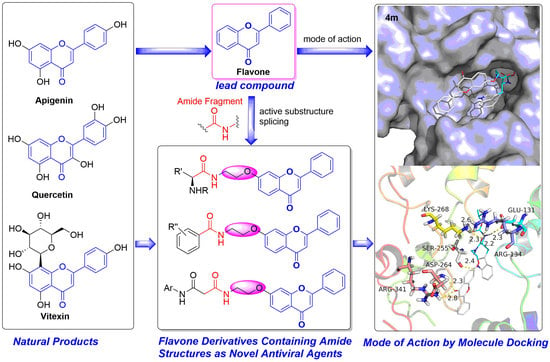
Discovery of Flavone Derivatives Containing Carboxamide Fragments as Novel Antiviral Agents
Abstract
:1. Introduction
2. Results
2.1. Chemistry
2.2. Phytotoxic Activity
2.3. Antiviral Activity
In Vivo Anti-TMV Activity
2.4. Mode of Action Studies
Docking Studies
3. Discussion
3.1. Synthesis
3.2. Structure–Activity Relationship of the Antiviral Activity
3.3. Study on the Mechanism of Anti-TMV Activity
Molecular Docking Study
4. Materials and Methods
4.1. Synthetic Procedures
4.1.1. Reagents and Instruments
4.1.2. Synthesis of Compounds 2a–2f
- 7-Butoxy-2-phenyl-4H-chromen-4-one (2a). Light yellow solid, 92.5% yield, m.p. 82–85 °C; 1H NMR (400 MHz, CDCl3) δ 8.13 (d, J = 8.7 Hz, 1H), 8.01–7.83 (m, 2H), 7.59–7.45 (m, 3H), 6.98 (d, J = 11.1 Hz, 2H), 6.77 (s, 1H), 4.10 (s, 2H), 1.84 (d, J = 6.5 Hz, 2H), 1.53 (h, J = 7.3 Hz, 2H), 1.01 (t, J = 7.4 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 177.9, 163.8, 163.0, 158.0, 131.9, 131.4, 129.0, 127.0, 126.2, 117.7, 114.8, 107.5, 100.9, 68.5, 31.0, 19.2, 13.8; HR-MS (ESI): calcd for C19H18O3 [M + H]+ 295.1329, found (ESI+) 295.1336.
- 2-Phenyl-7-(prop-2-yn-1-yloxy)-4H-chromen-4-one (2b). Light brown solid, 95.0% yield, m.p. 164–169 °C; 1H NMR (400 MHz, CDCl3) δ 8.20 (d, J = 8.8 Hz, 1H), 7.97–7.91 (m, 2H), 7.59–7.52 (m, 3H), 7.14–7.05 (m, 2H), 6.81 (s, 1H), 4.86 (s, 2H), 2.65 (s, 1H); 13C NMR (100 MHz, CDCl3) δ 177.8, 163.2, 161.9, 157.7, 131.8, 131.5, 129.0, 127.2, 126.2, 118.5, 114.7, 107.6, 101.8, 77.4, 76.6, 56.2; HR-MS (ESI): calcd for C18H12O3 [M + H]+ 277.0859, found (ESI+) 277.0862.
- 7-(Benzyloxy)-2-phenyl-4H-chromen-4-one (2c). White solid, 95% yield, m.p. 173–180 °C; 1H NMR (400 MHz, CDCl3) δ 8.16 (d, J = 8.6 Hz, 1H), 7.96–7.88 (m, 2H), 7.52 (d, J = 5.7 Hz, 3H), 7.43–7.48 (m, 5H), 7.07 (d, J = 9.7 Hz, 2H), 6.78 (s, 1H), 5.20 (s, 2H); 13C NMR (100 MHz, CDCl3) δ 177.9, 163.4, 163.2, 158.0, 135.8, 131.9, 131.5, 129.1, 128.8, 128.5, 127.6, 127.2, 126.3, 118.0, 115.0, 107.6, 101.6, 70.6; HR-MS (ESI): calcd for C22H17O3 [M + H]+ 329.1172, found (ESI+) 329.1176.
- Ethyl 2-((4-oxo-2-phenyl-4H-chromen-7-yl)oxy)acetate (2d). Light brown solid, 85.3% yield, m.p. 89–93 °C; 1H NMR (400 MHz, CDCl3) δ 8.16 (d, J = 8.8 Hz, 1H), 7.97–7.71 (m, 2H), 7.52 (d, J = 5.5 Hz, 3H), 7.08–6.98 (m, 1H), 6.99–6.89 (m, 1H), 6.77 (s, 1H), 4.75 (s, 2H), 4.31 (q, J = 7.1 Hz, 2H), 1.32 (t, J = 7.1 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 177.8, 168.0, 163.3, 162.2, 157.8, 131.8, 131.6, 129.1, 127.5, 126.2, 118.7, 114.3, 107.7, 101.7, 65.1, 61.8, 14.2; HR-MS (ESI): calcd for C19H16O5 [M + H]+ 325.1071, found (ESI+) 325.1069.
- Tert-butyl(3-((4-oxo-2-phenyl-4H-chromen-7-yl)oxy)propyl)carbamate (2e). White solid, 90.2% yield, m.p. 144–150 °C; 1H NMR (400 MHz, CDCl3) δ 8.13 (d, J = 9.0 Hz, 1H), 7.92 (d, J = 6.4 Hz, 2H), 7.53 (s, 3H), 6.99 (d, J = 7.7 Hz, 2H), 6.86 (s, 1H), 4.15 (t, J = 5.6 Hz, 2H), 3.36 (s, 2H), 2.11–2.01 (m, 2H), 1.45 (s, 9H); 13C NMR (100 MHz, CDCl3) δ 177.9, 163.4, 163.0, 157.9, 156.1, 131.8, 131.4, 129.0, 127.0, 126.2, 117.8, 114.7, 107.5, 100.9, 79.3, 66.4, 37.7, 29.5, 28.4; HR-MS (ESI): calcd for C23H25NO5 [M + H]+ 396.1806, found (ESI+) 396.1802.
- Tert-butyl (2-((4-oxo-2-phenyl-4H-chromen-7-yl)oxy)ethyl)carbamate (2f). White solid, 98.0% yield, m.p. 129–132 °C; 1H NMR (400 MHz, CDCl3) δ 8.13 (d, J = 8.4 Hz, 1H), 7.93–7.87 (m, 2H), 7.52 (d, J = 4.9 Hz, 3H), 6.98 (d, J = 9.0 Hz, 2H), 6.76 (s, 1H), 5.02 (s, 1H), 4.16 (s, 2H), 3.60 (s, 2H), 1.46 (s, 9H); 13C NMR (100 MHz, CDCl3) δ 177.8, 163.1, 163.1, 157.9, 155.9, 131.7, 131.5, 129.0, 127.1, 126.2, 118.0, 114.7, 107.5, 101.0, 79.8, 67.8, 39.9, 28.4; HR-MS (ESI): calcd for C19H16O5 [M + H]+ 382.1649, found (ESI+) 382.1648.
