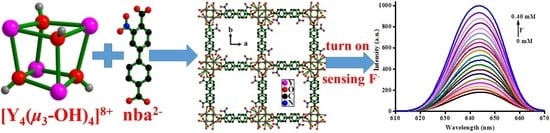
A 12-Connected [Y4((μ3-OH)4]8+ Cluster-Based Luminescent Metal-Organic Framework for Selective Turn-on Detection of F− in H2O
Abstract
:1. Introduction
2. Results and Discussion
2.1. Description of the Structure of {[Y(μ3-OH)]4[Y(μ3-OH)(μ2-H2O)0.25(H2O)0.5]4[μ4-nba]8}n (1)
2.2. Luminescent Properties
2.2.1. Fluorescence Properties of 1 and H2nba in Aqueous Solution
2.2.2. Detection of Cations
2.2.3. Detection of F− Anion
2.2.4. Stability and Cyclic Use Test
2.2.5. IR Spectrum Analysis
2.2.6. The Scalability of the Synthesis for 1
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Apparatus
3.3. Synthesis of {[Y(μ3-OH)]4[Y(μ3-OH)(μ2-H2O)0.25(H2O)0.5]4[μ4-nba]8}n (1)
3.4. Photoluminescent Sensing Experiments
3.5. Fluorescence Titration Experiments for F−
3.6. Recyclable Luminescence Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Snyder, B.E.R.; Turkiewicz, A.B.; Furukawa, H.; Paley, M.V.; Velasquez, E.O.; Dods, M.N.; Long, J.R. A ligand insertion mechanism for cooperative NH3 capture in metal–organic frameworks. Nature 2023, 613, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Datta, S.J.; Zhou, S.; Jia, J.; Shekhah, O.; Eddaoudi, M. Advances in metal-organic framework-based membranes. Chem. Soc. Rev. 2022, 51, 8300–8350. [Google Scholar] [CrossRef] [PubMed]
- Knebel, A.; Caro, J. Metal-organic frameworks and covalent organic frameworks as disruptive membrane materials for energy-efficient gas separation. Nat. Nanotechnol. 2022, 17, 911–923. [Google Scholar] [CrossRef]
- Datta, S.J.; Mayoral, A.; Bettahalli, N.M.S.; Bhatt, P.M.; Karunakaran, M.; Carja, I.D.; Fan, D.; Mileo, P.G.M.; Semino, R.; Maurin, G.; et al. Rational design of mixed-matrix metal-organic framework membranes for molecular separations. Science 2022, 376, 1080–1087. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Shekhah, O.; Ramírez, A.; Lyu, P.; Abou-Hamad, E.; Jia, J.; Li, J.; Bhatt, P.M.; Huang, Z.; Jiang, H.; et al. Asymmetric pore windows in MOF membranes for natural gas valorization. Nature 2022, 606, 706–712. [Google Scholar] [CrossRef]
- Wang, J.X.; Wang, Y.; Nadinov, I.; Yin, J.; Gutierrez-Arzaluz, L.; Healing, G.; Alkhazragi, O.; Cheng, Y.; Jia, J.; Alsadun, N. Metal-Organic Frameworks in Mixed-Matrix Membranes for High-Speed Visible-Light Communication. J. Am. Chem. Soc. 2022, 144, 6813–6820. [Google Scholar] [CrossRef]
- Lu, J.; Xu, H.; Yu, H.; Hu, X.; Xia, J.; Zhu, Y.; Wang, C.F.; Wu, H.A.; Jiang, L.; Wang, H. Ultrafast rectifying counter-directional transport of proton and metal ions in metal-organic framework–based nanochannels. Sci. Adv. 2022, 18, eabl5070. [Google Scholar] [CrossRef]
- Ye, C.R.; Wang, W.J.; Chen, W.; Xiao, Y.; Zhang, H.F.; Dai, B.L.; Chen, S.H.; Wu, X.D.; Li, M.; Huang, X.C. Harnessing Shape Complementarity for Upgraded Cyclohexane Purification through Adaptive Bottlenecked Pores in an Imidazole-Containing MOF. Angew. Chem. Int. Ed. 2022, 60, 23590–23595. [Google Scholar] [CrossRef]
