Microbial Hyaluronic Acid Production: A Review
Abstract
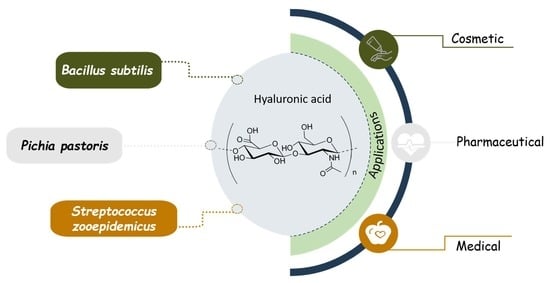
:1. Introduction
2. Hyaluronic Acid Production Using the Fermentation Process
3. Selection of a Microorganism Producer and Its Cultivation Media
3.1. Alternative Sources as Substrates for the Culture Media
- Agricultural waste: By-products of agriculture, such as sugar beet pulp, corn steep liquor, and wheat bran, have been found to be suitable substrates for hyaluronic acid production. These waste products are readily available and have the added benefit of being environmentally friendly [35].
- Synthetic substrates: Some researchers have also investigated synthetic substrates, such as hydrolysates of starch and cellulose, as an alternative substrate for hyaluronic acid production. These substrates are relatively cheap and easy to produce, making them an attractive option for commercial production [37].
3.2. Supplementation of Culture Media
- Carbon source supplements: Carbon sources such as glucose, fructose, and maltose can be added to the culture medium to provide an additional energy source for the microorganisms. This can help increase the rate of hyaluronic acid production [46].
- Nitrogen source supplements: Nitrogen sources such as ammonium sulfate and yeast extract can be added to the culture medium to provide essential amino acids and other nutrients for the microorganisms. This can help increase the rate of hyaluronic acid production [33].
- Vitamin supplements: Vitamins such as thiamine, riboflavin, and pyridoxine can be added to the culture medium to promote the growth of microorganisms and increase the rate of hyaluronic acid production [47].
- Mineral supplements: Minerals such as sodium, potassium, and calcium can be added to the culture medium to provide essential minerals for the microorganisms and promote hyaluronic acid production [34].
- Growth factors: Growth factors such as yeast extract, soy peptone, and tryptone can be added to the culture medium to provide additional nutrients and growth factors for the microorganisms [33]. This can help increase the rate of hyaluronic acid production.
4. Determination of Culture Conditions
- Temperature: The optimal temperature for the growth of the microorganisms and the production of hyaluronic acid will vary depending on the species used. The temperature range that is most suitable to produce HA is usually between 30 and 37 °C [50].
- pH: The pH of the culture medium is another important culture condition that must be determined. The pH must be maintained within a specific range that is suitable for the growth of the microorganisms and the production of hyaluronic acid. The optimal pH range for HA production is usually between 6.5 and 7.5 [57,58].
- Aeration: Adequate aeration is necessary for the growth of the microorganisms and the production of hyaluronic acid. The amount of air supplied to the culture medium can be adjusted to achieve the optimal aeration for the microorganisms used [59].
- Agitation: Agitation is the process of physically stirring the culture medium to ensure that the microorganisms are well-mixed and have access to nutrients and oxygen. The optimal agitation rate will depend on the microorganisms used and the culture medium [49].
- Substrate concentration: The concentration of the substrate in the culture medium will affect the rate of hyaluronic acid production. The optimal substrate concentration will depend on the microorganisms used and the culture medium [51].
5. Fermenter Configuration
- The fermenter vessel: This is the main container where the fermentation process takes place. The vessel is typically made of stainless steel and can be designed in a variety of shapes and sizes depending on the scale of the production.
- Agitator: This is a mechanical device that is used to mix the culture medium and provide oxygen to the microorganisms. Agitators can be designed in a variety of forms, such as propellers, impellers, and turbines, and can be adjusted to provide the optimal agitation rate for the microorganisms used.
- Aeration system: This is a device that is used to supply oxygen to the culture medium. Aeration systems can be designed in a variety of forms such as spargers, air bubblers, and air diffusers.
- pH and temperature control: These devices are used to monitor and maintain the pH and temperature of the culture medium within the optimal range for the growth and production of hyaluronic acid.
- Control system: This is a device that is used to control and monitor the various parameters of the fermentation process such as temperature, pH, agitation rate, and aeration rate.
