Profiling and Quantification of Anthocyanins in Purple-Pericarp Sweetcorn and Purple-Pericarp Maize
Abstract
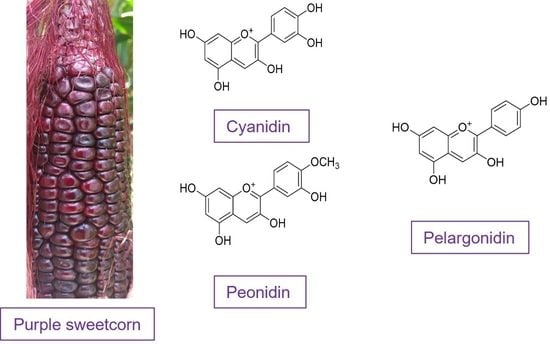
:1. Introduction
2. Results
2.1. Anthocyanin Quantification of Mature Round (Starchy) Kernels
2.2. Anthocyanin Quantification of Mature Shrunken (Non-Starchy) Kernels
2.3. Quantification of Anthocyanin at Eating-Stage (25 DAP) of Parental and F6 ‘Tim’ Purple Sweetcorn Accessions
2.4. Objective Colour Measurement of the Mature Round (Starchy) and Shrunken (Non-Starchy) Kernels
2.5. Kernel Anatomy Regarding Anthocyanin Development
2.6. Kernel Maturity and Anthocyanin Development
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Anthocyanin Extraction, Identification and Quantification
4.2.1. Chemicals
4.2.2. Anthocyanin Extraction
4.2.3. Anthocyanin Identification
4.2.4. Anthocyanin Quantification
4.3. Objective Colour Measurement
4.4. Statistical Analysis
4.5. Kernel Anatomy
4.6. Kernel Physiology
4.7. Moisture Content
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Civardi, L.; Xia, Y.; Edwards, K.J.; Schnable, P.S.; Nikolau, B.J. The relationship between genetic and physical distances in the cloned a1-sh2 interval of the Zea mays L. genome. Proc. Natl. Acad. Sci. USA 1994, 91, 8268–8272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, H.; Zhou, Q.; Li, J.; Smith, H.; Yandeau, M.; Nikolau, B.J.; Schnable, P.S. Molecular characterization of meiotic recombination across the 140-kb multigenic a1-sh2 interval of maize. Proc. Natl. Acad. Sci. USA 2002, 99, 6157–6162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kramer, V.; Shaw, J.R.; Senior, M.L.; Hannah, L.C. The sh2-R allele of the maize shrunken-2 locus was caused by a complex chromosomal rearrangement. Theor. Appl. Genet. 2014, 128, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Anirban, A.; O’Hare, T. Super-Sweet Purple Sweetcorn: Breaking the Genetic Link. Multidiscip. Digit. Publ. Inst. Proc. 2020, 36, 6134. [Google Scholar] [CrossRef] [Green Version]
- Anirban, A.; Hayward, A.; Hong, H.T.; Masouleh, A.K.; Henry, R.J.; O’Hare, T.J. Breaking the tight genetic linkage between the a1 and sh2 genes led to the development of anthocyanin-rich purple-pericarp super-sweetcorn. Sci. Rep. 2023, 13, 1–13. [Google Scholar] [CrossRef]
- Emerson, R.A. The Genetic Relations of Plant Colors in Maize; Cornell University: Ithaca, NY, USA, 1921. [Google Scholar]
- Paulsmeyer, M.; Chatham, L.; Becker, T.; West, M.; West, L.; Juvik, J. Survey of Anthocyanin Composition and Concentration in Diverse Maize Germplasms. J. Agric. Food Chem. 2017, 65, 4341–4350. [Google Scholar] [CrossRef]
- Hong, H.; Netzel, M.; O’Hare, T. Anthocyanin composition and changes during kernel development in purple-pericarp supersweet sweetcorn. Food Chem. 2020, 315, 126284. [Google Scholar] [CrossRef]
- Hong, H.T.; Phan, A.D.T.; O’Hare, T.J. Temperature and Maturity Stages Affect Anthocyanin Development and Phenolic and Sugar Content of Purple-Pericarp Supersweet Sweetcorn during Storage. J. Agric. Food Chem. 2021, 69, 922–931. [Google Scholar] [CrossRef]
- Styles, E.; Ceska, O. Flavonoid pigments in genetic strains of maize. Phytochemistry 1972, 11, 3019–3021. [Google Scholar] [CrossRef]
- De Pascual-Teresa, S.; Santos-Buelga, C. LC-MS analysis of anthocyanins from purple corn cob. J. Sci. Food Agric. 2002, 82, 1003–1006. [Google Scholar] [CrossRef]