- Bellis, J.D.; Dell’Amico, D.B.; Ciancaleoni, G.; Labella, L.; Marchetti, F.; Samaritani, S. Interconversion of lanthanide-organic frameworks based on the anions of 2,5-dihydroxyterephthalic acid as connectors. Inorg. Chim. Acta 2019, 495, 118937. [Google Scholar] [CrossRef]
- Li, J.X.; Xia, Y.Q.; Cheng, L.M.; Feng, X. One-pot hydrothermal synthesis of a mononuclear cobalt(II) complex and an organic-inorganic supramolecular adduct: Structures, properties and hirshfeld surface analyses. J. Solid State Chem. 2022, 313, 123271. [Google Scholar] [CrossRef]
- Li, J.X.; Xiong, L.Y.; Fu, L.L.; Bo, W.B.; Du, Z.X.; Feng, X. Structural diversity of Mn(II) and Cu(II) complexes based on 2-carboxyphenoxyacetate linker: Syntheses, conformation comparison and magnetic properties. J. Solid State Chem. 2022, 305, 122636. [Google Scholar] [CrossRef]
- Li, J.X.; Zhang, Y.H.; Du, Z.X.; Feng, X. One-pot solvothermal synthesis of mononuclear and oxalate-bridged binuclear nickel compounds: Structural analyses, conformation alteration and magnetic properties. Inorg. Chim. Acta 2022, 530, 120697. [Google Scholar] [CrossRef]
- Li, J.X.; Xiong, L.Y.; Xu, X.J.; Liu, C.; Wang, Z.G. The synthesis, crystal structure and conformation analysis of triclopyr ethyl ester. Z. Kristallogr.-Cryst. Mater. 2022, 237, 385–391. [Google Scholar] [CrossRef]
- Li, J.X.; Du, Z.X.; Xiong, L.Y.; Fu, L.L.; Bo, W.B. Supramolecular isomerism in two nickel(II) coordination polymers constructed with the flexible 2-carboxyphenoxyacetate linker: Syntheses, structure analyses and magnetic properties. J. Solid State Chem. 2021, 293, 121799. [Google Scholar] [CrossRef]
- Li, J.X.; Zhang, T.; Chen, H.J.; Du, Z.X. A (4,4)-connected zinc(II) coordination polymer constructed with the flexible 2-carboxy phenoxyacetate ligand: Synthesis, conformation alteration and fluorescent properties. Z. Kristallogr.-Cryst. Mater. 2021, 236, 251–259. [Google Scholar] [CrossRef]
- Abdelbaky, M.S.M.; Amghouz, Z.; García-Granda, S.; García, J.R. A metal-organic framework assembled from Y(III), Li(I), and terephthalate: Hydrothermal synthesis, crystal structure, thermal decomposition and topological studies. Dalton Trans. 2014, 43, 5739–5746. [Google Scholar] [CrossRef]
- Surblé, S.; Serre, C.; Millange, F.; Pelle, F.; Férey, G. Synthesis, characterisation and properties of a new three-dimensional Yttrium-Europium coordination polymer. Solid State Sci. 2005, 7, 1074–1082. [Google Scholar] [CrossRef]
- Wang, J.; Suffren, Y.; Daiguebonne, C.; Bernot, K.; Calvez, G.; Freslona, S.; Guillou, O. Lanthanide-based molecular alloys with hydroxyterephthalate: A versatile system. CrystEngComm 2021, 23, 100–118. [Google Scholar] [CrossRef]
- Amghouz, Z.; Roces, L.; Garcia-Granda, S.; Garcia, J.R. Metal Organic Frameworks Assembled from Y(III), Na(I), and Chiral Flexible-Achiral Rigid Dicarboxylates. Inorg. Chem. 2010, 49, 7917–7926. [Google Scholar] [CrossRef]
- Amiaud, T.; Stephant, N.; Dessapt, R.; Serier-Brault, H. Microwave-assisted synthesis of anhydrous lanthanide-based coordination polymers built upon benzene-1,2,4,5-tetracarboxylic acid. Polyhedron 2021, 204, 115261. [Google Scholar] [CrossRef]