6. Establishment of Culture Modes
- Batch culture: In batch culture, the microorganisms are grown in a closed vessel for a specific period of time, after which the culture is harvested, and the hyaluronic acid is extracted. Batch culture is simple and easy to set up, but it has the disadvantage of being less efficient and more costly than other modes of culture [31,56].
- Fed-batch culture: In fed-batch culture, the microorganisms are grown in a closed vessel and are periodically fed with a specific substrate or supplement. This allows for a higher concentration of microorganisms and a higher rate of hyaluronic acid production. Fed-batch culture is more efficient than batch culture, but it is more complex to set up and control [67].
- Continuous culture: In continuous culture, the microorganisms are grown in a closed vessel and are continuously supplied with a fresh medium. This allows for a high concentration of microorganisms and a high rate of hyaluronic acid production. Continuous culture is the most efficient mode of culture, but it is also the most complex to set up and control [56].
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhai, P.; Peng, X.; Li, B.; Liu, Y.; Sun, H.; Li, X. The application of hyaluronic acid in bone regeneration. Int. J. Biol. Macromol. 2020, 151, 1224–1239. [Google Scholar] [CrossRef]
- Necas, J.; Bartosikova, L.; Brauner, P.; Kolar, J. Hyaluronic acid (hyaluronan): A review. Vet. Med. 2008, 53, 397–411. [Google Scholar] [CrossRef] [Green Version]
- Fallacara, A.; Baldini, E.; Manfredini, S.; Vertuani, S. Hyaluronic acid in the third millennium. Polymers 2018, 10, 701. [Google Scholar] [CrossRef] [Green Version]
- Salwowska, N.M.; Bebenek, K.A.; Żądło, D.A.; Wcisło-Dziadecka, D.L. Physiochemical properties and application of hyaluronic acid: A systematic review. J. Cosmet. Dermatol. 2016, 15, 520–526. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Y.; Li, J.; Du, G.; Chen, J. Microbial production of hyaluronic acid: Current state, challenges, and perspectives. Microb. Cell Fact. 2011, 10, 99. [Google Scholar] [CrossRef] [Green Version]
- Gallo, N.; Nasser, H.; Salvatore, L.; Natali, M.L.; Campa, L.; Mahmoud, M.; Capobianco, L.; Sannino, A.; Madaghiele, M. Hyaluronic acid for advanced therapies: Promises and challenges. Eur. Polym. J. 2019, 117, 134–147. [Google Scholar] [CrossRef]
- Chong, B.F.; Blank, L.M.; Mclaughlin, R.; Nielsen, L.K. Microbial hyaluronic acid production. Appl. Microbiol. Biotechnol. 2005, 66, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Carlomagno, F.; Roveda, G.; Michelotti, A.; Ruggeri, F.; Tursi, F. Anti-Skin-Aging Effect of a Treatment with a Cosmetic Product and a Food Supplement Based on a New Hyaluronan: A Randomized Clinical Study in Healthy Women. Cosmetics 2022, 9, 54. [Google Scholar] [CrossRef]
- Boeriu, C.G.; Springer, J.; Kooy, F.K.; van den Broek, L.A.M.; Eggink, G. Production Methods for Hyaluronan. Int. J. Carbohydr. Chem. 2013, 2013, 624967. [Google Scholar] [CrossRef] [Green Version]
- Tan, J.; Spada, J.; Kerscher, M.; Anfilova, M.; Abdulla, S.; Altmeyer, A.; Delva, C.; Kerob, D.; Araviiskaia, E. 26268 M89, a combination of 89% of Vichy volcanic mineralizing water and hyaluronic acid improves signs and symptoms of subjects with rosacea/sensitive/reactive skin: Results from an open-label, observational, international study. J. Am. Acad. Dermatol. 2021, 85, AB93. [Google Scholar] [CrossRef]
