Application of Green Technology to Extract Clean and Safe Bioactive Compounds from Tetradesmus obliquus Biomass Grown in Poultry Wastewater
Abstract
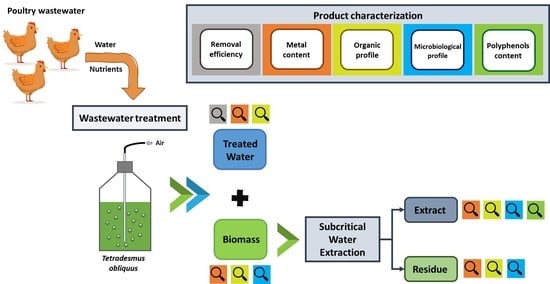
:1. Introduction
2. Results and Discussion
2.1. Wastewater Treatment
2.2. Biomass Composition
2.2.1. Content of Polyphenols and Antioxidant Activity of Biomass Extract
2.2.2. Organic Profile of Whole Biomass, Extract, and Residue
2.2.3. Metal Content of Whole Biomass, Extract, and Residue
2.2.4. Microbiological Profile
3. Materials and Methods
3.1. Poultry Wastewater Treatment
3.2. Microalga, Cultivation Conditions
3.3. Extraction of Tetradesmus Obliquus Biomass
3.4. Determination of Total Phenols and Total Flavonoids Content
3.5. Determination of Antioxidant Activity
3.6. Gas Chromatography-Mass Spectrometry (GC-MS) Screening Analysis
3.7. Determination of Metal Content
3.8. Microbiological Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- López-Sánchez, A.; Silva-Gálvez, A.L.; Aguilar-Juárez, Ó.; Senés-Guerrero, C.; Orozco-Nunnelly, D.A.; Carrillo-Nieves, D.; Gradilla-Hernández, M.S. Microalgae-based livestock wastewater treatment (MbWT) as a circular bioeconomy approach: Enhancement of biomass productivity, pollutant removal and high-value compound production. J. Environ. Manag. 2022, 308, 114612. [Google Scholar] [CrossRef] [PubMed]
- FAO. Food Outlook—Biannual Report on Global Food Markets; The Food and Agriculture Organization: Rome, Italy, 2020. [Google Scholar]
- Ferreira, A.; Reis, A.; Vidovic, S.; Vladic, J.; Gkelis, S.; Melkonyan, L.; Avetisova, G.; Congestri, R.; Acién, G.; Muñoz, R.; et al. Combining Microalgae-Based Wastewater Treatment with Biofuel and Bio-Based Production in the Frame of a Biorefinery; Springer: Cham, Switzerland, 2019; pp. 319–369. [Google Scholar]
- Martinelli, G.; Vogel, E.; Decian, M.; Farinha, M.J.U.S.; Bernardo, L.V.M.; Borges, J.A.R.; Gimenes, R.M.T.; Garcia, R.G.; Ruviaro, C.F. Assessing the eco-efficiency of different poultry production systems: An approach using life cycle assessment and economic value added. Sustain. Prod. Consum. 2020, 24, 181–193. [Google Scholar] [CrossRef]
- Oryschak, M.A.; Beltranena, E. Reconsidering the contribution of Canadian poultry production to anthropogenic greenhouse gas emissions: Returning to an integrated crop–poultry production system paradigm. Poult. Sci. 2020, 99, 3777–3783. [Google Scholar] [CrossRef] [PubMed]
- Fridrich, B.; Krčmar, D.; Dalmacija, B.; Molnar, J.; Pešić, V.; Kragulj, M.; Varga, N. Impact of wastewater from pig farm lagoons on the quality of local groundwater. Agric. Water Manag. 2014, 135, 40–53. [Google Scholar] [CrossRef]
- Eurostat 2013–2015. Available online: http://ec.europa.eu/eurostat (accessed on 1 February 2022).
