scholar] [CrossRef]
- Ifthikar, J.; Wang, J.; Wang, Q.; Wang, T.; Wang, H.; Khan, A.; Jawad, A.; Sun, T.; Jiao, X.; Chen, Z. Highly Efficient Lead Distribution by Magnetic Sewage Sludge Biochar: Sorption Mechanisms and Bench Applications. Bioresour. Technol. 2017, 238, 399–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choy, K.K.H.; Porter, J.F.; McKay, G. Langmuir isotherm models applied to the multicomponent sorption of acid dyes from effluent onto activated carbon. J. Chem. Eng. Data 2000, 45, 575–584. [Google Scholar] [CrossRef]
- Zhao, L.-X.; Song, S.-E.; Du, N.; Hou, W.-G. A sorbent concentration-dependent Freundlich isotherm. Colloid Polym. Sci. 2013, 291, 541–550. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Wang, S.; Wang, S.; Tang, J.; Chen, Y.; Zhang, L. Adenosine-functionalized UiO-66-NH2 to efficiently remove Pb(II) and Cr(VI) from aqueous solution: Thermodynamics, kinetics and isothermal adsorption. J. Hazard. Mater. 2022, 425, 127771. [Google Scholar] [CrossRef]
- Taşar, Ş.; Kaya, F.; Özer, A. Biosorption of lead(II) ions from aqueous solution by peanut shells: Equilibrium, thermodynamic and kinetic studies. J. Environ. Chem. Eng. 2014, 2, 1018–1026. [Google Scholar] [CrossRef]
- Yousif, E.; Ahmed, D.S.; Ahmed, A.; Abdallh, M.; Yusop, R.M.; Mohammed, S.A. Impact of stabilizer on the environmental behavior of PVC films reinforced 1,2,4-triazole moiety. Environ. Sci. Pollut. Res. 2019, 26, 26381–26388. [Google Scholar] [CrossRef] [PubMed]
- Pagacz-Kostrzewa, M.; Bronisz, R.; Wierzejewska, M. Theoretical and matrix isolation FTIR studies of 3-amino-1,2,4-triazole and its isomers. Chem. Phys. Lett. 2009, 473, 238–246. [Google Scholar] [CrossRef]
- Chen, B.; Chen, S.; Zhao, H.; Liu, Y.; Long, F.; Pan, X. A versatile β-cyclodextrin and polyethyleneimine bi-functionalized magnetic nanoadsorbent for simultaneous capture of methyl orange and Pb(II) from complex wastewater. Chemosphere 2019, 216, 605–616. [Google Scholar] [CrossRef]
- He, J.; Li, Y.; Wang, C.; Zhang, K.; Lin, D.; Kong, L.; Liu, J. Rapid adsorption of Pb, Cu and Cd from aqueous solutions by β-cyclodextrin polymers. Appl. Surf. Sci. 2017, 426, 29–39. [Google Scholar] [CrossRef]
- Li, X.-G.; Ma, X.-L.; Sun, J.; Huang, M.-R. Powerful Reactive Sorption of Silver(I) and Mercury(II) onto Poly(o-phenylenediamine) Microparticles. Langmuir 2009, 25, 1675–1684. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Wang, L.; Zhang, D.; Yan, P.; Nie, J.; Sharma, V.K.; Wang, C. Highly efficient and selective removal of mercury ions using hyperbranched polyethylenimine functionalized carboxymethyl chitosan composite adsorbent. Chem. Eng. J. 2019, 358, 253–263. [Google Scholar] [CrossRef]
- Xiao, D.; Ding, W.; Zhang, J.; Ge, Y.; Wu, Z.; Li, Z. Fabrication of a versatile lignin-based nano-trap for heavy metal ion capture and bacterial inhibition. Chem. Eng. J. 2019, 358, 310–320. [Google Scholar] [CrossRef]
- Swiatkowski, A.; Pakula, M.; Biniak, S.; Walczyk, M. Influence of the surface chemistry of modified activated carbon on its electrochemical behaviour in the presence of lead(II) ions. Carbon 2004, 42, 3057–3069. [Google Scholar] [CrossRef]
- Huang, Z.; Xiong, C.; Ying, L.; Wang, W.; Wang, S.; Ding, J.; Lu, J. Facile synthesis of a MOF-derived magnetic CoAl-LDH@chitosan composite for Pb (II) and Cr (VI) adsorption. Chem. Eng. J. 2022, 449, 137722. [Google Scholar] [CrossRef]







| Heavy Metal Ion | Langmuir Isotherm | Freundlich Isotherm | ||||
|---|---|---|---|---|---|---|
| KL (L mg−1) | qm (mg g−1) | R2 | KF (mg g−1 (L mg−1)1/n) | 1/n | R2 | |
| Pb(II) | 0.1784 | 609.7 | 0.9972 | 118,700 | 0.2872 | 0.9304 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leng, Y.; Jin, K.; Wang, T.; Lai, X.; Sun, H. Efficient Removal of Pb(Ⅱ) by Highly Porous Polymeric Sponges Self-Assembled from a Poly(Amic Acid). Molecules 2023, 28, 2897. https://doi.org/10.3390/molecules28072897
Leng Y, Jin K, Wang T, Lai X, Sun H. Efficient Removal of Pb(Ⅱ) by Highly Porous Polymeric Sponges Self-Assembled from a Poly(Amic Acid). Molecules. 2023; 28(7):2897. https://doi.org/10.3390/molecules28072897
Chicago/Turabian StyleLeng, Ying, Kai Jin, Tian Wang, Xiaoyong Lai, and Hui Sun. 2023. "Efficient Removal of Pb(Ⅱ) by Highly Porous Polymeric Sponges Self-Assembled from a Poly(Amic Acid)" Molecules 28, no. 7: 2897. https://doi.org/10.3390/molecules28072897