- Goa, K.L.; Benfield, P. Hyaluronic Acid: A Review of its Pharmacology and Use as a Surgical Aid in Ophthalmology, and its Therapeutic Potential in Joint Disease and Wound Healing. Drugs 1994, 47, 536–566. [Google Scholar] [CrossRef] [PubMed]
- Kogan, G.; Šoltés, L.; Stern, R.; Gemeiner, P. Hyaluronic acid: A natural biopolymer with a broad range of biomedical and industrial applications. Biotechnol. Lett. 2007, 29, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Cordero, A.; Leon-Dorantes, G.; Pons-Guiraud, A.; Di Pietro, A.; Asensi, S.V.; Walkiewicz-Cyraska, B.; Litvik, R.; Turlier, V.; Mery, S.; Merial-Kieny, C. Retinaldehyde/hyaluronic acid fragments: A synergistic association for the management of skin aging. J. Cosmet. Dermatol. 2011, 10, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Neves, J.R.; Grether-Beck, S.; Krutmann, J.; Correia, P.; Gonçalves, J.E., Jr.; Sant’Anna, B.; Kerob, D. Efficacy of a topical serum containing L-ascorbic acid, neohesperidin, pycnogenol, tocopherol, and hyaluronic acid in relation to skin aging signs. J. Cosmet. Dermatol. 2022, 21, 4462–4469. [Google Scholar] [CrossRef]
- Becker, L.C.; Bergfeld, W.F.; Belsito, D.V.; Klaassen, C.D.; Marks, J.G., Jr.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; Andersen, F.A. Final Report of the Safety Assessment of Hyaluronic Acid, Potassium Hyaluronate, and Sodium Hyaluronate. Int. J. Toxicol. 2009, 28, 5–67. [Google Scholar] [CrossRef]
- Ferreira, R.G.; Azzoni, A.R.; Santana, M.H.A.; Petrides, D. Techno-economic analysis of a hyaluronic acid production process utilizing streptococcal fermentation. Processes 2021, 9, 241. [Google Scholar] [CrossRef]
- Yatmaz, E.; Turhan, İ. Hyaluronic acid and production by fermentation. Gıda 2015, 40, 233–240. [Google Scholar]
- Rangaswamy, V.; Santosh, V.; Dharmendra, J.; Nataraj, V.; Velankar, H.; Kapat, A. Process for producing high molecular weight hyaluronic acid. European Patent EP2046969B1, 2011. [Google Scholar]
- Jia, Y.; Zhu, J.; Chen, X.; Tang, D.; Su, D.; Yao, W.; Gao, X. Metabolic engineering of Bacillus subtilis for the efficient biosynthesis of uniform hyaluronic acid with controlled molecular weights. Bioresour. Technol. 2013, 132, 427–431. [Google Scholar] [CrossRef]
- Lai, Z.-W.; Rahim, R.A.; Ariff, A.B.; Mohamad, R. Comparison of Hyaluronic Acid Biosynthesis by the recombinant Escherichia Coli Strains in different mode of bioreactor operation. J. Microbiol. Biotechnol. Food Sci. 2016, 6, 905–910. [Google Scholar] [CrossRef] [Green Version]
- Woo, J.E.; Seong, H.J.; Lee, S.Y.; Jang, Y.S. Metabolic Engineering of Escherichia coli for the Production of Hyaluronic Acid from Glucose and Galactose. Front. Bioeng. Biotechnol. 2019, 7, 375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy, K.J.; Karunakaran, K.T. Purification and characterization of hyaluronic acid produced by Streptococcus zooepidemicus. J. BioSci. Biotech 2013, 2, 173–179. [Google Scholar]
- Westbrook, A.W.; Ren, X.; Moo-Young, M.; Chou, C.P. Engineering of cell membrane to enhance heterologous production of hyaluronic acid in Bacillus subtilis. Biotechnol. Bioeng. 2018, 115, 216–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, H.; Stephanopoulos, G. Metabolic engineering of Escherichia coli for biosynthesis of hyaluronic acid. Metab. Eng. 2008, 10, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Jeong, E.; Shim, W.Y.; Kim, J.H. Metabolic engineering of Pichia pastoris for production of hyaluronic acid with high molecular weight. J. Biotechnol. 2014, 185, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.C.; Cooney, M.J.; Johns, M.R. Growth and amino acid requirements of hyaluronic-acid producing Streptococcus zooepidemicus. Appl. Microbiol. Biotechnol. 1997, 47, 309–312. [Google Scholar] [CrossRef]