- Patel, A.; Gami, B.; Patel, P.; Patel, B. Microalgae: Antiquity to era of integrated technology. Renew. Sustain. Energy Rev. 2017, 71, 535–547. [Google Scholar] [CrossRef]
- Moreno-Garcia, L.; Adjallé, K.; Barnabé, S.; Raghavan, G.S.V. Microalgae biomass production for a biorefinery system: Recent advances and the way towards sustainability. Renew. Sustain. Energy Rev. 2017, 76, 493–506. [Google Scholar] [CrossRef]
- Zhou, W.; Chen, P.; Min, M.; Ma, X.; Wang, J.; Griffith, R.; Hussain, F.; Peng, P.; Xie, Q.; Li, Y.; et al. Environment-enhancing algal biofuel production using wastewaters. Renew. Sustain. Energy Rev. 2014, 36, 256–269. [Google Scholar] [CrossRef]
- Maroneze, M.M.; Barin, J.S.; De Menezes, C.R.; Queiroz, M.I.; Queiroz, L.; Jacob-lopes, E. Treatment of cattle-slaughterhouse wastewater and the reuse of sludge for biodiesel production by microalgal heterotrophic bioreactors. Sci. Agric. 2014, 4, 521–524. [Google Scholar] [CrossRef] [Green Version]
- Napan, K.; Teng, L.; Quinn, J.C.; Wood, B.D. Impact of heavy metals from flue gas integration with microalgae production. Algal Res. 2015, 8, 83–88. [Google Scholar] [CrossRef] [Green Version]
- Napan, K.; Kumarasamy, K.; Quinn, J.C.; Wood, B. Contamination levels in biomass and spent media from algal cultivation system contaminated with heavy metals. Algal Res. 2016, 19, 39–47. [Google Scholar] [CrossRef] [Green Version]
- Guédon, D.; Brum, M.; Seigneuret, J.M.; Bizet, D.; Bizot, S.; Bourny, E.; Compagnon, P.A.; Kergosien, H.; Quintelas, L.G.; Respaud, J.; et al. Impurities in herbal substances, herbal preparations and herbal medicinal products, IV. Heavy (Toxic) Met. 2008, 3, 2107–2122. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, A.; Marques, P.; Ribeiro, B.; Assemany, P.; de Mendonça, H.V.; Barata, A.; Oliveira, A.C.; Reis, A.; Pinheiro, H.M.; Gouveia, L. Combining biotechnology with circular bioeconomy: From poultry, swine, cattle, brewery, dairy and urban wastewaters to biohydrogen. Environ. Res. 2018, 164, 32–38. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, A.; Ribeiro, B.; Ferreira, A.F.; Tavares, M.L.A.; Vladic, J.; Vidović, S.; Cvetkovic, D.; Melkonyan, L.; Avetisova, G.; Goginyan, V.; et al. Scenedesmus obliquus microalga-based biorefinery—From brewery effluent to bioactive compounds, biofuels and biofertilizers—Aiming at a circular bioeconomy. Biofuels Bioprod. Biorefining 2019, 13, 1169–1186. [Google Scholar] [CrossRef]
- Zhang, L.; Pei, H.; Yang, Z.; Wang, X.; Chen, S.; Li, Y.; Xie, Z. Microalgae nourished by mariculture wastewater aids aquaculture self-reliance with desirable biochemical composition. Bioresour. Technol. 2019, 278, 205–213. [Google Scholar] [CrossRef]
- Oliveira, A.C.; Barata, A.; Batista, A.P.; Gouveia, L. Scenedesmus obliquus in poultry wastewater bioremediation. Environ. Technol. 2019, 40, 3735–3744. [Google Scholar] [CrossRef] [Green Version]
- Gouveia, L.; Graça, S.; Sousa, C.; Ambrosano, L.; Ribeiro, B.; Botrel, E.P.; Neto, P.C.; Ferreira, A.F.; Silva, C.M. Microalgae biomass production using wastewater: Treatment and costs: Scale-up considerations. Algal Res. 2016, 16, 167–176. [Google Scholar] [CrossRef]