4.1.3. Synthesis of Compounds 4a–4n, 5a–5e, and 6a–6d
- 7-(2-Aminoethoxy)-2-phenyl-4H-chromen-4-one (3). A solution of compound 2f (0.381 g, 1.0 mmol, 1.0 equiv.) in CH2Cl2 (3 mL) was added to CF3COOH (TFA, 3 mL) and stirred at room temperature for 2 h. After the completion of the reaction, the solvent was removed in vacuum, and the crude product was purified by flash chromatography to obtain crude product 3·CF3COOH. The crude product was washed by saturated NaHCO3 solution to obtain compound 3. White solid, 98% yield; 1H NMR (400 MHz, DMSO-d6) δ 8.10 (d, J = 6.7 Hz, 2H), 7.94 (d, J = 8.8 Hz, 1H), 7.59 (d, J = 6.4 Hz, 3H), 7.32 (s, 1H), 7.07 (d, J = 8.4 Hz, 1H), 6.96 (s, 1H), 4.09 (t, J = 5.4 Hz, 2H), 3.17 (s, 2H), 2.94 (s, 2H); 13C NMR (100 MHz, DMSO-d6) δ 176.9, 163.2, 162.7, 157.9, 132.2, 129.6, 126.8, 126.7, 117.9, 115.6, 107.3, 102.1, 68.0.
- Isopropyl(2-oxo-2-((2-((4-oxo-2-phenyl-4H-chromen-7-yl)oxy)ethyl)amino)ethyl)carbamate (4a). White solid, 56.7% yield, m.p. 143–146 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.09 (d, J = 7.8 Hz, 3H), 7.94 (d, J = 8.8 Hz, 1H), 7.58 (d, J = 6.7 Hz, 3H), 7.34 (s, 1H), 7.17 (t, J = 5.4 Hz, 1H), 7.06 (d, J = 8.8 Hz, 1H), 6.97 (s, 1H), 4.83–4.63 (m, 1H), 4.17 (t, J = 4.9 Hz, 2H), 3.60 (d, J = 5.8 Hz, 2H), 3.51 (d, J = 5.3 Hz, 2H), 1.16 (d, J = 6.1 Hz, 6H); 13C NMR (100 MHz, DMSO-d6) δ 176.9, 170.2, 163.5, 162.7, 157.9, 156.7, 132.2, 131.7, 129.6, 126.7, 117.7, 115.5, 107.3, 102.1, 67.7, 67.5, 43.9, 38.5, 22.5; HR-MS (ESI): calcd for C23H24N2O6 [M + H]+ 425.1707, found (ESI+) 425.1704.
- Isobutyl(2-oxo-2-((2-((4-oxo-2-phenyl-4H-chromen-7-yl)oxy)ethyl)amino)ethyl)carbamate (4b). White solid, 53.5% yield, m.p. 141–143 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.10 (d, J = 7.3 Hz, 3H), 7.95 (d, J = 8.8 Hz, 1H), 7.59 (d, J = 6.6 Hz, 3H), 7.35 (s, 1H), 7.28 (t, J = 5.8 Hz, 1H), 7.07 (d, J = 8.7 Hz, 1H), 6.98 (s, 1H), 4.17 (t, J = 4.7 Hz, 2H), 3.72 (d, J = 6.5 Hz, 2H), 3.60 (d, J = 5.7 Hz, 2H), 3.51 (d, J = 5.1 Hz, 2H), 1.82 (dt, J = 13.6, 6.7 Hz, 1H), 0.87 (d, J = 6.5 Hz, 6H); 13C NMR (100 MHz, DMSO-d6) δ 177.0, 170.2, 163.5, 162.8, 157.9, 157.3, 129.6, 126.8, 117.7, 115.6, 107.3, 102.1, 70.5, 67.7, 43.9, 38.5, 28.1, 19.4; HR-MS (ESI): calcd for C24H26N2O6 [M + H]+ 439.1864, found (ESI+) 439.1898.
- Tert-butyl(2-oxo-2-((2-((4-oxo-2-phenyl-4H-chromen-7-yl)oxy)ethyl)amino)ethyl)carbamate (4c). White solid, 43.5% yield, m.p. 151–153 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.09 (t, J = 8.0 Hz, 3H), 7.95 (d, J = 8.8 Hz, 1H), 7.59 (d, J = 6.5 Hz, 3H), 7.35 (s, 1H), 7.07 (d, J = 8.6 Hz, 1H), 6.97 (d, J = 5.4 Hz, 2H), 4.17 (s, 2H), 3.53 (dd, J = 15.0, 5.5 Hz, 4H), 1.37 (s, 9H); 13C NMR (100 MHz, DMSO-d6) δ 177.0, 170.3, 163.5, 162.7, 158.0, 156.3, 132.2, 131.6, 129.6, 126.7, 117.7, 115.6, 107.2, 102.0, 78.6, 67.7, 43.7, 38.5, 28.7; HR-MS (ESI): calcd for C24H26N2O6 [M + H]+ 439.1864, found (ESI+) 439.1893.
- (S)-Isopropyl(1-oxo-1-((2-((4-oxo-2-phenyl-4H-chromen-7-yl)oxy)ethyl)amino)propan-2-yl)carbamate (4d). White solid, 37.5% yield, m.p. 134–136 °C; = +0.6° (c 1.0, CH3OH); 1H NMR (400 MHz, DMSO-d6) δ 8.08 (d, J = 6.8 Hz, 3H), 7.94 (d, J = 8.7 Hz, 1H), 7.58 (d, J = 6.4 Hz, 3H), 7.32 (s, 1H), 7.13 (d, J = 6.9 Hz, 1H), 7.05 (d, J = 8.8 Hz, 1H), 6.96 (s, 1H), 4.87 –4.54 (m, 1H), 4.16 (s, 2H), 4.10–3.94 (m, 1H), 3.61–3.43 (m, 2H), 1.18 (d, J = 7.0 Hz, 3H), 1.14 (d, J = 5.7 Hz, 6H); 13C NMR (100 MHz, DMSO-d6) δ 176.9, 173.6, 163.5, 162.7, 157.9, 155.9, 132.1, 131.6, 129.5, 126.7, 126.6, 117.7, 115.5, 107.2, 102.0, 67.6, 67.4, 50.4, 38.5, 22.5, 18.7; HR-MS (ESI): calcd for C24H26N2O6 [M + H]+ 439.1864, found (ESI+) 439.1891.
- (S)-Isopropyl(3-methyl-1-oxo-1-((2-((4-oxo-2-phenyl-4H-chromen-7-yl)oxy)ethyl)amino) butan-2-yl) carbamate (4e). White solid, 41.3% yield, m.p. 164–168 °C; = +3.2° (c 1.0, CH3OH); 1H NMR (400 MHz, DMSO-d6) δ 8.17 (s, 1H), 8.10 (d, J = 6.9 Hz, 2H), 7.95 (d, J = 8.8 Hz, 1H), 7.59 (d, J = 6.4 Hz, 3H), 7.34 (s, 1H), 7.04 (d, J = 8.8 Hz, 1H), 6.97 (s, 1H), 6.92 (d, J = 8.4 Hz, 1H), 4.71 (p, J = 5.9 Hz, 1H), 4.17 (s, 2H), 3.81 (t, J = 7.6 Hz, 1H), 3.52 (ddd, J = 45.7, 14.0, 5.8 Hz, 2H), 2.01–1.81 (m, 1H), 1.14 (d, J = 5.1 Hz, 6H), 0.81 (d, 6H); 13C NMR (100 MHz, DMSO-d6) δ 176.4, 171.7, 163.0, 162.2, 157.4, 155.8, 131.6, 131.1, 129.1, 126.2, 117.2, 115.0, 106.7, 101.5, 66.9, 60.1, 37.9, 30.2, 22.0, 19.1, 18.2; HR-MS (ESI): calcd for C26H30N2O6 [M + H]+ 467.2177, found (ESI+) 467.2179.