- Calvez, G.; Daiguebonne, C.; Guillou, O. Unprecedented Lanthanide-Containing Coordination Polymers Constructed from Hexanuclear Molecular Building Blocks: {[Ln6O(OH)8](NO3)2(bdc)(Hbdc)2·2NO3·H2bdc}∞. Inorg. Chem. 2011, 50, 2851–2858. [Google Scholar] [CrossRef] [PubMed]
- Weng, D.; Zheng, X.; Jin, L. Assembly and Upconversion Properties of Lanthanide Coordination Polymers Based on Hexanuclear Building Blocks with (μ3-OH) Bridges. Eur. J. Inorg. Chem. 2006, 2006, 4184–4190. [Google Scholar] [CrossRef]
- Bürgstein, M.R.; Gamer, M.T.; Roesky, P.W. Nitrophenolate as a Building Block for Lanthanide Chains, Layers, and Clusters. J. Am. Chem. Soc. 2004, 126, 5213–5218. [Google Scholar] [CrossRef]
- Bünzli, J.-C.G.; Piguet, C. Lanthanide-Containing Molecular and Supramolecular Polymetallic Functional Assemblies. Chem. Rev. 2002, 102, 1897–1928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, X.K.; Anderson, T.M.; Benelli, C.; Hill, C.L. Polyoxometalate-Supported Y- and YbIII-Hydroxo/Oxo Clusters from Carbonate-Assisted Hydrolysis. Chem. Eur. J. 2005, 11, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.J.; Jin, L.P.; Gao, S. Synthesis and Characterization of Two Novel Lanthanide Coordination Polymers with an Open Framework Based on an Unprecedented [Ln7(μ3-OH)8]13+ Cluster. Inorg. Chem. 2004, 43, 1600–1602. [Google Scholar] [CrossRef]
- Wang, R.Y.; Zheng, Z.P.; Jin, T.Z.; Staples, R.J. Coordination Chemistry of Lanthanides at “High” pH: Synthesis and Structure of the Pentadecanuclear Complex of Europium(III) with Tyrosine. Angew. Chem. Int. Ed. 1999, 38, 1813–1815. [Google Scholar] [CrossRef]
- Wang, R.Y.; Song, D.T.; Wang, S.N. Toward constructing nanoscale hydroxo-lanthanide clusters: Syntheses and characterizations of novel tetradecanuclear hydroxo-lanthanide clusters. Chem. Commun. 2002, 4, 368–369. [Google Scholar] [CrossRef]
- Xiong, R.G.; Zuo, J.L.; Yu, Z.; You, X.Z.; Chen, W. Eu5(μ4-OH)(μ3-OH)4(μ-DBM)4(DBM)6 (DBM=dibenzoylmethide): A novel Eu5 square-pyramid polynuclear complex with a rare μ4-OH bridging mode. Inorg. Chem. Commun. 1999, 2, 490–494. [Google Scholar] [CrossRef]
- Mahé, N.; Guillou, O.; Daiguebonne, C.; Gérault, Y.; Caneschi, A.; Sangregorio, C.; Chane-Ching, J.Y.; Car, P.E.; Roisnel, T. Polynuclear Lanthanide Hydroxo Complexes: New Chemical Precursors for Coordination Polymers. Inorg. Chem. 2005, 44, 7743–7750. [Google Scholar] [CrossRef]
- Hubert-Pfalzgraf, L.G.; Miele-Pajot, N.; Papiernik, R.; Vaissermann, J.J. A novel example of self-assembly in lanthanide chemistry: Synthesis and molecular structure of [Na(EtOH)6][Y9(µ4-O)2(µ3-OH)8{µ-η2-MeC(O)CHC(O)OEt}8{η2-MeC(O)CHC(O)OEt}8]+. J. Chem. Soc. Dalton Trans. 1999, 23, 4127–4130. [Google Scholar] [CrossRef]
- Bürgstein, M.R.; Roesky, P.W. Nitrophenolate as a Building Block for Lanthanide Chains and Clusters. Angew. Chem. Int. Ed. 2000, 39, 549–550. [Google Scholar] [CrossRef]
- Chen, S.-S.; Su, H.-F.; Long, L.-S.; Zheng, L.-S.; Kong, X.-J. Hydrolysis-Promoted Building Block Assembly: Structure Transformation from Y12 Wheel and Y34 Ship to Y60 Cage. Inorg. Chem. 2021, 60, 16922–16926. [Google Scholar] [CrossRef]
