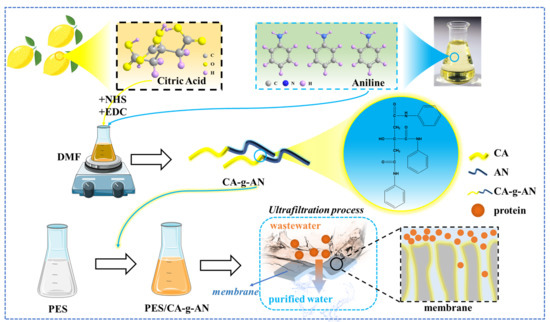
Amphiphilic Grafted Polymers Based on Citric Acid and Aniline Used to Enhance the Antifouling and Permeability Properties of PES Membranes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of CA–g–AN
2.1.1. 1H NMR Analysis of CA–g–AN
2.1.2. FT-IR Analysis of CA–g–AN
2.1.3. TG Analysis of CA–g–AN
2.1.4. XPS Analysis of CA–g–AN
2.2. Hydrophilicity of the Membranes
2.3. AFM Observation and XPS Analysis of the Membrane Surface
2.4. Membrane Morphology, Porosity, and Mean Pore Size Analysis of the Membranes
2.5. Ultrafiltration Performance of the CA–g–AN Modified Membrane
3. Materials and Methods
3.1. Materials
3.2. Synthesis of CA–g–AN
3.3. Preparation of the Membranes
3.4. Characterization of CA–g–AN
3.5. Characterization of the Membranes
3.6. Porosity and Mean Pore Size Analysis of the Membranes
3.7. Ultrafiltration Experiment of the Membranes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tan, H.-F.; Ooi, B.S.; Leo, C.P. Future perspectives of nanocellulose-based membrane for water treatment. J. Water. Process. Eng. 2020, 37, 101502. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, J.; Li, L.; Zhen, G.; Lu, X.; Zhang, J.; Liu, H.; Zhou, Z.; Wu, Z.; Zhang, X. Prospects for humic acids treatment and recovery in wastewater: A review. Chemosphere 2023, 312, 137193. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Shi, Y.; Liu, G.; Fan, X.; Yu, Y. Recent development of graphene oxide based forward osmosis membrane for water treatment: A critical review. Desalination 2020, 491, 114452. [Google Scholar] [CrossRef]
- Lan, D.; Zhu, H.; Zhang, J.; Li, S.; Chen, Q.; Wang, C.; Wu, T.; Xu, M. Adsorptive removal of organic dyes via porous materials for wastewater treatment in recent decades: A review on species, mechanisms and perspectives. Chemosphere 2022, 293, 133464. [Google Scholar] [CrossRef]
- Khajvand, M.; Mostafazadeh, A.K.; Drogui, P.; Tyagi, R.D.; Brien, E. Greywater characteristics, impacts, treatment, and reclamation using adsorption processes towards the circular economy. Environ. Sci. Pollut. Res. 2022, 29, 10966–11003. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, H.; Jiang, Z.; Cai, T.; Li, H.; Li, H.; Li, A.; Cheng, R. Flocculation of both anionic and cationic dyes in aqueous solutions by the amphoteric grafting flocculant carboxymethyl chitosan-graft-polyacrylamide. J. Hazard. Mater. 2013, 254–255, 36–45. [Google Scholar] [CrossRef]
- Amor, C.; Torres-Socías, E.D.; Peres, J.A.; Maldonado, M.I.; Oller, I.; Malato, S.; Lucas, M.S. Mature landfill leachate treatment by coagulation/flocculation combined with Fenton and solar photo-Fenton processes. J. Hazard. Mater. 2015, 286, 261–268. [Google Scholar] [CrossRef]
- Cseri, L.; Topuz, F.; Abdulhamid, M.A.; Alammar, A.; Budd, P.M.; Szekely, G. Electrospun Adsorptive Nanofibrous Membranes from Ion Exchange Polymers to Snare Textile Dyes from Wastewater. Adv. Mater. Technol. 2021, 6, 2000955. [Google Scholar] [CrossRef]
- Goyal, P.; Tiwary, C.S.; Misra, S.K. Ion exchange based approach for rapid and selective Pb(II) removal using iron oxide decorated metal organic framework hybrid. J. Environ. Manage. 2021, 277, 111469. [Google Scholar] [CrossRef]
- Camacho, L.M.; Dumée, L.; Zhang, J.; Li, J.-d.; Duke, M.; Gomez, J.; Gray, S. Advances in Membrane Distillation for Water Desalination and Purification Applications. Water 2013, 5, 94–196. [Google Scholar] [CrossRef] [Green Version]