- (S)-Isopropyl(4-methyl-1-oxo-1-((2-((4-oxo-2-phenyl-4H-chromen-7-yl)oxy)ethyl)amino) pentan-2-yl) carbamate (4f). White solid, 40.5% yield, m.p. 132–136 °C; = −7.6° (c 1.0, CH3OH); 1H NMR (400 MHz, DMSO-d6) δ 8.19–8.04 (m, 3H), 7.95 (d, J = 8.8 Hz, 1H), 7.59 (d, J = 6.3 Hz, 3H), 7.33 (s, 1H), 7.06 (t, J = 9.9 Hz, 2H), 6.97 (s, 1H), 4.70 (dt, J = 11.9, 5.9 Hz, 1H), 4.17 (s, 2H), 3.99 (d, J = 6.0 Hz, 1H), 3.49 (dd, J = 20.4, 3.9 Hz, 2H), 1.61–1.51 (m, 1H), 1.38 (dt, J = 14.0, 7.8 Hz, 2H), 1.13 (d, J = 5.7 Hz, 6H), 0.81 (t, J = 6.5 Hz, 6H); 13C NMR (100 MHz, DMSO-d6) δ 176.4, 172.9, 163.0, 162.2, 157.4, 155.6, 131.6, 131.1, 129.1, 126.2, 117.2, 115.0, 106.7, 101.5, 67.1, 66.9, 53.0, 40.8, 38.0, 24.2, 22.8, 21.5; HR-MS (ESI): calcd for C27H32N2O6 [M + H]+ 481.2333, found (ESI+) 481.2343.
- Isopropyl((2S,3S)-3-methyl-1-oxo-1-((2-((4-oxo-2-phenyl-4H-chromen-7-yl)oxy)ethyl) amino)pentan-2-yl)carbamate (4g). White solid, 37.5% yield, m.p. 180–187 °C; = +1.6° (c 1.0, CH3OH); 1H NMR (400 MHz, DMSO-d6) δ 8.18 (s, 1H), 8.10 (d, J = 6.8 Hz, 2H), 7.95 (d, J = 8.9 Hz, 1H), 7.59 (d, J = 6.4 Hz, 3H), 7.34 (s, 1H), 7.04 (d, J = 8.7 Hz, 1H), 7.00–6.89 (m, 2H), 4.71 (dt, J = 11.7, 5.8 Hz, 1H), 4.17 (s, 2H), 3.84 (t, J = 7.8 Hz, 1H), 3.51 (dd, J = 35.8, 4.9 Hz, 2H), 1.66 (s, 1H), 1.39 (s, 2H), 1.13 (d, J = 5.1 Hz, 6H), 0.82–0.74 (m, 6H); 13C NMR (100 MHz, DMSO-d6) δ 176.9, 172.2, 163.5, 162.7, 157.9, 156.2, 132.2, 131.6, 129.6, 126.7, 117.7, 115.5, 107.2, 102.0, 67.6, 67.4, 59.5, 38.4, 36.8, 24.9, 22.5, 15.7, 11.4; HR-MS (ESI): calcd for C27H32N2O6 [M + H]+ 481.2333, found (ESI+) 481.2338.
- (Isopropyl((2S,3R)-3-hydroxy-1-oxo-1-((2-((4-oxo-2-phenyl-4H-chromen-7-yl)oxy)ethyl) amino)butan-2-yl)carbamate (4h). White solid, 33.0% yield, m.p. 134–137 °C; = +4.5° (c 1.0, CH3OH); 1H NMR (400 MHz, DMSO-d6) δ 8.12 (d, J = 5.8 Hz, 3H), 7.97 (d, J = 8.8 Hz, 1H), 7.62 (s, 3H), 7.36 (s, 1H), 7.08 (d, J = 8.5 Hz, 1H), 6.99 (s, 1H), 6.58 (d, J = 7.4 Hz, 1H), 4.92–4.65 (m, 2H), 4.20 (s, 2H), 3.93 (d, J = 6.2 Hz, 2H), 3.54 (dd, J = 18.9, 5.0 Hz, 2H), 1.28–1.12 (m, 6H), 1.05 (d, J = 4.6 Hz, 3H); 13C NMR (100 MHz, DMSO-d6) δ 171.3, 163.5, 162.7, 156.3, 132.2, 131.7, 129.6, 126.7, 115.6, 107.3, 67.7, 67.7, 67.2, 61.1, 38.6, 22.5, 20.5; HR-MS (ESI): calcd for C25H28N2O7 [M + H]+ 469.1970, found (ESI+) 469.1977.
- (S)-Isopropyl(2-oxo-2-((2-((4-oxo-2-phenyl-4H-chromen-7-yl)oxy)ethyl)amino)-1-phenylethyl)carbamate (4i).White solid, 47.5% yield, m.p. 131–134 °C; = +17.0° (c 1.0, CH3OH); 1H NMR (400 MHz, DMSO-d6) δ 8.48 (s, 1H), 8.09 (s, 2H), 7.94 (d, J = 8.7 Hz, 1H), 7.60 (s, 4H), 7.41 (d, J = 7.0 Hz, 2H), 7.32–7.21 (m, 4H), 7.02 (d, J = 8.7 Hz, 2H), 5.25 (d, J = 8.0 Hz, 1H), 4.92–4.53 (m, 1H), 4.16 (s, 2H), 3.50 (s, 2H), 1.20–1.07 (m, 6H); 13C NMR (100 MHz, DMSO-d6) δ 177.0, 170.9, 163.4, 162.7, 157.9, 155.8, 139.1, 131.6, 129.6, 128.7, 128.0, 127.6, 126.7, 126.6, 117.7, 115.5, 107.2, 101.9, 67.7, 67.5, 58.4, 38.7, 22.4; HR-MS (ESI): calcd for C29H28N2O6 [M + H]+ 501.2020, found (ESI+) 501.2023.
- (S)-Isobutyl(2-oxo-2-((2-((4-oxo-2-phenyl-4H-chromen-7-yl)oxy)ethyl)amino)-1-phenylethyl)carbamate (4j). White solid, 49.0% yield, m.p. 130–133 °C; = +26.0° (c 1.0, CH3OH); 1H NMR (400 MHz, DMSO-d6) δ 8.52 (s, 1H), 8.10 (d, J = 5.9 Hz, 2H), 7.94 (d, J = 8.7 Hz, 1H), 7.69 (d, J = 7.7 Hz, 1H), 7.60 (s, 3H), 7.42 (d, J = 7.1 Hz, 2H), 7.33–7.19 (m, 4H), 7.01 (d, J = 8.5 Hz, 1H), 6.97 (s, 1H), 5.25 (d, J = 8.2 Hz, 1H), 4.16 (s, 2H), 3.72 (d, J = 6.1 Hz, 2H), 3.51 (s, 2H), 1.98–1.68 (m, 1H), 0.86 (d, J = 5.5 Hz, 6H); 13C NMR (100MHz, DMSO-d6) δ 176.92, 170.85, 163.41, 162.68, 157.88, 156.35, 139.04, 132.15, 131.61, 129.56, 128.66, 127.62, 126.66, 117.67, 115.48, 107.23, 102.01, 70.56, 67.51, 58.53, 38.69, 28.05, 19.31; HR-MS (ESI): calcd for C30H30N2O6 [M + H]+ 515.2177, found (ESI+) 515.2170.