- Abdelbaky, S.M.M.; Amghouz, Z.; Fernández-Zapico, E.; García-Granda, S.; Garcí, J.R. Metal–organic frameworks assembled from lanthanide and 2,5-pyridinedicaboxylate with cubane-like [Ln4(OH)4] building units. J. Solid State Chem. 2015, 229, 197–207. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Li, X.; Zhang, H.; Weng, L.; Zhou, X. Synthesis and controlled hydrolysis of organolanthanide complexes with mono- and dianionic benzimidazole-2-thiolate ligands. Dalton Trans. 2010, 39, 11053–11059. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Hubert-Pfalzgraf, L.G.; Daniele, S.; Rolland, M.; Jeanneau, E.; Jouguet, B. Thermal dehydration of Y(TFA)3(H2O)3: Synthesis and molecular structures of [Y(μ,η1:η1-TFA)3(THF)(H2O)]11.THF and [Y4(μ3-OH)4(μ,η1:η1-TFA)6(η1-TFA)(η2-TFA)(THF)3(DMSO)(H2O)].6THF (TFA = trifluoroacetate). Inorg. Chem. Commun. 2009, 12, 97–100. [Google Scholar] [CrossRef]
- Pearson, R.G. Hard and soft acids and bases-the evolution of a chemical concept. Coord. Chem. Rev. 1990, 100, 403–425. [Google Scholar] [CrossRef]
- Grishko, A.Y.; Utochnikova, V.V.; Averin, A.A.; Mironov, A.V.; Kuzmina, N.P. Unusual Luminescence Properties of Heterometallic REE Terephthalates. Eur. J. Inorg. Chem. 2015, 10, 1660–1664. [Google Scholar] [CrossRef]
- Yan, X.; Li, Y.-P.; Lei, J.; Wang, Y.; Li, S.-N.; Zhai, Q.-G. Introduction of continuous excited-state intermolecular proton transfer process into open yttrium-0 framework for ratiometric fluorescent fluorion detection. J. Solid State Chem. 2021, 300, 122212. [Google Scholar] [CrossRef]
- Chen, W.; Li, L.; Li, X.-X.; Lin, L.-D.; Wang, G.; Zhang, Z.; Li, L.; Yu, Y. Layered Rare Earth-Organic Framework as Highly Efficient Luminescent Matrix: The Crystal Structure, Optical Spectroscopy, Electronic Transition, and Luminescent Sensing Properties. Cryst. Growth Des. 2019, 19, 4754–4764. [Google Scholar] [CrossRef]
- Daga, P.; Sarkar, S.; Majee, P.; Singha, D.K.; Hui, S.; Mahata, P.; Mondal, S.K. Selective detection of nanomolar-range noxious nions in water by a luminescent metal-organic framework. Mater. Adv. 2021, 2, 985–995. [Google Scholar] [CrossRef]
- Dietzel, P.D.C.; Blom, R.; Fjellvåg, H. Variability in the Formation and Framework Polymorphism of Metal-organic Frameworks based on Yttrium(III) and the Bifunctional Organic Linker 2,5-Dihydroxyterephthalic Acid. Z. Anorg. Allg. Chem. 2021, 647, 15–25. [Google Scholar] [CrossRef]
- Gong, H.-Y.; Rambo, B.M.; Nelson, C.A.; Cho, W.; Lynch, V.M.; Zhu, X.; Oh, M.; Sessler, J.L. Multi component self-assembly: Supramolecular organic frameworks containing metal-rotaxane subunits (RSOFs). Dalton Trans. 2012, 41, 1134–1137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guillou, O.; Daiguebonne, C.; Calvez, G.; Le Dret, F.; Car, P.-E. Structuring effects of [Ln6O(OH)8(NO3)6(H2O)12]2+ entities. J. Alloys Compd. 2008, 451, 329–333. [Google Scholar] [CrossRef]
- Han, Y.; Li, X.; Li, L.; Ma, C.; Shen, Z.; Song, Y.; You, X. Structures and Properties of Porous Coordination Polymers Based on Lanthanide Carboxylate Building Units. Inorg. Chem. 2010, 49, 10781–10787. [Google Scholar] [CrossRef]