- Duman, O.; Uğurlu, H.; Diker, C.Ö.; Tunç, S. Fabrication of highly hydrophobic or superhydrophobic electrospun PVA and agar/PVA membrane materials for efficient and selective oil/water separation. J. Environ. Chem. Eng. 2022, 10, 107405. [Google Scholar] [CrossRef]
- Wang, H.; Li, J.; Yu, X.; Zhao, X.; Zeng, X.; Xu, F.; Tang, X.; Sun, Y.; Lin, L. Facile fabrication of super-hydrophilic cellulose hydrogel-coated mesh using deep eutectic solvent for efficient gravity-driven oil/water separation. Cellulose 2021, 28, 949–960. [Google Scholar] [CrossRef]
- Li, R.; Li, J.; Rao, L.; Lin, H.; Shen, L.; Xu, Y.; Chen, J.; Liao, B.-Q. Inkjet printing of dopamine followed by UV light irradiation to modify mussel-inspired PVDF membrane for efficient oil-water separation. J. Membr. Sci. 2021, 619, 118790. [Google Scholar] [CrossRef]
- Zhao, C.; Xue, J.; Ran, F.; Sun, S. Modification of polyethersulfone membranes—A review of methods. Prog. Mater. Sci. 2013, 58, 76–150. [Google Scholar] [CrossRef]
- Van der Bruggen, B. Chemical modification of polyethersulfone nanofiltration membranes: A review. J. Appl. Polym. Sci. 2009, 114, 630–642. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, W.; Zhu, L.; Li, N.; Chen, X.; Tian, J.; Zhang, X. Simultaneously enhanced permeability and anti-fouling performance of polyethersulfone ultrafiltration membranes by structural control and mixed carbon quantum dots. J. Membr. Sci. 2022, 641, 119931. [Google Scholar] [CrossRef]
- Nie, Z.; Liu, C.; Jiang, X.; Zhou, Y.; Lin, X.; Zhao, X.; He, Q.; Chai, H.; Pang, X.; Ma, J. Dopamine-triggered one-step codeposition of zwitterionic surfactants for anti-fouling polyethersulfone ultrafiltration membrane modification. Appl. Surf. Sci. 2022, 598, 153871. [Google Scholar] [CrossRef]
- Zhang, P.; Li, R. Preparation and performance of acrylic acid grafted PES ultrafiltration membrane via plasma surface activation. High Perform. Polym. 2022, 34, 1131–1142. [Google Scholar] [CrossRef]
- Louie, J.S.; Pinnau, I.; Ciobanu, I.; Ishida, K.P.; Ng, A.; Reinhard, M. Effects of polyether–polyamide block copolymer coating on performance and fouling of reverse osmosis membranes. J. Membr. Sci. 2006, 280, 762–770. [Google Scholar] [CrossRef]
- Kallem, P.; Ibrahim, Y.; Hasan, S.W.; Show, P.L.; Banat, F. Fabrication of novel polyethersulfone (PES) hybrid ultrafiltration membranes with superior permeability and antifouling properties using environmentally friendly sulfonated functionalized polydopamine nanofillers. Sep. Purif. Technol. 2021, 261, 118311. [Google Scholar] [CrossRef]
- Li, R.; Liang, Y.; Li, Q.; Niu, H.; Zhang, Y.; Li, Q. Studies on surface properties of polyethersulfone membrane by remote argon plasma. Vacuum 2020, 175, 109276. [Google Scholar] [CrossRef]
- Abdul Rahman, A.F.H.B.; Abu Seman, M.N.B. Polyacrylic-polyethersulfone membrane modified via UV photografting for forward osmosis application. J. Environ. Chem. Eng. 2018, 6, 4368–4379. [Google Scholar] [CrossRef]
- Wang, H.; Yu, T.; Zhao, C.; Du, Q. Improvement of hydrophilicity and blood compatibility on polyethersulfone membrane by adding polyvinylpyrrolidone. Fibers Polym. 2009, 10, 1–5. [Google Scholar] [CrossRef]
- Wang, Y.-Q.; Wang, T.; Su, Y.-L.; Peng, F.-B.; Wu, H.; Jiang, Z.-Y. Protein-adsorption-resistance and permeation property of polyethersulfone and soybean phosphatidylcholine blend ultrafiltration membranes. J. Membr. Sci. 2006, 270, 108–114. [Google Scholar] [CrossRef]
- Li, J.-H.; Li, M.-Z.; Miao, J.; Wang, J.-B.; Shao, X.-S.; Zhang, Q.-Q. Improved surface property of PVDF membrane with amphiphilic zwitterionic copolymer as membrane additive. Appl. Surf. Sci. 2012, 258, 6398–6405. [Google Scholar] [CrossRef]