- (S)-Tert-butyl(1-oxo-1-((2-((4-oxo-2-phenyl-4H-chromen-7-yl)oxy)ethyl)amino)-3-phenylpropan-2-yl)carbamate (4k). White solid, 42.5% yield, m.p. 120–125 °C; = −8.8° (c 1.0, CH3OH); 1H NMR (400 MHz, DMSO-d6) δ 8.21 (s, 1H), 8.10 (d, J = 5.3 Hz, 2H), 7.96 (d, J = 8.7 Hz, 1H), 7.60 (s, 3H), 7.34 (s, 1H), 7.23 (s, 4H), 7.15 (s, 1H), 7.07 (d, J = 8.2 Hz, 1H), 6.97 (s, 1H), 6.91 (d, J = 8.1 Hz, 1H), 4.14 (s, 3H), 3.51 (d, J = 22.2 Hz, 2H), 3.04–2.62 (m, 2H), 1.28 (s, 9H); 13C NMR (100 MHz, DMSO-d6) δ 177.1, 172.6, 163.6, 162.8, 158.0, 155.7, 138.5, 132.3, 131.6, 129.6, 128.5, 126.7, 117.7, 115.6, 107.2, 102.0, 78.6, 67.6, 56.2, 38.6, 38.1, 28.6; HR-MS (ESI): calcd for C31H32N2O6 [M + H]+ 529.2333, found (ESI+) 529.2330.
- (S)-Isopropyl(3-(4-hydroxyphenyl)-1-oxo-1-((2-((4-oxo-2-phenyl-4H-chromen-7-yl)oxy)ethyl)amino)propan-2-yl)carbamate (4l). White solid, 37.2% yield, m.p. 160–164 °C; = −7.9° (c 1.0, CH3OH); 1H NMR (400 MHz, DMSO-d6) δ 8.30 (s, 1H), 8.20–8.07 (m, 2H), 7.98 (d, J = 8.8 Hz, 1H), 7.61 (d, J = 6.6 Hz, 3H), 7.36 (d, J = 2.1 Hz, 1H), 7.30 (d, J = 8.1 Hz, 2H), 7.22 (d, J = 8.5 Hz, 1H), 7.09 (d, J = 8.0 Hz, 3H), 7.00 (d, J = 1.7 Hz, 1H), 4.83 (p, J = 6.3 Hz, 1H), 4.65 (p, J = 6.4 Hz, 1H), 4.24 (s, 1H), 4.15 (q, J = 5.6 Hz, 2H), 3.35 (d, J = 1.9 Hz, 2H), 3.04–2.70 (m, 2H), 1.28 (d, J = 6.2 Hz, 6H); 13C NMR (100 MHz, DMSO-d6) δ 176.4, 163.0, 162.2, 157.4, 152.5, 149.2, 135.7, 131.7, 131.1, 130.2, 129.1, 126.2, 120.7, 117.2, 115.0, 106.7, 72.6, 66.9, 55.9, 21.3; HR-MS (ESI): calcd for C30H31N2O7 [M + H]+ 531.2126, found (ESI+) 531.2121.
- (S)-Isopropyl(3-(1H-indol-3-yl)-1-oxo-1-((2-((4-oxo-2-phenyl-4H-chromen-7-yl)oxy)ethyl) amino)propan-2-yl)carbamate (4m). White solid, 54.3% yield, m.p. 105–115 °C; = +2.5° (c 1.0, CH3OH); 1H NMR (400 MHz, DMSO-d6) δ 10.79 (s, 1H), 8.23 (s, 1H), 8.11 (d, J = 6.8 Hz, 2H), 7.95 (d, J = 8.7 Hz, 1H), 7.59 (d, J = 6.0 Hz, 4H), 7.30 (d, J = 6.7 Hz, 2H), 7.13 (s, 1H), 7.05 (d, J = 10.0 Hz, 3H), 6.96 (d, J = 10.6 Hz, 2H), 4.55–4.76 (m, 1H), 4.24 (d, J = 2.9 Hz, 1H), 4.10 (s, 2H), 3.47 (s, 2H), 3.11–2.84 (m, 2H), 1.08 (dd, J = 21.3, 5.8 Hz, 6H); 13C NMR (100 MHz, DMSO-d6) δ 176.5, 172.3, 163.0, 162.2, 157.4, 155.5, 136.0, 131.7, 131.1, 129.1, 127.2, 126.2, 123.7, 120.8, 118.4, 118.1, 117.2, 116.2, 115.0, 111.2, 110.0, 106.7, 101.5, 67.0, 66.9, 55.4, 38.0, 27.8, 21.9; HR-MS (ESI): calcd for C32H31N3O6 [M + H]+ 554.2286, found (ESI+) 554.2285.
- (S)-Diisopropyl(6-oxo-6-((2-((4-oxo-2-phenyl-4H-chromen-7-yl)oxy)ethyl)amino)hexane-1,5-diyl)dicarbamate (4n). White solid, 43.6% yield, m.p. 169–173 °C; = −15.3° (c 1.0, CH3OH); 1H NMR (400 MHz, DMSO-d6) δ 8.10 (d, J = 6.8 Hz, 3H), 7.95 (d, J = 8.8 Hz, 1H), 7.59 (d, J = 6.5 Hz, 3H), 7.34 (s, 1H), 7.05 (t, J = 8.0 Hz, 2H), 6.97 (s, 1H), 6.93 (s, 1H), 4.67–4.73 (m, 2H), 4.17 (s, 2H), 3.88–3.93 (m, 1H), 3.43–3.56 (m, 2H), 2.87–2.88 (m, 2H), 1.46–1.54 (m, 2H), 1.32 (d, J = 5.9 Hz, 2H), 1.28–1.17 (m, 2H), 1.12 (d, J = 6.2 Hz, 12H); 13C NMR (100 MHz, DMSO-d6) δ 177.0, 173.0, 163.5, 162.7, 157.9, 156.3, 156.2, 132.2, 131.6, 129.6, 126.7, 117.7, 115.5, 107.2, 102.0, 67.4, 66.9, 55.0, 38.4, 32.1, 29.6, 23.2, 22.5, 22.5; HR-MS (ESI): calcd for C31H39N3O8 [M + H]+ 582.2810, found (ESI+) 582.2807.
- N-(2-((4-Oxo-2-phenyl-4H-chromen-7-yl)oxy)ethyl)benzamide (5a). White solid, 42.2% yield, m.p. 132–144 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.76 (s, 1H), 8.09 (d, J = 6.2 Hz, 2H), 7.94 (d, J = 8.7 Hz, 1H), 7.87 (d, J = 7.4 Hz, 2H), 7.59 (d, J = 5.7 Hz, 3H), 7.52 (d, J = 7.1 Hz, 1H), 7.46 (t, J = 7.2 Hz, 2H), 7.39 (s, 1H), 7.09 (d, J = 8.8 Hz, 1H), 6.97 (s, 1H), 4.31 (d, J = 5.1 Hz, 2H), 3.71 (d, J = 5.1 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) δ 177.0, 167.2, 163.6, 162.7, 156.0, 134.6, 132.2, 131.8, 131.6, 129.6, 128.8, 127.7, 126.7, 117.7, 115.6, 107.2, 102.1, 67.4, 39.2; HR-MS (ESI): calcd for C24H19NO4 [M + H]+ 386.1387, found (ESI+) 386.1395.
- 2-Methoxy-N-(2-((4-oxo-2-phenyl-4H-chromen-7-yl)oxy)ethyl)benzamide (5b). White solid, 43.5% yield, m.p. 95–100 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.46 (s, 1H), 8.09 (d, J = 6.1 Hz, 2H), 7.95 (d, J = 8.7 Hz, 1H), 7.80 (d, J = 6.9 Hz, 1H), 7.59 (s, 3H), 7.47 (t, J = 7.2 Hz, 1H), 7.40 (s, 1H), 7.12 (t, J = 8.7 Hz, 2H), 7.03 (t, J = 7.3 Hz, 1H), 6.97 (s, 1H), 4.31 (s, 2H), 3.88 (s, 3H), 3.79–3.68 (m, 2H); 13C NMR (100 MHz, DMSO-d6) δ 177.1, 165.9, 163.6, 162.8, 158.0, 157.5, 133.1, 132.3, 131.6, 131.0, 129.6, 126.8, 126.7, 122.8, 121.1, 117.7, 115.6, 112.6, 107.2, 102.0, 67.6, 56.4, 38.9; HR-MS (ESI): calcd for C25H21NO5 [M + H]+ 416.1493, found (ESI+) 416.1485.