- Han, Y.; Fu, L.; Mafra, L.; Shi, F.-N. Hydrothermal synthesis, crystal structures and photoluminescence properties of mixed europium-yttrium organic frameworks. J. Solid State Chem. 2012, 186, 165–170. [Google Scholar] [CrossRef]
- Hernandez, A.; Jenkins, J.; Maslen, H.; Zeller, M.; Horner, G.; Dempsey, C.; Urteaga, J.; Dunlap, C.; Zehnder, R.A. Stress compensation in an extended series of lanthanide 2-sulfonatoterephthalates [Ln(TPSO3)(H2O)2]n (Ln = Ce-Lu, except Pm). Inorg. Chim. Acta 2018, 471, 104–112. [Google Scholar] [CrossRef]
- Kandiah, M.; Wragg, D.S.; Tilset, M.; Lillerud, K.P. Poly[tris(μ2-aminobenzene-1,4-dicarboxylato)tetrakis(N,N-dimethylformamide) diyttrium(III)]. Acta Cryst. 2011, E67, m44–m45. [Google Scholar]
- Kariem, M.; Yawer, M.; Sharma, S.; Sheikh, H.N. Syntheses, Crystal Structure, Luminescence, Porosity and Magnetic Properties of Three-Dimensional Lanthanide Coordination Polymers with 2-Aminoterepthalic Acid. ChemistrySelect 2016, 1, 4489–4501. [Google Scholar] [CrossRef]
- Petrosyants, S.; Dobrokhotova, Z.; Ilyukhin, A.; Gavrikov, A.; Efimov, N.; Novotortsev, V. Coordination Polymers of Rare-Earth Elements with 2-Aminoterephthalic Acid. Russ. J. Coord. Chem. 2017, 43, 770–779. [Google Scholar] [CrossRef]
- Thirumurugan, A.; Natarajan, S. Inorganic_Organic Hybrid Compounds: Synthesis and Structures of New Metal Organic Polymers Synthesized in the Presence of Mixed Dicarboxylates. Eur. J. Inorg. Chem. 2004, 2004, 762–770. [Google Scholar] [CrossRef]
- Thirumurugan, A.; Natarajan, S. Yttrium coordination polymers with layered structures. Solid State Sci. 2004, 6, 599–604. [Google Scholar] [CrossRef]
- Tan, B.; Xie, Z.-L.; Feng, M.-L.; Hu, B.; Wu, Z.-F.; Huang, X.-Y. Thermal Syntheses, Crystal Structures and Properties of Three-Dimensional Rare Earth Metal Organic Frameworks with 1,4-naphthalenedicarboxylic acid. Dalton Trans. 2012, 41, 10576–10584. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.-H.; Zhao, X.-X.; Zhao, D. Crystal structure of poly[tetraaqua-(μ4-oxalato-κ4O,O′:O″,O‴)-(μ8-benzene-1,2,4,5-tetracarboxylato-κ8O1:O2:O3:O4:O5:O6:O7:O8) yttrium(III)], C6H5O8Y. Z. Kristallogr. NCS 2018, 233, 583–584. [Google Scholar]
- Xia, C.-K.; Sun, W.; Min, Y.-Y.; Yang, K.; Wu, Y.-L. Low pH hydrothermal syntheses, structural characterization and properties of several lanthanide complexes constructed with 1,2,3,5-benzenetetracarboxylic acid. Polyhedron 2018, 141, 377–384. [Google Scholar] [CrossRef]
- Xu, X.; Wang, Z.; Yan, C.-C.; Hou, X.; Tang, S.-F. Structural variability of rare earth carboxylates based on polydentate carboxylate ligand containing pyridine group. J. Solid State Chem. 2020, 292, 121708. [Google Scholar] [CrossRef]
- Xue, D.-X.; Belmabkhout, Y.; Shekhah, O.; Jiang, H.; Adil, K.; Cairns, A.J.; Eddaoudi, M. Tunable Rare Earth fcu-MOF Platform: Access to Adsorption Kinetics Driven Gas/Vapor Separations via Pore Size Contraction. J. Am. Chem. Soc. 2015, 137, 5034–5040. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Wu, T.; Chen, S.; Feng, P.; Bu, H. Versatile Structure-Directing Roles of Deep-Eutectic Solvents and Their Implication in the Generation of Porosity and Open Metal Sites for Gas Storage. Angew. Chem. Int. Ed. 2009, 48, 3486–3490. [Google Scholar] [CrossRef]