- Batista, A.P.; Ambrosano, L.; Graça, S.; Sousa, C.; Marques, P.A.S.S.; Ribeiro, B.; Botrel, E.P.; Neto, P.C.; Gouveia, L. Combining urban wastewater treatment with biohydrogen production—An integrated microalgae-based approach. Bioresour. Technol. 2015, 184, 230–235. [Google Scholar] [CrossRef] [Green Version]
- Posadas, E.; Alcántara, C.; García-Encina, P.A.; Gouveia, L.; Guieysse, B.; Norvill, Z.; Acién, F.G.; Markou, G.; Congestri, R.; Koreiviene, J.; et al. Microalgae cultivation in wastewater. In Microalgae-Based Biofuels and Bioproducts; Gonzalez-Fernandez, C., Muñoz, R., Eds.; Woodhead Publishing: Lincolnshire, IL, USA, 2017; pp. 67–91. [Google Scholar]
- Acién, F.G.; Gómez-Serrano, C.; Morales-Amaral, M.M.; Fernández-Sevilla, J.M.; Molina-Grima, E. Wastewater treatment using microalgae: How realistic a contribution might it be to significant urban wastewater treatment? Appl. Microbiol. Biotechnol. 2016, 100, 9013–9022. [Google Scholar] [CrossRef]
- Markou, G.; Iconomou, D.; Muylaert, K. Applying raw poultry litter leachate for the cultivation of Arthrospira platensis and Chlorella vulgaris. Algal Res. 2016, 13, 79–84. [Google Scholar] [CrossRef]
- Oliveira, C.Y.B.; Oliveira, C.D.L.; Prasad, R.; Ong, H.C.; Araujo, E.S.; Shabnam, N.; Gálvez, A.O. A multidisciplinary review of Tetradesmus obliquus: A microalga suitable for large-scale biomass production and emerging environmental applications. Rev. Aquac. 2021, 13, 1594–1618. [Google Scholar] [CrossRef]
- Ferreira, A.F.; Ferreira, A.; Dias, A.P.S.; Gouveia, L. Pyrolysis of Scenedesmus obliquus biomass following the treatment of different wastewaters. Bioenergy Res. 2020, 13, 896–906. [Google Scholar] [CrossRef]
- Fredriksson, S.; Elwinger, K.; Pickova, J. Fatty acid and carotenoid composition of egg yolk as an effect of microalgae addition to feed formula for laying hens. Food Chem. 2006, 3, 530–537. [Google Scholar] [CrossRef]
- Saeid, A.; Chojnacka, K.; Opaliński, S.; Korczyński, M. Biomass of Spirulina maxima enriched by biosorption process as a new feed supplement for laying hens. Algal Res. 2016, 19, 342–347. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, J.; Sommerfeld, M. Biofertilizer and biostimulant properties of the microalga Acutodesmus dimorphus. J. Appl. Phycol. 2016, 28, 1051–1061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, R.; Peng, B.; Huang, K. The research progress of CO2 sequestration by algal bio-fertilizer in China. J. CO2 Util. 2015, 11, 67–70. [Google Scholar] [CrossRef]
- Ferreira, A.; Melkonyan, L.; Carapinha, S.; Ribeiro, B.; Figueiredo, D.; Avetisova, G.; Gouveia, L. Biostimulant and biopesticide potential of microalgae growing in piggery wastewater. Environ. Adv. 2021, 4, 100062. [Google Scholar] [CrossRef]
- Viegas, C.; Gouveia, L.; Gonçalves, M. Aquaculture wastewater treatment through microalgal. Biomass potential applications on animal feed, agriculture, and energy. J. Environ. Manag. 2021, 286, 112187. [Google Scholar] [CrossRef]
- Viegas, C.; Gouveia, L.; Gonçalves, M. Evaluation of microalgae as bioremediation agent for poultry effluent and biostimulant for germination. Environ. Technol. Innov. 2021, 24, 102048. [Google Scholar] [CrossRef]
- Ranglová, K.; Lakatos, G.E.; Câmara Manoel, J.A.; Grivalský, T.; Suárez Estrella, F.; Acién Fernández, F.G.; Molnár, Z.; Ördög, V.; Masojídek, J. Growth, biostimulant and biopesticide activity of the MACC-1 Chlorella strain cultivated outdoors in inorganic medium and wastewater. Algal Res. 2021, 53, 102136. [Google Scholar] [CrossRef]