- 2-Chloro-N-(2-((4-oxo-2-phenyl-4H-chromen-7-yl)oxy)ethyl)benzamide (5c). White solid, 39.8% yield, m.p. 148–154 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.74 (s, 1H), 8.10 (d, J = 6.0 Hz, 2H), 7.96 (d, J = 8.8 Hz, 1H), 7.58 (d, J = 6.1 Hz, 3H), 7.49 (d, J = 7.6 Hz, 1H), 7.45–7.41 (m, 2H), 7.37 (d, J = 8.1 Hz, 2H), 7.09 (d, J = 8.7 Hz, 1H), 6.97 (s, 1H), 4.30 (s, 2H), 3.68 (d, J = 5.1 Hz, 2H); 13C NMR (101 MHz, DMSO-d6) δ 177.0, 167.23, 163.5, 162.7, 158.0, 137.3, 132.2, 131.6, 131.3, 130.4, 130.1, 129.6, 129.3, 127.6, 126.7, 117.7, 115.6, 107.3, 102.0, 67.4, 39.0; HR-MS (ESI): calcd for C24H18ClNO4 [M + H]+ 420.0997, found (ESI+) 420.0995.
- 2-Bromo-N-(2-((4-oxo-2-phenyl-4H-chromen-7-yl)oxy)ethyl)benzamide (5d). White solid, 37.5% yield, m.p. 138–148 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.72 (s, 1H), 8.10 (d, J = 6.0 Hz, 2H), 7.96 (d, J = 8.8 Hz, 1H), 7.66–7.53 (m, 4H), 7.39 (q, J = 12.7, 10.6 Hz, 4H), 7.09 (d, J = 8.8 Hz, 1H), 6.97 (s, 1H), 4.30 (s, 2H), 3.81–3.57 (m, 2H); 13C NMR (100 MHz, DMSO-d6) δ 177.0, 168.1, 163.5, 162.7, 156.0, 139.4, 133.2, 132.2, 131.6, 131.4, 129.6, 129.3, 128.0, 126.7, 119.4, 117.7, 115.6, 107.3, 102.0, 67.4, 39.0; HR-MS (ESI): calcd for C24H18BrNO4 [M + H]+ 464.0492, found (ESI+) 464.0496.
- 2-Nitro-N-(2-((4-oxo-2-phenyl-4H-chromen-7-yl)oxy)ethyl)benzamide (5e). White solid, 40.1% yield, m.p. 166–172 °C; 1H NMR (400 MHz, DMSO-d6) δ 9.01 (s, 1H), 8.10 (d, J = 6.4 Hz, 2H), 8.04 (d, J = 7.9 Hz, 1H), 7.96 (d, J = 8.6 Hz, 1H), 7.78 (t, J = 7.2 Hz, 1H), 7.70 (d, J = 7.4 Hz, 1H), 7.61 (d, J = 7.6 Hz, 4H), 7.40 (d, J = 8.1 Hz, 1H), 7.10 (d, J = 8.6 Hz, 1H), 6.98 (s, 1H), 4.30 (s, 2H), 3.77–3.60 (m, 2H); 13C NMR (100 MHz, DMSO-d6) δ 177.0, 166.5, 163.6, 162.8, 158.0, 147.5, 134.2, 132.8, 132.2, 131.6, 131.3, 129.6, 129.5, 126.7, 124.6, 117.7, 115.6, 107.2, 102.0, 67.4, 39.2; HR-MS (ESI): calcd for C24H18N2O6 [M + H]+ 431.1238, found (ESI+) 431.1236.
- N1-(2-((4-Oxo-2-phenyl-4H-chromen-7-yl)oxy)ethyl)-N3-phenylmalonamide (6a). White solid, 44.5% yield, m.p. 173–178 °C; 1H NMR (400 MHz, DMSO-d6) δ 10.10 (s, 1H), 8.41 (s, 1H), 8.10 (d, J = 6.9 Hz, 2H), 7.96 (d, J = 8.8 Hz, 1H), 7.62–7.54 (m, 5H), 7.37 (s, 1H), 7.29 (t, J = 7.7 Hz, 2H), 7.06 (dt, J = 14.8, 8.0 Hz, 2H), 6.98 (s, 1H), 4.21 (s, 2H), 3.55 (d, J = 5.0 Hz, 2H), 3.30 (s, 2H); 13C NMR (100 MHz, DMSO-d6) δ 177.0, 167.6, 166.1, 163.5, 162.8, 158.0, 139.4, 132.2, 131.6, 129.6, 129.2, 126.7, 123.9, 119.6, 117.7, 115.6, 107.2, 102.1, 67.7, 45.0, 38.8; HR-MS (ESI): calcd for C26H22N2O5 [M + H]+ 443.1602, found (ESI+) 443.1605.
- N1-(2-((4-Oxo-2-phenyl-4H-chromen-7-yl)oxy)ethyl)-N3-(o-tolyl)malonamide (6b). White solid, 46.3% yield, m.p. 166–169 °C; 1H NMR (400 MHz, DMSO-d6) δ 9.64 (s, 1H), 8.50 (s, 1H), 8.10 (d, J = 7.1 Hz, 2H), 7.95 (d, J = 8.8 Hz, 1H), 7.57 (dd, J = 14.6, 7.5 Hz, 4H), 7.36 (s, 1H), 7.19 (d, J = 7.3 Hz, 1H), 7.14 (t, J = 7.5 Hz, 1H), 7.06 (q, J = 8.4, 7.3 Hz, 2H), 6.97 (s, 1H), 4.22 (s, 2H), 3.57 (d, J = 5.0 Hz, 2H), 3.36 (s, 2H), 2.21 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 176.9, 168.2, 165.9, 163.4, 162.7, 158.0, 136.7, 132.2, 131.7, 130.9, 130.8, 129.6, 126.7, 126.5, 125.3, 124.2, 117.8, 115.5, 107.3, 102.1, 67.7, 44.0, 38.8, 18.2; HR-MS (ESI): calcd for C27H24N2O5 [M + H]+ 457.1758, found (ESI+) 457.1755.
- N1-(2-Chlorophenyl)-N3-(2-((4-oxo-2-phenyl-4H-chromen-7-yl)oxy)ethyl)malonamide (6c). White solid, 39.0% yield, m.p. 184–189 °C; 1H NMR (400 MHz, DMSO-d6) δ 10.18 (s, 1H), 8.60 (s, 1H), 8.10 (d, J = 6.8 Hz, 2H), 8.02 (d, J = 8.0 Hz, 1H), 7.94 (d, J = 8.8 Hz, 1H), 7.59 (d, J = 6.9 Hz, 3H), 7.48 (d, J = 8.0 Hz, 1H), 7.36 (s, 1H), 7.31 (t, J = 7.7 Hz, 1H), 7.13 (t, J = 7.6 Hz, 1H), 7.08 (d, J = 8.8 Hz, 1H), 6.97 (s, 1H), 4.30–4.15 (m, 2H), 3.58 (d, J = 4.9 Hz, 2H), 3.45 (s, 2H). 13C NMR (100 MHz, DMSO-d6) δ 177.0, 168.3, 166.1, 163.5, 162.8, 158.0, 135.2, 132.2, 131.6, 129.9, 129.6, 128.1, 126.7, 126.1, 124.7, 124.1, 117.7, 115.6, 107.2, 102.1, 67.7, 43.6, 38.8; HR-MS (ESI): calcd for C26H21ClN2O5 [M + H]+ 477.1212, found (ESI+) 477.1205.