- Hoffmann, J.; Altenbuchner, J. Hyaluronic acid production with Corynebacterium glutamicum: Effect of media composition on yield and molecular weight. J. Appl. Microbiol. 2014, 117, 663–678. [Google Scholar] [CrossRef]
- Cheng, F.; Gong, Q.; Yu, H.; Stephanopoulos, G. High-titer biosynthesis of hyaluronic acid by recombinant Corynebacterium glutamicum. Biotechnol. J. 2016, 11, 574–584. [Google Scholar] [CrossRef]
- Cheng, F.; Luozhong, S.; Guo, Z.; Yu, H.; Stephanopoulos, G. Enhanced Biosynthesis of Hyaluronic Acid Using Engineered Corynebacterium glutamicum Via Metabolic Pathway Regulation. Biotechnol. J. 2017, 12, 1700191. [Google Scholar] [CrossRef]
- Sunguroğlu, C.; Sezgin, D.E.; Çelik, P.A.; Çabuk, A. Higher titer hyaluronic acid production in recombinant Lactococcus lactis. Prep. Biochem. Biotechnol. 2018, 48, 734–742. [Google Scholar] [CrossRef]
- Jeeva, P.; Doss, S.S.; Sundaram, V.; Jayaraman, G. Production of controlled molecular weight hyaluronic acid by glucostat strategy using recombinant Lactococcus lactis cultures. Appl. Microbiol. Biotechnol. 2019, 103, 4363–4375. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.M.V.; Netto, J.H.C.M.; Carvalho, L.S.; Parachin, N.S. Heterologous hyaluronic acid production in kluyveromyces lactis. Microorganisms 2019, 7, 294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, M.V.; Badle, S.S.; Ramachandran, K.B. Hyaluronic acid production and molecular weight improvement by redirection of carbon flux towards its biosynthesis pathway. Biochem. Eng. J. 2013, 80, 53–60. [Google Scholar] [CrossRef]
- Im, J.H.; Song, J.M.; Kang, J.H.; Kang, D.J. Optimization of medium components for high-molecular-weight hyaluronic acid production by Streptococcus sp. ID9102 via a statistical approach. J. Ind. Microbiol. Biotechnol. 2009, 36, 1337–1344. [Google Scholar] [CrossRef] [PubMed]
- Amado, I.R.; Vázquez, J.A.; Pastrana, L.; Teixeira, J.A. Microbial production of hyaluronic acid from agro-industrial by-products: Molasses and corn steep liquor. Biochem. Eng. J. 2017, 117, 181–187. [Google Scholar] [CrossRef] [Green Version]
- Amado, I.R.; Vázquez, J.A.; Pastrana, L.; Teixeira, J.A. Cheese whey: A cost-effective alternative for hyaluronic acid production by Streptococcus zooepidemicus. Food Chem. 2016, 198, 54–61. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Ding, X.; Yang, L.; Kong, Z. A serum-free medium for colony growth and hyaluronic acid production by Streptococcus zooepidemicus NJUST01. Appl. Microbiol. Biotechnol. 2006, 72, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Arslan, N.P.; Aydogan, M.N. Evaluation of Sheep Wool Protein Hydrolysate and Molasses as Low-Cost Fermentation Substrates for Hyaluronic Acid Production by Streptococcus zooepidemicus ATCC 35246. Waste Biomass Valorization 2021, 12, 925–935. [Google Scholar] [CrossRef]
- Pan, N.C.; Pereira, H.C.B.; da Silva, M.L.C.; Vasconcelos, A.F.D.; Celligoi, M.A.P.C. Improvement Production of Hyaluronic Acid by Streptococcus zooepidemicus in Sugarcane Molasses. Appl. Biochem. Biotechnol. 2017, 182, 276–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pires, A.M.B.; Macedo, A.C.; Eguchi, S.Y.; Santana, M.H.A. Microbial production of hyaluronic acid from agricultural resource derivatives. Bioresour. Technol. 2010, 101, 6506–6509. [Google Scholar] [CrossRef]