- Navarro-López, E.; Ruíz-Nieto, A.; Ferreira, A.; Gabriel Acién, F.; Gouveia, L. Biostimulant potential of Scenedesmus obliquus grown in brewery wastewater. Molecules 2020, 25, 664. [Google Scholar] [CrossRef] [Green Version]
- Navarro-López, E.; Cerón-García, M. del C.; López-Rodríguez, M.; Acién-Fernández, F.G.; Molina-Grima, E. Biostimulants obtained after pilot-scale high-pressure homogenization of Scenedesmus sp. grown in pig manure. Algal Res. 2020, 52, 102123. [Google Scholar] [CrossRef]
- Morales-Jiménez, M.; Gouveia, L.; Yáñez-Fernández, J.; Castro-Muñoz, R.; Barragán-Huerta, B.E. Production, Preparation and Characterization Microalgae-Based Biopolymer as a Potential Bioactive Film. Coatings 2020, 10, 120. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, A.; Figueiredo, D.; Ferreira, F.; Ribeiro, B.; Reis, A.; da Silva, T.L.; Gouveia, L. Impact of high-pressure homogenization on the cell integrity of Tetradesmus obliquus and seed germination. Molecules 2022, 27, 2275. [Google Scholar] [CrossRef]
- Machmudah, S.; Wahyudiono; Kanda, H.; Goto, M. Hydrolysis of biopolymers in near-critical and subcritical water. In Water Extraction of Bioactive Compounds; Elsevier: Amsterdam, The Netherlands, 2017; pp. 69–107. [Google Scholar]
- Plaza, M.; Turner, C. Pressurized hot water extraction of bioactives. TrAC Trends Anal. Chem. 2015, 71, 39–54. [Google Scholar] [CrossRef] [Green Version]
- Vladić, J.; Jakovljević, M.; Molnar, M.; Vidović, S.; Tomić, M.; Drinić, Z.; Jokić, S. Valorization of yarrow (Achillea millefolium L.) by-product through application of subcritical water extraction. Molecules 2020, 25, 1878. [Google Scholar] [CrossRef] [Green Version]
- Essien, S.O.; Young, B.; Baroutian, S. Recent advances in subcritical water and supercritical carbon dioxide extraction of bioactive compounds from plant materials. Trends Food Sci. Technol. 2020, 97, 156–169. [Google Scholar] [CrossRef]
- Vladić, J.; Canli, O.; Pavlić, B.; Zeković, Z.; Vidović, S.; Kaplan, M. Optimization of Satureja montana subcritical water extraction process and chemical characterization of volatile fraction of extracts. J. Supercrit. Fluids 2017, 120, 86–94. [Google Scholar] [CrossRef]
- Jay, J.; Loessner, M.; Golden, D. Food Protection with High Temperatures, and Characteristics of Thermophilic Microorganisms. In Modern Food Microbiology; Springer: Boston, MA, USA, 2005; pp. 415–441. [Google Scholar]
- Heubeck, S.; Craggs, R.J.; Shilton, A. Influence of CO2 scrubbing from biogas on the treatment performance of a high rate algal pond. Water Sci. Technol. 2007, 55, 193–200. [Google Scholar] [CrossRef]
- European Commission. Council Directive of 21 May 1991 concerning urban waste water treatment (91/271/EEC). Off. J. Eur. Communities 1991, 34, 40–52. [Google Scholar]
- European Commission. Commission Implementing Decision (EU) 2016/902 for common waste water and waste gas treatment/management systems in the chemical sector. Off. J. Eur. Union 2016, 75, 1–20. [Google Scholar]
- Wiyarno, Y.; Widyastuti, S. Isolation and identification odorous chemical markers of wastewater poultry slaughterhouse. Procedia Environ. Sci. 2015, 23, 400–406. [Google Scholar] [CrossRef] [Green Version]