- N1-(4-Bromophenyl)-N3-(2-((4-oxo-2-phenyl-4H-chromen-7-yl)oxy)ethyl)malonamide (6d). White solid, 42.2% yield, m.p. 181–186 °C; 1H NMR (400 MHz, DMSO-d6) δ 10.30 (d, J = 5.0 Hz, 1H), 8.50–8.40 (m, 1H), 8.10 (d, J = 6.2 Hz, 2H), 7.95 (d, J = 8.8 Hz, 1H), 7.64–7.53 (m, 5H), 7.46 (d, J = 8.5 Hz, 2H), 7.36 (s, 1H), 7.08 (d, J = 8.7 Hz, 1H), 6.97 (s, 1H), 4.20 (s, 2H), 3.54 (d, J = 4.8 Hz, 2H), 3.33 (s, 2H); 13C NMR (100 MHz, DMSO-d6) δ 176.9, 167.3, 166.3, 163.5, 162.7, 158.0, 138.8, 132.2, 132.0, 131.7, 129.6, 126.7, 121.5, 117.7, 115.5, 115.4, 107.3, 102.1, 67.7, 45.1, 38.8; HR-MS (ESI): calcd for C26H21BrN2O5 [M + H]+ 521.0707, found (ESI+) 521.0713.
4.2. Biological Assays
4.2.1. Extraction of TMV
4.2.2. Inactivation Effect of Compounds against TMV In Vivo
4.2.3. Cultivation Effect of Compounds against TMV In Vivo
4.2.4. Protective Effect of Compounds against TMV In Vivo
4.3. Calculation Procedures for Molecular Docking Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; Mcroberts, N.; Nelson, A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Abraham, A.; Philip, S.; Jacob, M.K.; Narayanan, S.P.; Jacob, C.K.; Kochupurackal, J. Phenazine-1-carboxylic acid mediated anti-oomycete activity of the endophytic Alcaligenes sp. EIL-2 against Phytophthora meadii. Microbiol. Res. 2015, 170, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Wang, S.; Song, D.; Cao, X.; Huang, W.; Ke, S. Discovery of γ-Lactam alkaloid derivatives as potential fungicidal agents targeting steroid biosynthesis. J. Agric. Food Chem. 2020, 68, 14438–14451. [Google Scholar] [CrossRef]
- Guo, J.; Hao, Y.; Ji, X.; Wang, Z.; Liu, Y.; Ma, D.; Li, Y.; Pang, H.; Ni, J.; Wang, Q. Optimization, structure–activity relationship, and mode of action of nortopsentin analogues containing thiazole and oxazole moieties. J. Agric. Food Chem. 2019, 67, 10018–10031. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wang, T.; Zhou, Y.; Shi, L.; Lu, A.; Wang, Z. Discovery of cysteine and its derivatives as novel antiviral and antifungal agents. Molecules 2021, 26, 383. [Google Scholar] [CrossRef]
- Guo, W.; Yan, H.; Ren, X.; Tang, R.; Sun, Y.; Wang, Y.; Feng, J. Berberine induces resistance against tobacco mosaic virus in tobacco. Pest Manag. Sci. 2019, 76, 1804–1813. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Yang, S.; Li, H.; Lu, A.; Wang, Z.; Yao, Y.; Wang, Q. Discovery, structural optimization, and mode of action of essramycin alkaloid and its derivatives as anti-tobacco mosaic virus and anti-phytopathogenic fungus agents. J. Agric. Food Chem. 2019, 68, 471–484. [Google Scholar] [CrossRef]
- Scholthof, K.-B.G. Tobacco mosaic virus: A model system for plant biology. Annu. Rev. Phytopathol. 2004, 42, 13–34. [Google Scholar] [CrossRef] [Green Version]
- Reichman, M.; Devash, Y.; Suhadolnik, R.J.; Sela, I. Human leukocyte interferon and the antiviral factor (AVF) from virus-infected plants stimulate plant tissues to produce nucleotides with antiviral activity. Virology 1983, 128, 240–244. [Google Scholar] [CrossRef]
- Gan, X.; Wang, Y.; Hu, D.; Song, B. Design, synthesis, and antiviral activity of novel chalcone derivatives containing a purine moiety. Chin. J. Chem. 2017, 35, 665–672. [Google Scholar] [CrossRef]
- Cai, L.; Zhang, W.; Jia, H.; Feng, H.; Wei, X.; Chen, H.; Wang, D.; Xue, Y.; Sun, X. Plant-derived compounds: A potential source of drugs against Tobacco mosaic virus. Pestic. Biochem. Physiol. 2020, 169, 104589. [Google Scholar] [CrossRef]
- Cantrell, C.L.; Dayan, F.E.; Duke, S.O. Natural products as sources for new pesticides. J. Nat. Prod. 2012, 75, 1231–1242. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues, T.; Reker, D.; Schneider, P.; Schneider, G. Counting on natural products for drug design. Nat. Chem. 2016, 8, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Liu, J.; Xu, S.; Zhu, Z.; Xu, J. The structural modification of natural products for novel drug discovery. Expert Opin. Drug Discov. 2016, 12, 121–140. [Google Scholar] [CrossRef] [PubMed]
- Mihorianu, M.; Franz, M.H.; Jones, P.G.; Freytag, M.; Kelter, G.; Fiebig, H.-H.; Tamm, M.; Neda, I. N-Heterocyclic carbenes derived from imidazo-[1,5-a]pyridines related to natural products: Synthesis, structure and potential biological activity of some corresponding gold(I) and silver(I) complexes. Appl. Organomet. Chem. 2016, 30, 581–589. [Google Scholar] [CrossRef]
- Sparks, T.C.; Duke, S.O. Structure simplification of natural products as a lead generation approach in agrochemical discovery. J. Agric. Food Chem. 2021, 69, 8324–8346. [Google Scholar] [CrossRef] [PubMed]
- Song, B.A.; Zhang, G.P.; Hu, D.Y.; Pang, L.; Yang, S.; Liu, G.; Wang, H. N-Substituted Benzothiazolyl-1-substitutedphenyl-O,O-dialkyl-α-aminophosphonate Ester Derivatives Preparation and Application. China Patent CN 1687088, 2005. [Google Scholar]
- Chen, Z.; Li, G.J.; Fan, H.T.; Liu, J.J.; Bi, L.; Yang, S.; Song, B.A.; Hu, D.Y. The study of efficiency of dufulin against southern rice black-streaked dwarf virus. Chin. Agr. Sci. Bull. 2011, 27, 250–254. [Google Scholar]
- Martens, S.; Mithöfer, A. Flavones and flavone synthases. Phytochemistry 2005, 66, 2399–2407. [Google Scholar] [CrossRef]