- Vázquez, J.A.; Montemayor, M.I.; Fraguas, J.; Murado, M.A. Hyaluronic acid production by Streptococcus zooepidemicus in marine by-products media from mussel processing wastewaters and tuna peptone viscera. Microb. Cell Fact. 2010, 9, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vázquez, J.A.; Pastrana, L.; Piñeiro, C.; Teixeira, J.A.; Pérez-Martín, R.I.; Amado, I.R. Production of hyaluronic acid by Streptococcus zooepidemicus on protein substrates obtained from Scyliorhinus canicula discards. Mar. Drugs 2015, 13, 6537–6549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benedini, L.J.; Santana, M.H.A. Effects of soy peptone on the inoculum preparation of Streptococcus zooepidemicus for production of hyaluronic acid. Bioresour. Technol. 2013, 130, 798–800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghodke, R.S.; Kakati, J.P.; Tadi, S.R.R.; Mohan, N.; Silvaprakasam, S. Kinetic modeling of hyaluronic acid production in palmyra palm (Borassus flabellifer) based medium by Streptococcus zooepidemicus MTCC 3523. Biochem. Eng. J. 2018, 137, 284–293. [Google Scholar] [CrossRef]
- Duffeck, H.C.B.P.; Pan, N.C.; Saikawa, G.I.A.; da Rocha, S.P.D.; Baldo, C.; Celligoi, M.A.P.C. Biomedical Potential of Hyaluronic Acid from Streptococcus zooepidemicus Produced in Sugarcane Molasses. Braz. J. Dev. 2020, 6, 49963–49980. [Google Scholar] [CrossRef]
- Aroskar, V.J.; Kamat, S.D.; Kamat, D. Effect of Various Nutritional Supplements on Hyaluronic Acid Production. IIOAB Lett. 2013, 2, 16–24. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Yoo, S.J.; Oh, D.K.; Kweon, Y.G.; Park, D.W.; Lee, C.H.; Gil, G.H. Selection of a Streptococcus equi mutant and optimization of culture conditions for the production of high molecular weight hyaluronic acid. Enzyme Microb. Technol. 1996, 19, 440–445. [Google Scholar] [CrossRef]
- Lai, Z.W.; Rahim, R.A.; Ariff, A.B.; Mohamad, R. Biosynthesis of high molecular weight hyaluronic acid by Streptococcus zooepidemicus using oxygen vector and optimum impeller tip speed. J. Biosci. Bioeng. 2012, 114, 286–291. [Google Scholar] [CrossRef]
- Liu, L.; Du, G.; Chen, J.; Wang, M.; Sun, J. Comparative study on the influence of dissolved oxygen control approaches on the microbial hyaluronic acid production of Streptococcus zooepidemicus. Bioprocess Biosyst. Eng. 2009, 32, 755–763. [Google Scholar] [CrossRef]
- Armstrong, D.C.; Johns, M.R. Culture conditions affect the molecular weight properties of hyaluronic acid produced by Streptococcus zooepidemicus. Appl. Environ. Microbiol. 1997, 63, 2759–2764. [Google Scholar] [CrossRef] [Green Version]
- Don, M.M.; Shoparwe, N.F. Kinetics of hyaluronic acid production by Streptococcus zooepidemicus considering the effect of glucose. Biochem. Eng. J. 2010, 49, 95–103. [Google Scholar] [CrossRef]
- Zakeri, A.; Rasaee, M.J. Identification of wild type Streptococcus Zooepidemicus and optimization of culture medium and fermentation conditions for production of hyaluronic acid. Biosci. Biotechnol. Res. Asia 2016, 13, 189–198. [Google Scholar] [CrossRef] [Green Version]
- Gedikli, S.; Güngör, G.; Toptaş, Y.; Sezgin, D.E.; Demirbilek, M.; Yazıhan, N.; Çelik, P.A.; Denkbaş, E.B.; Bütün, V.; Çabuk, A. Optimization of hyaluronic acid production and its cytotoxicity and degradability characteristics. Prep. Biochem. Biotechnol. 2018, 48, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Rangaswamy, V.; Jain, D. An efficient process for production and purification of hyaluronic acid from Streptococcus equi subsp. zooepidemicus. Biotechnol. Lett. 2008, 30, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Kakema, T.; Fujii, K.; Ikemi, M. Method for producing hyaluronic acid. US patent 8927234B2, 2015. [Google Scholar]