- Njoya, M.; Basitere, M.; Ntwampe, S.K.O. Analysis of the characteristics of poultry slaughterhouse wastewater (PSW) and its treatability. Water Pract. Technol. 2019, 14, 959–970. [Google Scholar] [CrossRef]
- Chalima, A.; Oliver, L.; De Castro, L.F.; Karnaouri, A.; Dietrich, T.; Topakas, E. Utilization of volatile fatty acids from microalgae for the production of high added value compounds. Fermentation 2017, 3, 54. [Google Scholar] [CrossRef] [Green Version]
- Al-Jabri, H.; Das, P.; Khan, S.; Thaher, M.; Abdulquadir, M. Treatment of wastewaters by microalgae and the potential applications of the produced biomass—a review. Water 2020, 13, 27. [Google Scholar] [CrossRef]
- Chan, S.S.; Khoo, K.S.; Chew, K.W.; Ling, T.C.; Show, P.L. Recent advances biodegradation and biosorption of organic compounds from wastewater: Microalgae-bacteria consortium—A review. Bioresour. Technol. 2022, 344, 126159. [Google Scholar] [CrossRef]
- Vahabisani, A.; An, C. Use of biomass-derived adsorbents for the removal of petroleum pollutants from water: A mini-review. Environ. Syst. Res. 2021, 10, 25. [Google Scholar] [CrossRef]
- Marques, I.M.; Oliveira, A.C.V.; de Oliveira, O.M.C.; Sales, E.A.; Moreira, Í.T.A. A photobioreactor using Nannochloropsis oculata marine microalgae for removal of polycyclic aromatic hydrocarbons and sorption of metals in produced water. Chemosphere 2021, 281, 130775. [Google Scholar] [CrossRef]
- Zhang, C.; Lu, J.; Wu, J. Adsorptive removal of polycyclic aromatic hydrocarbons by detritus of green tide algae deposited in coastal sediment. Sci. Total Environ. 2019, 670, 320–327. [Google Scholar] [CrossRef]
- Gouveia, L.; Molnar Jazić, J.; Ferreira, A.; Maletić, S.; Cvetković, D.; Vidović, S.; Vladić, J. Green approach for the valorization of microalgae Tetradesmus obliquus. Sustain. Chem. Pharm. 2021, 24, 100556. [Google Scholar] [CrossRef]
- Ferreira, A.; Jazić, J.M.; Gouveia, L.; Maletić, S.; Tomić, M.; Agbaba, J.; Vladic, J. Valorisation of microalga Tetradesmus obliquus grown in brewery wastewater using subcritical water extraction towards zero waste. Chem. Eng. J. 2022, 437, 135324. [Google Scholar] [CrossRef]
- Tomšik, A.; Pavlić, B.; Vladić, J.; Cindrić, M.; Jovanov, P.; Sakač, M.; Mandić, A.; Vidović, S. Subcritical water extraction of wild garlic (Allium ursinum L.) and process optimization by response surface methodology. J. Supercrit. Fluids 2017, 128, 79–88. [Google Scholar] [CrossRef]
- Güneş, F.E. Medical Use of Squalene as a Natural Antioxidant. J. Marmara Univ. Inst. Health Sci. 2013, 3, 3. [Google Scholar]
- Sánchez-Quesada, C.; López-Biedma, A.; Toledo, E.; Gaforio, J.J. Squalene stimulates a key innate immune cell to foster wound healing and tissue repair. Evid. Based. Complement. Alternat. Med. 2018, 2018, 9473094. [Google Scholar] [CrossRef]
- Narayan Bhilwade, H.; Tatewaki, N.; Nishida, H.; Konishi, T. Squalene as novel food factor. Curr. Pharm. Biotechnol. 2010, 11, 875–880. [Google Scholar] [CrossRef]
- Kelly, G.S. Squalene and its potential clinical uses. Altern. Med. Rev. 1999, 4, 29–36. [Google Scholar]
- Lou-Bonafonte, J.M.; Martínez-Beamonte, R.; Sanclemente, T.; Surra, J.C.; Herrera-Marcos, L.V.; Sanchez-Marco, J.; Arnal, C.; Osada, J. Current insights into the biological action of squalene. Mol. Nutr. Food Res. 2018, 62, 1800136. [Google Scholar] [CrossRef]