- Shamsudin, N.F.; Ahmed, Q.U.; Mahmood, S.; Shah, S.A.A.; Khatib, A.; Mukhtar, S.; Alsharif, M.A.; Parveen, H.; Zakaria, Z.A. Antibacterial effects of flavonoids and their structure-activity relationship study: A comparative interpretation. Molecules 2022, 27, 1149. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.-S. Recent advances in natural antifungal flavonoids and their derivatives. Bioorganic Med. Chem. Lett. 2019, 29, 126589. [Google Scholar] [CrossRef]
- Orhan, D.D.; Özçelik, B.; Özgen, S.; Ergun, F. Antibacterial, antifungal, and antiviral activities of some flavonoids. Microbiol. Res. 2010, 165, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Al-Khayri, J.M.; Sahana, G.R.; Nagella, P.; Joseph, B.V.; Alessa, F.M.; Al-Mssallem, M.Q. Flavonoids as potential anti-inflammatory molecules: A review. Molecules 2022, 27, 2901. [Google Scholar] [CrossRef] [PubMed]
- Benavente-García, O.; Castillo, J. Update on uses and properties of Citrus flavonolds: New findings in anticancer, cardiovascular, and anti-inflammatory activity. J. Agric. Food Chem. 2008, 56, 6185–6205. [Google Scholar] [CrossRef]
- Jia, L.-G.; Sheng, Z.-W.; Xu, W.-F.; Li, Y.-X.; Liu, Y.-G.; Xia, Y.-J.; Zhang, J.-H. Modulation of anti-oxidation ability by proanthocyanidins during germination of Arabidopsis thaliana seeds. Mol. Plant 2012, 5, 472–481. [Google Scholar] [CrossRef] [Green Version]
- Kashiwada, Y.; Aoshima, A.; Ikeshiro, Y.; Chen, Y.-P.; Furukawa, H.; Itoigawa, M.; Fujioka, T.; Mihashi, K.; Cosentino, L.M.; Morris-Natschke, S.L.; et al. Anti-HIV benzylisoquinoline alkaloids and flavonoids from the leaves of Nelumbo nucifera, and structure–activity correlations with related alkaloids. Bioorganic Med. Chem. 2005, 13, 443–448. [Google Scholar] [CrossRef]
- Krcatović, E.; Rusak, G.; Bezić, N.; Krajacić, M. Inhibition of tobacco mosaic virus infection by quercetin and vitexin. Acta Virol. 2008, 52, 119–124. [Google Scholar]
- Zhao, W.; Zeng, X.; Zhang, T.; Wang, L.; Yang, G.; Chen, Y.-K.; Hu, Q.; Miao, M. Flavonoids from the bark and stems of Cassia fistula and their anti-tobacco mosaic virus activities. Phytochem. Lett. 2013, 6, 179–182. [Google Scholar] [CrossRef]
- Li, Y.; Ye, S.; Hu, Z.; Hao, N.; Bo, X.; Liang, H.; Tian, X. Identification of anti- TMV active flavonoid glycosides and their mode of action on virus particles from Clematis lasiandra Maxim. Pest Manag. Sci. 2021, 77, 5268–5277. [Google Scholar] [CrossRef]
- Chen, Z.; Tan, J.; Yang, G.; Miao, M.; Chen, Y.; Li, T. Isoflavones from the roots and stems of Nicotiana Tabacum and their anti-tobacco mosaic virus activities. Phytochem. Lett. 2012, 5, 233–235. [Google Scholar] [CrossRef]
- Kong, W.-S.; Xing, H.-H.; Li, J.; Ye, L.; Liu, X.; Li, Y.-P.; Rao, G.-X.; Zhou, M.; Yang, G.; Hu, Q.-F.; et al. Two new flavones from Cassia pumila and their anti-tobacco mosaic virus activity. Chem. Nat. Compd. 2018, 54, 1048–1051. [Google Scholar] [CrossRef]
- Yao, J.M.; Pu, L.L.; Rao, J.R.; Wang, M.; Guo, C.; Lei, Z.W. Research progress of anti-plant virus agents based on structural diversification. Agrochemicals 2021, 60, 859–865. [Google Scholar] [CrossRef]
- Ma, Y.C.; Wang, L.; Lu, A.D.; Xue, W. Synthesis and biological activity of novel oxazinyl flavonoids as antiviral and anti-phytopathogenic fungus agents. Molecules 2022, 27, 6875. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Gao, M.Q.; Zhu, X.L.; Yang, G.F. Research progress on carboxamide fungicides targeting succinate dehydrogenase. Chin. J. Pestic. Sci. 2019, 21, 673–680. [Google Scholar]
- Luo, B.; Ning, Y. Comprehensive overview of carboxamide derivatives as succinate dehydrogenase inhibitors. J. Agric. Food Chem. 2022, 70, 957–975. [Google Scholar] [CrossRef]
- Chaparro, S.; Rojas, H.A.; Castillo, J.C.; Portilla, J.; Romanelli, G.P.; Pineda, A.; Elsharif, A.M.; Martinez, J.J.; Luque, R. Solventless amide synthesis catalyzed by biogenic CaCO3 materials. ACS Sustain. Chem. Eng. 2020, 8, 13139–13146. [Google Scholar] [CrossRef]
- Zeng, S.; Liu, J.; Anankanbil, S.; Chen, M.; Guo, Z.; Adams, J.P.; Snajdrova, R.; Li, Z. Amide synthesis via aminolysis of ester or acid with an intracellular lipase. ACS Catal. 2018, 8, 8856–8865. [Google Scholar] [CrossRef]
- Serdiuk, I.E.; Roshal, A.D. Single and double intramolecular proton transfers in the electronically excited state of flavone derivatives. RSC Adv. 2015, 5, 102191–102203. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Taylor, S.F.; Dupart, P.S.; Arnold, C.L.; Sridhar, J.; Jiang, Q.; Wang, Y.; Skripnikova, E.V.; Zhao, M.; Maryam Foroozesh, M. Pyranoflavones: A group of small-molecule probes for exploring the active site cavities of cytochrome P450 enzymes 1A1, 1A2, and 1B1. J. Med. Chem. 2013, 56, 4082–4092. [Google Scholar] [CrossRef] [Green Version]
- Du, X.-J.; Bian, Q.; Wang, H.-X.; Yu, S.-J.; Kou, J.-J.; Wang, Z.-P.; Li, Z.-M.; Zhao, W.-G. Design, synthesis, and fungicidal activity of novel carboxylic acid amides represented by N-benzhydryl valinamode carbamates. Org. Biomol. Chem. 2014, 12, 5427–5434. [Google Scholar] [CrossRef]
- Sun, M.; Yang, H.-H.; Tian, L.; Li, J.-Q.; Zhao, W.-G. Design, synthesis, and fungicidal activities of imino diacid analogs of valine amide fungicides. Bioorganic Med. Chem. Lett. 2015, 25, 5729–5731. [Google Scholar] [CrossRef]