- Orooji, Y.; Faghih, M.; Razmjou, A.; Hou, J.; Moazzam, P.; Emami, N.; Aghababaie, M.; Nourisfa, F.; Chen, V.; Jin, W. Nanostructured mesoporous carbon polyethersulfone composite ultrafiltration membrane with significantly low protein adsorption and bacterial adhesion. Carbon 2017, 111, 689–704. [Google Scholar] [CrossRef]
- Wang, J.; Qiu, M.; He, C. A zwitterionic polymer/PES membrane for enhanced antifouling performance and promoting hemocompatibility. J. Membr. Sci. 2020, 606, 118119. [Google Scholar] [CrossRef]
- Shalaby, M.; Mansor, E.S.; Abdallah, H.; Shaban, A.M.; Zhu, B.-K.; Fang, L.-F. Antiviral amphiphilic membranes based on the organometallic compound for protein removal from wastewater with fouling-resistant. J. Polym. Res. 2021, 28, 150. [Google Scholar] [CrossRef]
- Kang, D.; Shao, H.; Chen, G.; Dong, X.; Qin, S. Microstructure manipulation in PVDF/styrene-maleic anhydride copolymer composite membranes: Effects of miscibility on the phase separation. Sep. Purif. Technol. 2021, 263, 118371. [Google Scholar] [CrossRef]
- Gaalken, J.; Ulbricht, M. Polyethyleneoxide-b-poly(isopropyl methacrylate) diblock copolymers as novel material for ultrafiltration membranes. J. Polym. Sci. 2020, 58, 2561–2574. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Hashim, N.A.; Liu, Y.; Abed, M.R.M.; Li, K. Progress in the production and modification of PVDF membranes. J. Membr. Sci. 2011, 375, 1–27. [Google Scholar] [CrossRef]
- Wang, C.; Song, X.; Liu, Y.; Zhang, C. PVC-g-PVP amphiphilic polymer synthesis by ATRP and its membrane separation performance for silicone-containing wastewater. Polymer 2021, 229, 123965. [Google Scholar] [CrossRef]
- Wu, H.; Li, T.; Liu, B.; Chen, C.; Wang, S.; Crittenden, J.C. Blended PVC/PVC-g-PEGMA ultrafiltration membranes with enhanced performance and antifouling properties. Appl. Surf. Sci. 2018, 455, 987–996. [Google Scholar] [CrossRef]
- Mahdavi, H.; Zeinalipour, N.; Kerachian, M.A.; Heidari, A.A. Preparation of high-performance PVDF mixed matrix membranes incorporated with PVDF-g-PMMA copolymer and GO@SiO2 nanoparticles for dye rejection applications. J. Water Process Eng. 2022, 46, 102560. [Google Scholar] [CrossRef]
- Zhao, Y.-F.; Zhang, P.-B.; Sun, J.; Liu, C.-J.; Zhu, L.-P.; Xu, Y.-Y. Electrolyte-responsive polyethersulfone membranes with zwitterionic polyethersulfone-based copolymers as additive. J. Membr. Sci. 2016, 510, 306–313. [Google Scholar] [CrossRef]
- Ji, Y.; Yang, X.; Ji, Z.; Zhu, L.; Ma, N.; Chen, D.; Jia, X.; Tang, J.; Cao, Y. DFT-Calculated IR Spectrum Amide I, II, and III Band Contributions of N-Methylacetamide Fine Components. ACS Omega 2020, 5, 8572–8578. [Google Scholar] [CrossRef] [Green Version]
- Yan, J.; Huang, Y.; Wei, C.; Zhang, N.; Liu, P. Covalently bonded polyaniline/graphene composites as high-performance electromagnetic (EM) wave absorption materials. Compos. Part A 2017, 99, 121–128. [Google Scholar] [CrossRef]
- Liu, L.; Jiao, L.; Zhang, Y.; Sun, J.; Yang, L.; Miao, Y.; Yuan, H.; Wang, Y. Synthesis of LiV3O8 by an improved citric acid assisted sol–gel method at low temperature. Mater. Chem. Phys. 2008, 111, 565–569. [Google Scholar] [CrossRef]
- Lee, S.; Noh, J.; Hong, S.; Kim, Y.K.; Jang, J. Dual stimuli-responsive smart fluid of graphene oxide-coated iron oxide/silica core/shell nanoparticles. Chem. Mater. 2016, 28, 2624–2633. [Google Scholar] [CrossRef]
- Hsine, Z.; Bizid, S.; Mlika, R.; Sauriat-Dorizon, H.; Haj Said, A.; Korri-Youssoufi, H. Nanocomposite Based on Poly (para-phenylene)/Chemical Reduced Graphene Oxide as a Platform for Simultaneous Detection of Ascorbic Acid, Dopamine and Uric Acid. Sensors 2020, 20, 1256. [Google Scholar] [CrossRef] [Green Version]