- Lozano-Grande, M.A.; Gorinstein, S.; Espitia-Rangel, E.; Dávila-Ortiz, G.; Martínez-Ayala, A.L. Plant Sources, extraction methods, and uses of squalene. Int. J. Agron. 2018, 2018, 1829160. [Google Scholar] [CrossRef] [Green Version]
- Gohil, N.; Bhattacharjee, G.; Khambhati, K.; Braddick, D.; Singh, V. Engineering strategies in microorganisms for the enhanced production of squalene: Advances, challenges and opportunities. Front. Bioeng. Biotechnol. 2019, 7, 50. [Google Scholar] [CrossRef] [Green Version]
- Zhao, F.; Wang, P.; Lucardi, R.D.; Su, Z.; Li, S. Natural sources and bioactivities of 2,4-di-tert-butylphenol and its analogs. Toxins 2020, 12, 35. [Google Scholar] [CrossRef] [Green Version]
- Varsha, K.K.; Devendra, L.; Shilpa, G.; Priya, S.; Pandey, A.; Nampoothiri, K.M. 2,4-Di-tert-butyl phenol as the antifungal, antioxidant bioactive purified from a newly isolated Lactococcus sp. Int. J. Food Microbiol. 2015, 211, 44–50. [Google Scholar] [CrossRef]
- Abdullah, A.S.H.; Mirghani, M.E.S.; Jamal, P. Antibacterial activity of Malaysian mango kernel. Afr. J. Biotechnol. 2011, 10, 18739–18748. [Google Scholar]
- Malek, S.N.A.; Shin, S.K.; Wahab, N.A.; Yaacob, H. Cytotoxic components of Pereskia bleo (Kunth) DC. (Cactaceae) leaves. Molecules 2009, 14, 1713–1724. [Google Scholar] [CrossRef]
- Rangel-Sánchez, G.; Castro-Mercado, E.; García-Pineda, E. Avocado roots treated with salicylic acid produce phenol-2,4-bis (1,1-dimethylethyl), a compound with antifungal activity. J. Plant Physiol. 2014, 171, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Wang, J.; Karthikeyan, S.; Liu, H.; Cai, J. Natural anti-phytopathogenic fungi compound phenol, 2, 4-bis (1, 1-dimethylethyl) from Pseudomonas fluorescens TL-1. Indian J. Biochem. Biophys. 2019, 56, 162–168. [Google Scholar]
- Huynh, L.H.; Tran Nguyen, P.L.; Ho, Q.P.; Ju, Y.H. Catalyst-free fatty acid methyl ester production from wet activated sludge under subcritical water and methanol condition. Bioresour. Technol. 2012, 123, 112–116. [Google Scholar] [CrossRef]
- Saeed, N.M.; El-Demerdash, E.; Abdel-Rahman, H.M.; Algandaby, M.M.; Al-Abbasi, F.A.; Abdel-Naim, A.B. Anti-inflammatory activity of methyl palmitate and ethyl palmitate in different experimental rat models. Toxicol. Appl. Pharmacol. 2012, 264, 84–93. [Google Scholar] [CrossRef]
- El-Demerdash, E. Anti-inflammatory and antifibrotic effects of methyl palmitate. Toxicol. Appl. Pharmacol. 2011, 254, 238–244. [Google Scholar] [CrossRef]
- Kumar, P.P.; Kumaravel, S.; Lalitha, C. Screening of antioxidant activity, total phenolics and GC-MS study of Vitex negundo. Afr. J. Biochem. Res. 2010, 4, 191–195. [Google Scholar]
- Yu, F.R.; Lian, X.Z.; Guo, H.Y.; McGuire, P.M.; De Li, R.; Wang, R.; Yu, F.H. Isolation and characterization of methyl esters and derivatives from Euphorbia kansui (Euphorbiaceae) and their inhibitory effects on the human SGC-7901 cells. J. Pharm. Pharm. Sci. 2005, 8, 528–535. [Google Scholar]
- Directive 2002/32/EC of The European Parliament and of the Council of 7 May 2002 on Undesirable Substances in Animal Feed; European Parliament and the Council of the EU: Strasbourg, France, 2002; pp. 1–15. Available online: https://www.legislation.gov.uk/eudr/2002/32 (accessed on 1 February 2022).