- Casas, M.I.; Duarte, S.; Doseff, A.I.; Grotewold, E. Flavone-rich maize: An opportunity to improve the nutritional value of an important commodity crop. Front. Plant Sci. 2014, 5, 440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lao, F.; Sigurdson, G.T.; Giusti, M.M. Health Benefits of Purple Corn (Zea mays L.) Phenolic Compounds. Compr. Rev. Food Sci. Food Saf. 2017, 16, 234–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finkel, M.L.; Sanchez, S.E.; Mak, T.; Granstein, J.; Lefkowitz, A. Anthocyanin-Rich Purple Corn Extract and Its Effects on the Blood Pressure of Adults. J. Evid.-Based Complement. Altern. Med. 2013, 18, 237–242. [Google Scholar] [CrossRef]
- Hagiwara, A.; Miyashita, K.; Nakanishi, T.; Sano, M.; Tamano, S.; Kadota, T.; Koda, T.; Nakamura, M.; Imaida, K.; Ito, N.; et al. Pronounced inhibition by a natural anthocyanin, purple corn color, of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP)-associated colorectal carcinogenesis in male F344 rats pretreated with 1,2-dimethylhydrazine. Cancer Lett. 2001, 171, 17–25. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, C.-L.; Yu, Y.-Q.; Chen, Z.-J.; Wen, G.-S.; Wei, F.-G.; Zheng, Q.; Wang, C.-D.; Xiao, X.-L. Stability-increasing effects of anthocyanin glycosyl acylation. Food Chem. 2017, 214, 119–128. [Google Scholar] [CrossRef]
- Chatham, L.A.; Paulsmeyer, M.; Juvik, J.A. Prospects for economical natural colorants: Insights from maize. Theor. Appl. Genet. 2019, 132, 2927–2946. [Google Scholar] [CrossRef]
- Khanduri, A.; Hossain, F.; Lakhera, P.C.; Prasanna, B.M. Effect of harvest time on kernel sugar concentration in sweet corn. Indian J. Genet. Plant Breed. 2011, 71, 231–234. [Google Scholar]
- Lago, C.; Landoni, M.; Cassani, E.; Atanassiu, S.; Canta-Luppi, E.; Pilu, R. Development and characterization of a coloured sweet corn line as a new functional food. Maydica 2014, 59, 191–200. [Google Scholar]
- Wang, B.; Brewbaker, J.L. Quantitative trait loci affecting pericarp thickness of corn kernels. Maydica 2001, 46, 159–165. [Google Scholar]
- Petrussa, E.; Braidot, E.; Zancani, M.; Peresson, C.; Bertolini, A.; Patui, S.; Vianello, A. Plant Flavonoids—Biosynthesis, Transport and Involvement in Stress Responses. Int. J. Mol. Sci. 2013, 14, 14950–14973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, M.; Cortes-Cruz, M.; Ahern, K.R.; McMullen, M.; Brutnell, T.P.; Chopra, S. Identification of the Pr1 Gene Product Completes the Anthocyanin Biosynthesis Pathway of Maize. Genetics 2011, 188, 69–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatham, L.A.; Juvik, J.A. Linking anthocyanin diversity, hue, and genetics in purple corn. G3 Genes|Genomes|Genet. 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zhang, W.; Yang, H.; Dong, Q.; Ren, J.; Fan, H.; Zhang, X.; Zhou, Y. Comparative transcriptome analysis reveals differentially expressed genes related to the tissue-specific accumulation of anthocyanins in pericarp and aleurone layer for maize. Sci. Rep. 2019, 9, 2485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brenes, P.G.C. Factors affecting carotenoids in zeaxanthin-biofortified sweet-corn (Zea mays L.). 2019. Available online: https://espace.library.uq.edu.au/data/UQ_059e428/s4333314_final_thesis.pdf?Expires=1678962567&Key-Pair-Id=APKAJKNBJ4MJBJNC6NLQ&Signature=ZLX~e4hklXq0dXf8CZMVRfwyKhOOdu0-pUVJ4docWO3W3D0CiHaNqX4Lx8uA-SlrG7XpVrpOoX~9TGFuSXTpwEjl1b1IFs6q0YOR0R3GCYkXB05tJNmjDLJJgrpbGRiSvKbO84rb4DxQ4gBQWQFhbf~ca954F6R1WNOmWbFa3scc1RX2KwiJ0mzRJ1n9ZKAV1w19aBIqoLBWzIB6JxXUtXoB6MqasDBy0t94Zp4p0TP4Op-fqhmnZEwzhPQWjlJde6Y45JWRK6us2hS~QJpgcXkLol3HJaKIahUzwjtEuSPh920Yu4uZ3QTnFusLUvmQAfpWaakoJVtPP0DPxkqnxQ (accessed on 12 February 2023). [CrossRef]