- Zhang, J.; Bu, J.T.; Chen, S.; Wu, T.; Zheng, S.; Chen, Y.; Nieto, R.A.; Feng, P.; Bu, H. Urothermal Synthesis of Crystalline Porous Materials. Angew. Chem. Int. Ed. 2010, 49, 8876–8879. [Google Scholar] [CrossRef]
- Zhao, C.-Y.; Zhao, D.; Nie, C.-K. Crystal structure of poly[tetraaqua-bis(μ6-benzene-1,2,4,5-tetracarboxylato)nickel(II) diyttrium(III)]dihydrate, C20H16NiO22Y2. Z. Kristallogr. NCS 2017, 232, 935–936. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Zhu, G.; Fang, Q.; Xue, M.; Tian, G.; Sun, J.; Li, X.; Qiu, S. Synthesis, Structure and Luminescent Properties of Rare Earth Coordination Polymers Constructed from Paddle-Wheel Building Blocks. Inorg. Chem. 2005, 44, 3850–3855. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wei, N.; Xing, Z.; Han, Z.-B. Functional hexanuclear Y(III) cluster-based MOFs supported Pd(II) single site catalysts for aerobic selective oxidation of styrene. Appl. Catal. A-Gen. 2020, 602, 117668. [Google Scholar] [CrossRef]
- Chen, J.; Chen, Z.; Yu, T.; Weng, L.; Tu, B.; Zhao, D. Synthesis, structure, and adsorption properties of a three-dimensional porous yttrium-organic coordination network. Micropor. Mesopor. Mat. 2007, 98, 16–20. [Google Scholar] [CrossRef]
- Xu, T.Y.; Li, J.M.; Han, Y.H.; Wang, A.R.; He, K.H.; Shi, Z.F. A new 3D four-fold interpenetrated dia-like luminescent Zn(II)-based metal-organic framework: The sensitive detection of Fe3+, Cr2O72−, and CrO42− in water, and nitrobenzene in ethanol. New J. Chem. 2020, 44, 4011–4022. [Google Scholar] [CrossRef]
- Goodenough, I.; Devulapalli, V.S.D.; Xu, W.; Boyanich, M.C.; Luo, T.-Y.; Souza, M.D.; Richard, M.; Rosi, N.L.; Borguet, E. Interplay between Intrinsic Thermal Stability and Expansion Properties of Functionalized UiO-67 Metal-Organic Frameworks. Chem. Mater. 2021, 33, 910–920. [Google Scholar] [CrossRef]
- Xu, D.; Lou, W.-G.; Xu, J.; Pan, S. Two Co(II) coordination polymers: Crystal structures and important role in bone healing. J. Mol. Struct. 2021, 1242, 130726. [Google Scholar] [CrossRef]
- Gu, J.-Z.; Cai, Y.; Qian, Z.-Y.; Wen, M.; Shi, Z.-F.; Lv, D.-Y.; Kirillov, A.M. A new series of Co, Ni, Zn, and Cd metal-organic architectures driven by an unsymmetrical biphenyl-tricarboxylic acid: Hydrothermal assembly, structural features and properties. Dalton Trans. 2018, 47, 7431–7444. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Duan, W.; Song, J.; Guo, X.; Wang, Z.; Shi, X.; Liang, J.; Wang, J.; Cheng, P.; Chen, A.; et al. Self-Healing Hyper-Cross-Linked Metal-Organic Polyhedra (HCMOPs) Membranes with Antimicrobial Activity and Highly Selective Separation Properties. J. Am. Chem. Soc. 2019, 141, 12064–12070. [Google Scholar] [CrossRef]
- Luo, T.-Y.; Liu, C.; Eliseeva, S.V.; Muldoon, P.F.; Petoud, S.; Rosi, N.L. Rare Earth pcu Metal-Organic Framework Platform Based on RE4(μ3-OH)4(COO)62+ Clusters: Rational Design, Directed Synthesis, and Deliberate Tuning of Excitation Wavelengths. J. Am. Chem. Soc. 2017, 139, 9333–9340. [Google Scholar] [CrossRef]