- Council of the European Communities Protection of the Environment, and in particular of the soil, when sewage sludge is used in agriculture. Off. J. Eur. Communities 1986, 4, 6–12.
- AWWA-APHA-WEF. Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association/American Water Works Association/Water Environment Federation: Washington, DC, USA, 2012. [Google Scholar]
- ISO 6878:2004; Water Quality—Determination of Phosphorus—Ammonium Molybdate Spectrometric Method. International Organization for Standardization: Geneva, Switzerland, 2004.
- Kähkönen, M.P.; Hopia, A.I.; Vuorela, H.J.; Rauha, J.P.; Pihlaja, K.; Kujala, T.S.; Heinonen, M. Antioxidant activity of plant extracts containing phenolic compounds. J. Agric. Food Chem. 1999, 47, 3954–3962. [Google Scholar] [CrossRef]
- Markham, K.R. Flavones, Flavonols and their Glycosides. In Methods in Plant Biochemistry; Harborne, J.B., Ed.; Elsevier, Academic Press: San Diego, CA, USA, 1989; Volume 1, pp. 197–235. [Google Scholar]
- Espín, J.C.; Soler-Rivas, C.; Wichers, H.J. Characterization of the total free radical scavenger capacity of vegetable oils and oil fractions using 2,2-diphenyl-1-picrylhydrazyl radical. J. Agric. Food Chem. 2000, 48, 648–656. [Google Scholar] [CrossRef]
- USEPA. EPA Method 9071B: N-Hexane Extractable Material (HEM) for Sludge, Sediment, and Solid Samples, Revision 2.0; U.S. Environmental Protection Agency: Washington, DC, USA, 1998; pp. 1–13. [Google Scholar]
- Gani, P.; Sunar, N.M.; Matias-Peralta, H.; Mohamed, R.M.S.R.; Latiff, A.A.A.; Parjo, U.K. Extraction of hydrocarbons from freshwater green microalgae (Botryococcus sp.) biomass after phycoremediation of domestic wastewater. Int. J. Phytoremediation 2017, 19, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Sunar, N.M.; Fatin, N.; Abdullah, N.; Gani, P.A.; Matias, H.M. FMC Potential of biofuel from extraction of microalgae biomass via domestic wastewater phycoremediation. Fuel Mix. Form. Combust. Process 2019, 1, 1–6. [Google Scholar]
- USEPA. EPA Method 3550B: Ultrasonic Extraction, Revision 2.0; U.S. Environmental Protection Agency: Washington, DC, USA, 1996; Volume 52, pp. 13837–13866. [Google Scholar]
- USEPA. EPA Method 3051A: Microwave Assisted Acid Digestion of Sediments, Sludges, Soils, and Oils, Revision 1; U.S. Environmental Protection Agency: Washington, DC, USA, 2007; pp. 119–122. [Google Scholar]
- ISO 4833-1:2013; Microbiology of the Food Chain—Horizontal method for the enumeration of microorganisms—part 1: Colony count at 30 °C by the pour plate technique. International Organization for Standardization: Geneva, Switzerland, 2013.
- ISO 21527-1:2008; Microbiology of food and animal feeding stuffs—Horizontal method for the enumeration of yeasts and moulds—part 1: Colony count technique in products with water activity greater than 0.95. International Organization for Standardization: Geneva, Switzerland, 2008.
- ISO 21528-2:2017; Microbiology of the food chain—Horizontal method for the detection and enumeration of enterobacteriaceae—part 2: Colony-count technique. International Organization for Standardization: Geneva, Switzerland, 2017.