- Chen, S.J.; Chen, J.L.; Huang, W.C.; Chen, H.L. Fermentation process development for hyaluronic acid production by Streptococcus zooepidemicus ATCC 39920. Korean J. Chem. Eng. 2009, 26, 428–432. [Google Scholar] [CrossRef]
- Johns, M.R.; Goh, L.; Oeggeru, A. Effect of pH, agitation and aeration on Hyaluronic Acid production by Streptococcus Zooepidemicus. Biotechnol. Lett. 1994, 16, 507–512. [Google Scholar] [CrossRef]
- Liu, L.; Wang, M.; Du, G.; Chen, J. Enhanced hyaluronic acid production of Streptococcus zooepidemicus by an intermittent alkaline-stress strategy. Soc. Appl. Microbiol. Lett. Appl. Microbiol. 2008, 46, 383–388. [Google Scholar] [CrossRef]
- Huang, W.C.; Chen, S.J.; Chen, T.L. The role of dissolved oxygen and function of agitation in hyaluronic acid fermentation. Biochem. Eng. J. 2006, 32, 239–243. [Google Scholar] [CrossRef]
- Saharkhiz, S.; Babaeipour, V. The dilution effect of media culture on mixing time, Kla O2, and hyaluronic acid production in S. zooepidemicus fed-batch culture. Biotechnol. Lett. 2021, 43, 2217–2222. [Google Scholar] [CrossRef]
- Li, Y.; Li, G.; Zhao, X.; Shao, Y.; Wu, M.; Ma, T. Regulation of hyaluronic acid molecular weight and titer by temperature in engineered Bacillus subtilis. 3 Biotech 2019, 9, 225. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.J.; Niu, H.X.; Tan, W.S.; Zhang, X. Mechanism analysis of effect of oxygen on molecular weight of hyaluronic acid produced by Streptococcus zooepidemicus. J. Microbiol. Biotechnol. 2009, 19, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, Y.; Li, Z.; Ren, Y.; Zhao, Y.; Zhao, G. Efficient production of high-molecular-weight hyaluronic acid with a two-stage fermentation. RSC Adv. 2018, 8, 36167–36171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Shi, Z.; Shao, Y.; Wu, M.; Li, G.; Ma, T. Temperature-controlled molecular weight of hyaluronic acid produced by engineered Bacillus subtilis. Biotechnol. Lett. 2021, 43, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Stanburry, P.F.; Whitaker, A.; Hall, S. Microbial Growth Kinetics. In Principles of Fermentation Technology; Butterworth Heinemann: Oxford, UK, 1995. [Google Scholar]
- Widner, B.; Behr, R.; Von Dollen, S.; Tang, M.; Heu, T.; Sloma, A.; Sternberg, D.; DeAngelis, P.L.; Weigel, P.H.; Brown, S. Hyaluronic Acid Production in Bacillus subtilis. Appl. Environ. Microbiol. 2005, 71, 3747–3752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Du, G.; Chen, J.; Wang, M.; Sun, J. Influence of culture modes on the microbial production of hyaluronic acid by Streptococcus zooepidemicus. Biotechnol. Bioprocess Eng. 2008, 13, 269–273. [Google Scholar] [CrossRef]
- Liu, L.; Du, G.; Chen, J.; Wang, M.; Sun, J. Enhanced hyaluronic acid production by a two-stage culture strategy based on the modeling of batch and fed-batch cultivation of Streptococcus zooepidemicus. Bioresour. Technol. 2008, 99, 8532–8536. [Google Scholar] [CrossRef]
- Huang, W.C.; Chen, S.J.; Chen, T.L. Production of hyaluronic acid by repeated batch fermentation. Biochem. Eng. J. 2008, 40, 460–464. [Google Scholar] [CrossRef]
- Badle, S.S.; Jayaraman, G.; Ramachandran, K.B. Ratio of intracellular precursors concentration and their flux influences hyaluronic acid molecular weight in Streptococcus zooepidemicus and recombinant Lactococcus lactis. Bioresour. Technol. 2014, 163, 222–227. [Google Scholar] [CrossRef]