- Atalay, S.S.; Assad, M.Y.; Amagata, T.; Wu, W. Mild, efficient, and solvent-free synthesis of 4-hydroxy-2-quinolinones. Tetrahedron Lett. 2020, 61, 151778. [Google Scholar] [CrossRef]
- Lamberth, C. Amino acid chemistry in crop protection. Tetrahedron 2010, 66, 7239–7256. [Google Scholar] [CrossRef]
- Ryzhkov, V.L. Effect of amino acids and related substances on reproduction of the tobacco mosaic virus. Dokl. Akad. Nauk SSSR 1951, 80, 677–680. [Google Scholar]
- Zhang, B.; Li, L.; Liu, Y.; Wang, Q. Antiviral mechanism study of gossypol and its Schiff base derivatives based on reactive oxygen species (ROS). RSC Adv. 2016, 6, 87637–87648. [Google Scholar] [CrossRef]
- Seyedi, S.S.; Shukri, M.; Hassandarvish, P.; Oo, A.; Shankar, E.M.; Abubakar, S.; Zandi, K. Computational approach towards exploring potential anti-chikungunya activity of selected flavonoids. Sci. Rep. 2016, 6, 24027. [Google Scholar] [CrossRef] [PubMed]
- Bhyravbhatla, B.; Watowich, S.J.; Caspar, D.L. Refined atomic model of the four-layer aggregate of the tobacco mosaic virus coat protein at 2.4-Å resolution. Biophys. J. 1998, 74, 604–615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takamatsu, Y.; Sugiyama, A.; Purqon, A.; Nagao, H.; Nishikawa, K. Bingding free energy calculation and structural analysis for antigen-antibody complex. AIP Conf. Proc. 2006, 832, 566–569. [Google Scholar] [CrossRef]
- Wang, Z.; Wei, P.; Wang, L.; Wang, Q. Design, synthesis, and anti-tobacco mosaic virus (TMV) activity of phenanthroindolizidines and their analogues. J. Agric. Food Chem. 2012, 60, 10212–10219. [Google Scholar] [CrossRef] [PubMed]
- Gooding, G.V., Jr.; Hebert, T.T. A simple technique for purification of tobacco mosaic virus in large quantities. Phytopathology 1967, 57, 1285–1290. [Google Scholar] [PubMed]
- Li, S.Z.; Wang, D.M.; Jiao, S.M. Pesticide Experiment Methods-Fungicide Sector; Li, S.Z., Ed.; Agriculture Press of China: Beijing, China, 1991; pp. 93–94. [Google Scholar]





| Compounds | Concentrations (mg/L) | Anti-TMV Activities (%) a | ||
|---|---|---|---|---|
| Inactivation Effect | Curative Effect | Protection Effect | ||
| 1 | 500 | 32 ± 1 | 30 ± 3 | 30 ± 3 |
| 100 | 0 | 0 | 0 | |
| 2a | 500 | 44 ± 2 | 40 ± 2 | 41 ± 1 |
| 100 | 23 ± 1 | 16 ± 2 | 18 ± 2 | |
| 2b | 500 | 47 ± 2 | 42 ± 1 | 43 ± 1 |
| 100 | 23 ± 2 | 15 ± 2 | 18 ± 3 | |
| 2c | 500 | 43 ± 2 | 41 ± 2 | 41 ± 1 |
| 100 | 17 ± 1 | 14 ± 2 | 16 ± 2 | |
| 2d | 500 | 53 ± 1 | 50 ± 2 | 52 ± 2 |
| 100 | 20 ± 2 | 18 ± 3 | 20 ± 3 | |
| 2e | 500 | 49 ± 2 | 50 ± 1 | 53 ± 2 |
| 100 | 24 ± 2 | 22 ± 3 | 21 ± 1 | |
| 2f | 500 | 50 ± 3 | 47 ± 1 | 48 ± 3 |
| 100 | 18 ± 2 | 16 ± 2 | 19 ± 3 | |
| 4a | 500 | 55 ± 2 | 53 ± 2 | 53 ± 1 |
| 100 | 19 ± 2 | 21 ± 3 | 23 ± 2 | |
| 4b | 500 | 52 ± 2 | 50 ± 2 | 50 ± 1 |
| 100 | 36 ± 2 | 33 ± 1 | 35 ± 2 | |
| 4c | 500 | 50 ± 1 | 47 ± 1 | 48 ± 2 |
| 100 | 18 ± 1 | 16 ± 1 | 19 ± 2 | |
| 4d | 500 | 48 ± 2 | 45 ± 1 | 47 ± 1 |
| 100 | 15 ± 2 | 14 ± 2 | 14 ± 2 | |
| 4e | 500 | 39 ± 1 | 37 ± 2 | 39 ± 3 |
| 100 | 0 | 0 | 0 | |
| 4f | 500 | 38 ± 1 | 36 ± 2 | 36 ± 1 |
| 100 | 0 | 0 | 0 | |
| 4g | 500 | 43 ± 2 | 40 ± 1 | 41 ± 1 |
| 100 | 16 ± 2 | 14 ± 2 | 15 ± 1 | |
| 4h | 500 | 52 ± 3 | 50 ± 2 | 50 ± 1 |
| 100 | 34 ± 3 | 31 ± 2 | 32 ± 3 | |
| 4i | 500 | 48 ± 2 | 45 ± 1 | 47 ± 2 |
| 100 | 16 ± 1 | 14 ± 2 | 16 ± 2 | |
| 4j | 500 | 42 ± 3 | 41 ± 1 | 43 ± 2 |
| 100 | 12 ± 2 | 13 ± 3 | 11 ± 1 | |
| 4k | 500 | 47 ± 1 | 45 ± 1 | 46 ± 2 |
| 100 | 19 ± 2 | 17 ± 3 | 16 ± 1 | |
| 4l | 500 | 44 ± 3 | 40 ± 2 | 41 ± 2 |
| 100 | 14 ± 1 | 13 ± 2 | 15 ± 2 | |
| 4m | 500 | 58 ± 2 | 57 ± 2 | 59 ± 3 |
| 100 | 23 ± 1 | 24 ± 3 | 27 ± 1 | |
| 4n | 500 | 52 ± 1 | 48 ± 2 | 51 ± 2 |
| 100 | 29 ± 1 | 26 ± 1 | 27 ± 2 | |
| 5a | 500 | 53 ± 2 | 55 ± 1 | 54 ± 3 |
| 100 | 20 ± 1 | 21 ± 2 | 20 ± 1 | |
| 5b | 500 | 45 ± 4 | 44 ± 2 | 46 ± 2 |
| 100 | 18 ± 3 | 16 ± 1 | 17 ± 1 | |
| 5c | 500 | 49 ± 3 | 52 ± 2 | 51 ± 2 |
| 100 | 20 ± 1 | 19 ± 3 | 18 ± 3 | |
| 5d | 500 | 48 ± 2 | 52 ± 4 | 50 ± 4 |
| 100 | 15 ± 1 | 21 ± 2 | 18 ± 2 | |
| 5e | 500 | 53 ± 3 | 54 ± 4 | 52 ± 3 |
| 100 | 20 ± 4 | 21 ± 3 | 19 ± 2 | |
| 6a | 500 | 43 ± 3 | 45 ± 4 | 43 ± 3 |
| 100 | 19 ± 2 | 17 ± 3 | 15 ± 2 | |
| 6b | 500 | 54 ± 4 | 57 ± 3 | 54 ± 3 |
| 100 | 20 ± 2 | 23 ± 2 | 19 ± 2 | |
| 6c | 500 | 46 ± 3 | 47 ± 1 | 45 ± 4 |
| 100 | 15 ± 2 | 17 ± 2 | 15 ± 3 | |
| 6d | 500 | 45 ± 3 | 47 ± 2 | 49 ± 3 |
| 100 | 20 ± 1 | 21 ± 3 | 22 ± 3 | |
| Ningnanmycin | 500 | 61 ± 2 | 57 ± 2 | 58 ± 2 |
| 100 | 27 ± 1 | 24 ± 3 | 26 ± 2 | |
| Ribavirin | 500 | 39 ± 1 | 40 ± 2 | 39 ± 2 |
| 100 | 44 ± 2 | 40 ± 2 | 41 ± 1 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, B.; Wang, J.; Wang, L.; Wang, Z.; Lu, A. Discovery of Flavone Derivatives Containing Carboxamide Fragments as Novel Antiviral Agents. Molecules 2023, 28, 2179. https://doi.org/10.3390/molecules28052179
Zhao B, Wang J, Wang L, Wang Z, Lu A. Discovery of Flavone Derivatives Containing Carboxamide Fragments as Novel Antiviral Agents. Molecules. 2023; 28(5):2179. https://doi.org/10.3390/molecules28052179
Chicago/Turabian StyleZhao, Bobo, Jiali Wang, Lu Wang, Ziwen Wang, and Aidang Lu. 2023. "Discovery of Flavone Derivatives Containing Carboxamide Fragments as Novel Antiviral Agents" Molecules 28, no. 5: 2179. https://doi.org/10.3390/molecules28052179