- ISO 21528-2:2004; Microbiology of food and animal feeding stuffs—Horizontal method for the detection and enumeration of Enterobacteriaceae—part 2: Colony-count method. International Organization for Standardization: Geneva, Switzerland, 2004.
- ISO 16649-2:2001; Microbiology of food and animal feeding stuffs—Horizontal method for the enumeration of beta-glucuronidase-positive Escherichia coli—part 2: Colony-count technique at 44 °C using 5-bromo-4-chloro-3-indolyl beta-d-glucur. International Organization for Standardization: Geneva, Switzerland, 2001.

| Poultry WW | Treated Water | Removal (%) | |
|---|---|---|---|
| COD (mg O2/L) | 1950 | 268 1 | 84 |
| TKN (mg N/L) | 277 | 63.8 1 | 77 |
| PO43− (mg P/L) | 22.7 | 11.1 1 | 50 |
| Metals (µg/L) | |||
| Cr | 20.2 | 6.9 | 66 |
| Mn | 566 | 161 | 72 |
| Fe | 4040 | 511 | 87 |
| Co | <1 | <1 | - |
| Ni | 21.3 | 11.0 | 48 |
| Cu | 160 | 35 | 78 |
| Zn | 1140 | 128 | 89 |
| As | <1 | <1 | - |
| Cd | <0.5 | <0.5 | - |
| Pb | 7.83 | 3.98 | 49 |
| TP (mg GAE/mL) | TF (mg CE/mL) | IC50 (µg/mL) |
|---|---|---|
| 1.073 ± 0.015 | 0.111 ± 0.002 | 7.18 ± 0.34 |
| Metal | T. obliquus | ||
|---|---|---|---|
| Whole Biomass (mg/kg) | Extract (µg/L) | Residue (mg/kg) | |
| Cr | 16.2 | 145 | 16.14 |
| Mn | 88.3 | 369 | 90.74 |
| Fe | 2070 | 7890 | 1949.26 |
| Co | <1 | <1 | <0.5 |
| Ni | 14.8 | 155 | 21.05 |
| Cu | 91.9 | 201 | 79.82 |
| Zn | 3530 | 587 | 760.12 |
| As | <1 | <1 | <0.5 |
| Cd | 0.58 | <0.5 | <0.25 |
| Pb | 2.30 | 70.7 | 4.73 |
| T. obliquus | TAMC 1 | Molds and Yeasts | Enterobacteriaceae | E. coli | SAMB 2 |
|---|---|---|---|---|---|
| Whole biomass (cfu/g) | 190 × 105 | 300 (molds) <10 (yeasts) | <10 | <10 | 12 × 102 |
| Extract (cfu/mL) | <1 | <1 | <1 | <1 | <1 |
| Residue (cfu/g) | <10 | <10 | <10 | <10 | <10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vladić, J.; Jazić, J.M.; Ferreira, A.; Maletić, S.; Cvetković, D.; Agbaba, J.; Vidović, S.; Gouveia, L. Application of Green Technology to Extract Clean and Safe Bioactive Compounds from Tetradesmus obliquus Biomass Grown in Poultry Wastewater. Molecules 2023, 28, 2397. https://doi.org/10.3390/molecules28052397
Vladić J, Jazić JM, Ferreira A, Maletić S, Cvetković D, Agbaba J, Vidović S, Gouveia L. Application of Green Technology to Extract Clean and Safe Bioactive Compounds from Tetradesmus obliquus Biomass Grown in Poultry Wastewater. Molecules. 2023; 28(5):2397. https://doi.org/10.3390/molecules28052397
Chicago/Turabian StyleVladić, Jelena, Jelena Molnar Jazić, Alice Ferreira, Snežana Maletić, Dragoljub Cvetković, Jasmina Agbaba, Senka Vidović, and Luisa Gouveia. 2023. "Application of Green Technology to Extract Clean and Safe Bioactive Compounds from Tetradesmus obliquus Biomass Grown in Poultry Wastewater" Molecules 28, no. 5: 2397. https://doi.org/10.3390/molecules28052397