- Wann, E.V.; Brown, G.B.; Hills, W.A. Genetic Modifications of Sweet Corn Quality1. J. Am. Soc. Hortic. Sci. 1971, 96, 441–444. [Google Scholar] [CrossRef]
- Becraft, P.W.; Yi, G. Regulation of aleurone development in cereal grains. J. Exp. Bot. 2011, 62, 1669–1675. [Google Scholar] [CrossRef] [Green Version]
- Luna-Vital, D.A.; Li, Q.; West, L.; West, M.; de Mejia, E.G. Anthocyanin condensed forms do not affect color or chemical stability of purple corn pericarp extracts stored under different pHs. Food Chem. 2017, 232, 639–647. [Google Scholar] [CrossRef]
- Aaby, K.; Mazur, S.; Arnfinn Nes, A.; Skrede, G. Phenolic compounds in strawberry (Fragaria x ananassa Duch.) fruits: Composition in 27 cultivars and changes during ripening. Food Chem. 2012, 132, 86–97. [Google Scholar] [CrossRef]
- Proteggente, A.R.; Pannala, A.S.; Paganga, G.; Van Buren, L.; Wagner, E.; Wiseman, S.; Van De Put, F.; Dacombe, C.; Rice-Evans, C.A. The Antioxidant Activity of Regularly Consumed Fruit and Vegetables Reflects their Phenolic and Vitamin C Composition. Free Radic. Res. 2002, 36, 217–233. [Google Scholar] [CrossRef]
- Wu, X.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Gebhardt, S.E.; Prior, R.L. Concentrations of Anthocyanins in Common Foods in the United States and Estimation of Normal Consumption. J. Agric. Food Chem. 2006, 54, 4069–4075. [Google Scholar] [CrossRef] [PubMed]
- McGuire, R.G. Reporting of Objective Color Measurements. Hortscience 1992, 27, 1254–1255. [Google Scholar] [CrossRef] [Green Version]




| Anthocyanin-Based Compound (mg/100g FW) | ||||
|---|---|---|---|---|
| Accession (60 DAP) | Cyanidin | Peonidin | Pelargonidin | Total Anthocyanin |
| ‘Costa Rica’ A | 160.5x | 27.1x | 6.7x | 194.5x |
| ‘Tims-aleurone’ | 15.8y | 9.0y | 2.9x | 10.3y |
| ‘Tims-white’ | n.d. | n.d. | n.d. | n.d. |
| ‘Tim1’ B | 230.1a | 68.4b | 66.9b | 365.4a |
| ‘Tim2’ | 813.5c | 197.5d | 98.3c | 1109.3c |
| ‘Tim4’ | 225.7a | 50.3a | 59.4ab | 335.3a |
| ‘Tim5’ | 506.9b | 85.7c | 56.8a | 649.3b |
| Anthocyanin Proportion (% Total Peak Area) | ||||||
|---|---|---|---|---|---|---|
| Anthocyanin | m/z (Peak No.) | ‘Costa Rica’ | ‘Tim1’ | ‘Tim2’ | ‘Tim4’ | ‘Tim5’ |
| Cy3G | 449.2 (1) | 54.8 | 15.5 | 19.5 | 6.9 | 60.9 |
| Cy3MG | 535.2 (4) | 20.9 | 30.7 | 34.3 | 27.9 | 15.2 |
| Cy3DMG | 621.2 (6) | 3.7 | 24.7 | 27.4 | 28.3 | 5.1 |
| Pn3G | 463.2 (3) | 3.3 | 3.7 | 6.6 | 3.0 | 3.8 |
| Pn3MG | 549.2 (7) | 5.5 | 1.7 | 2.7 | 2.4 | 3.4 |
| Pn3DMG | 635.1 (9) | 2.4 | 1.4 | 2.6 | 15.2 | 2.4 |
| Pg3G | 433.2 (2) | 5.9 | 7.5 | 1.7 | 2.6 | 5.8 |
| Pg3MG | 519.1 (5) | 2.1 | 12.7 | 3.9 | 8.6 | 2.1 |
| Pg3DMG | 605.2 (8) | 1.4 | 2.1 | 1.4 | 5.1 | 1.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anirban, A.; Hong, H.T.; O’Hare, T.J. Profiling and Quantification of Anthocyanins in Purple-Pericarp Sweetcorn and Purple-Pericarp Maize. Molecules 2023, 28, 2665. https://doi.org/10.3390/molecules28062665
Anirban A, Hong HT, O’Hare TJ. Profiling and Quantification of Anthocyanins in Purple-Pericarp Sweetcorn and Purple-Pericarp Maize. Molecules. 2023; 28(6):2665. https://doi.org/10.3390/molecules28062665
Chicago/Turabian StyleAnirban, Apurba, Hung T. Hong, and Tim J. O’Hare. 2023. "Profiling and Quantification of Anthocyanins in Purple-Pericarp Sweetcorn and Purple-Pericarp Maize" Molecules 28, no. 6: 2665. https://doi.org/10.3390/molecules28062665