- Reddy, K.J.; Karunakaran, K.T.; Rao, K.R.S.S. Enhanced hyaluronic acid production by a mutant strain, 3523–3527 of Streptococcus zooepidemicus. Curr. Trends Biotechnol. Pharm. 2011, 5, 1473–1479. [Google Scholar]
- Mohan, N.; Balakrishnan, R.; Sivaprakasam, S. Optimization and effect of dairy industrial waste as media components in the production of hyaluronic acid by Streptococcus thermophilus. Prep. Biochem. Biotechnol. 2016, 46, 628–638. [Google Scholar] [CrossRef] [PubMed]

| Microorganism | Culture Media | Fermentation Conditions | Culture Mode | [HA]/MW | References |
|---|---|---|---|---|---|
| Streptococcus. Zooepidemicus ATCC 35246 | Cheese whey protein Glucose Yeast extract Tryptone | pH 6.7 T 37.00 °C 500 rpm 1.0 vvm | Batch mode 5 L bioreactor | 4.0 g/L -12 h 3.71 × 103 kDa 0.87 g/L h | [36] |
| Streptococcus zooepidemicus ATCC 35246 | Molasses Sheep Wool Peptone | pH 8 T 37.00 °C 200 rpm 1.0 vvm | 250 mL flasks | 3.54 g/L-48 h | [38] |
| Streptococcus zooepidemicus ATCC 35246 | Glucose Yeast extract Peptone from Scyliorhinus Canicula visceral treatment | pH 6.7 T 37 °C 500 rpm 0 vvm | Fed-batch mode 2 L bioreactor | 2.53 g/L-18 h 2.11 × 103 kDa | [42] |
| Streptococcus zooepidemicus NJUST01 | Starch (5%) Glucose Peptone Yeast extract | T 37 °C 220 rpm | Batch mode 500 mL flasks | 6.7 g/L-36 h | [37] |
| Streptococcus sp. ID9102 mutant strain KCTC 1139BP | Glucose Yeast extract Casein Peptone K2HPO4 MgCl2 Glutamine Glutamate Oxalic acid | pH 7 T 36 °C 400 rpm 0.5 vvm | Batch mode 75 L jar fermenter | 6.94 g/L-24 h 5.9 × 103 kDa | [34] |
| Streptococcus zooepidemicus WSH-24 | Sucrose Yeast extract 3% PFC as an oxygen vector | pH 7 T 37 °C 200 rpm 0.5 vvm | Batch mode 7 L bioreactor | 6.6 g/L-20 h | [49] |
| Streptococcus zooepidemicus WSH-24 | Sucrose Yeast extract | T 37 °C 200 rpm aeration 2 L/min alkaline-stress fermentation | Batch mode 7 L bioreactor | 6.5 g/L-16 h | [58] |
| Streptococcus zooepidemicus WSH-24 | Sucrose Yeast extract | pH 7 T 37 °C 200 rpm 0.5 vvm | fed-batch mode (0–8 h) batch mode (8–20 h) 7 L bioreactor | 6.6 g/L-20 h 0.023 g HA/g cell/h | [68] |
| Streptococcus zooepidemicus ATCC 39920 | Glucose Yeast extract BHI Ascorbic Acid | pH 7 T 37.00 °C 200 rpm 1 vvm dilution rate 0.066 h−1 | Chemostat mode 2,4 L Bioengineering reactor | 2.6 × 103 kDa | [70] |
| Streptococcus thermophilus NCIM 2904 | Whey Permeate Whey Protein hydrolysate | pH 8 T 37 °C 150 rpm | Batch mode 250 mL flasks | 0.34 g/L-20 h 9.22–9.46 kDa | [72] |
| Bacillus subtilis WmB | Sucrose | T 32 °C | 5.0 L fermenter | 3.65 g/L-54 h 3.92 × 102 kDa | [61] |
| Corynebacterium glutamicum/Δldh-AB | Corn Syrup Powder Glucose IPTG (isopropyl-β-D-thiogalactoside) | pH 7.2 T 28 °C 600 rpm 1 vvm | Fed-batch mode 5.0 L fermenter | 21.6 g/L-48 h 1.28 × 103 kDa | [29] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serra, M.; Casas, A.; Toubarro, D.; Barros, A.N.; Teixeira, J.A. Microbial Hyaluronic Acid Production: A Review. Molecules 2023, 28, 2084. https://doi.org/10.3390/molecules28052084
Serra M, Casas A, Toubarro D, Barros AN, Teixeira JA. Microbial Hyaluronic Acid Production: A Review. Molecules. 2023; 28(5):2084. https://doi.org/10.3390/molecules28052084
Chicago/Turabian StyleSerra, Mónica, Ana Casas, Duarte Toubarro, Ana Novo Barros, and José António Teixeira. 2023. "Microbial Hyaluronic Acid Production: A Review" Molecules 28, no. 5: 2084. https://doi.org/10.3390/molecules28052084