- Masrie, M.; Badaruddin, S.; Hussin, M.; Nor, N.; Joe, J. Rapid reduction of graphene oxide thin films on large-area silicon substrate. In Journal of Physics: Conference Series, 2020; IOP Publishing: Bristol, UK, 2020; p. 012027. [Google Scholar]
- Zhang, J.; Wang, H.; Xiao, Y.; Tang, J.; Liang, C.; Li, F.; Dong, H.; Xu, W. A Simple Approach for Synthesizing of Fluorescent Carbon Quantum Dots from Tofu Wastewater. Nanoscale Res. Lett. 2017, 12, 611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, X.; Zhang, L.; Duan, B. Polyphenol-mediated chitin self-assembly for constructing a fully naturally resourced hydrogel with high strength and toughness. Mater. Horiz. 2021, 8, 2503–2512. [Google Scholar] [CrossRef] [PubMed]
- Ahmad Daud, N.; Chieng, B.W.; Ibrahim, N.A.; Talib, Z.A.; Muhamad, E.N.; Abidin, Z.Z. Functionalizing graphene oxide with alkylamine by gamma-ray irradiation method. Nanomaterials 2017, 7, 135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dutta, M.; Bhattacharjee, S.; De, S. Separation of reactive dyes from textile effluent by hydrolyzed polyacrylonitrile hollow fiber ultrafiltration quantifying the transport of multicomponent species through charged membrane pores. Sep. Purif. Technol. 2020, 234, 116063. [Google Scholar] [CrossRef]
- Kumari, P.; Modi, A.; Bellare, J. Enhanced flux and antifouling property on municipal wastewater of polyethersulfone hollow fiber membranes by embedding carboxylated multi-walled carbon nanotubes and a vitamin E derivative. Sep. Purif. Technol. 2020, 235, 116199. [Google Scholar] [CrossRef]
- Sun, H.; Yang, X.; Zhang, Y.; Cheng, X.; Xu, Y.; Bai, Y.; Shao, L. Segregation-induced in situ hydrophilic modification of poly (vinylidene fluoride) ultrafiltration membranes via sticky poly (ethylene glycol) blending. J. Membr. Sci. 2018, 563, 22–30. [Google Scholar] [CrossRef]







| Membrane Name | Porosity (%) | Mean Pore Size (nm) |
|---|---|---|
| PES | 66.84 ± 2.60 | 47.07 ± 2.24 |
| PES/0.10–CA–g–AN | 70.83 ± 3.09 | 49.47 ± 0.89 |
| PES/0.15–CA–g–AN | 71.90 ± 9.10 | 77.52 ± 2.38 |
| PES/0.20–CA–g–AN | 73.46 ± 1.79 | 85.47 ± 3.30 |
| PES/0.25–CA–g–AN | 77.64 ± 9.19 | 98.22 ± 3.62 |
| Membrane Name | PES (wt%) | PVP (wt%) | CA–g–AN (wt%) | DMAc (wt%) |
|---|---|---|---|---|
| PES | 18.00 | 1.00 | 0.00 | 81.00 |
| PES/0.10–CA–g–AN | 18.00 | 1.00 | 0.10 | 80.90 |
| PES/0.15–CA–g–AN | 18.00 | 1.00 | 0.15 | 80.85 |
| PES/0.20–CA–g–AN | 18.00 | 1.00 | 0.20 | 80.80 |
| PES/0.25–CA–g–AN | 18.00 | 1.00 | 0.25 | 80.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Zhang, P.; Cao, L.; Huo, H.; Lin, H.; Wang, Q.; Vogel, F.; Li, W.; Lin, Z. Amphiphilic Grafted Polymers Based on Citric Acid and Aniline Used to Enhance the Antifouling and Permeability Properties of PES Membranes. Molecules 2023, 28, 1936. https://doi.org/10.3390/molecules28041936
Zhao J, Zhang P, Cao L, Huo H, Lin H, Wang Q, Vogel F, Li W, Lin Z. Amphiphilic Grafted Polymers Based on Citric Acid and Aniline Used to Enhance the Antifouling and Permeability Properties of PES Membranes. Molecules. 2023; 28(4):1936. https://doi.org/10.3390/molecules28041936
Chicago/Turabian StyleZhao, Jiahui, Peng Zhang, Lin Cao, Haoling Huo, Huaijun Lin, Qiwei Wang, Florian Vogel, Wei Li, and Zhidan Lin. 2023. "Amphiphilic Grafted Polymers Based on Citric Acid and Aniline Used to Enhance the Antifouling and Permeability Properties of PES Membranes" Molecules 28, no. 4: 1936. https://doi.org/10.3390/molecules28041936