- Zeng, X.; Hu, J.; Zhang, M.; Wang, F.; Hou, X. Visual Detection of Fluoride Anions Using Mixed Lanthanide Metal-Organic Frameworks with a Smartphone. Anal. Chem. 2020, 92, 2097–2102. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, J.F.; Yoon, J. Fluorescence and Colorimetric Chemosensors for Fluoride-Ion Detection. Chem. Rev. 2014, 114, 5511–5571. [Google Scholar] [CrossRef] [PubMed]
- Beer, P.D.; Gale, P.A. Anion Recognition and Sensing: The State of the Art and Future Perspectives. Angew. Chem. Int. Ed. 2001, 40, 486–516. [Google Scholar] [CrossRef]
- Ozsvath, D.L. Fluoride and Environmental Health: A Review. Rev. Environ. Sci. Bio. 2009, 8, 59–79. [Google Scholar] [CrossRef]
- Ayoob, S.; Gupta, A.K. Fluoride in Drinking Water: A Review on the Status and Stress Effects. Crit. Rev. Environ. Sci. Technol. 2006, 36, 433–487. [Google Scholar] [CrossRef]
- Mandinic, Z.; Curcic, M.; Antonijevic, B.; Carevic, M.; Mandic, J.; Djukic-Cosic, D.; Lekic, C.P. Fluoride in Drinking Water and Dental Fluorosis. Sci. Total Environ. 2010, 408, 3507–3512. [Google Scholar] [CrossRef]
- Hu, R.; Feng, J.; Hu, D.; Wang, S.; Li, S.; Li, Y.; Yang, G. A Rapid Aqueous Fluoride Ion Sensor with Dual Output Modes. Angew. Chem. Int. Ed. 2010, 49, 4915–4918. [Google Scholar] [CrossRef]
- Shen, J.; Gagliardi, S.; McCoustra, M.R.S.; Arrighi, V. Effect of Humic Substances Aggregation on the Determination of Fluoride in Water Using An Ion Selective Electrode. Chemosphere 2016, 159, 66–71. [Google Scholar] [CrossRef]
- van den Hoop, M.A.G.T.; Cleven, R.F.M.J.; van Staden, J.J.; Neele, J. Analysis of Fluoride in Rain Water Comparison of Capillary Electrophoresis with Ion Chromatography and Ion-Selective Electrode Potentiometry. J. Chromatogr. A 1996, 739, 241–248. [Google Scholar] [CrossRef]
- Hussain, I.; Ahamad, K.U.; Nath, P. Low-Cost, Robust, and Field Portable Smartphone Platform Photometric Sensor for Fluoride Level Detection in Drinking Water. Anal. Chem. 2017, 89, 767–775. [Google Scholar] [CrossRef]
- Ebrahim, F.M.; Nguyen, T.N.; Shyshkanov, S.; Gładysiak, A.; Favre, P.; Zacharia, A.; Itskos, G.; Dyson, P.J.; Stylianou, K.C. Selective, Fast-Response, and Regenerable Metal-Organic Framework for Sampling Excess Fluoride Levels in Drinking Water. J. Am. Chem. Soc. 2019, 141, 3052–3058. [Google Scholar] [CrossRef]
- Weng, Q.-Y.; Zhao, Y.-L.; Li, J.-M.; Ouyang, M. Construction of Two Stable Co(II)-Based Hydrogen-Bonded Organic Frameworks as a Luminescent Probe for Recognition of Fe3+ and Cr2O72− in H2O. Molecules 2021, 26, 5955. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.-Y.; Nie, H.-J.; Li, J.-M.; Shi, Z.-F. Highly selective sensing of Fe3+/Hg2+ and proton conduction using two fluorescent Zn(II) coordination polymers. Dalton Trans. 2020, 49, 11129–11141. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.-Y.; Nie, H.-J.; Li, J.-M.; Shi, Z.-F. Luminescent Zn(II)/Cd(II) coordination polymers based on 1-(tetrazol-5-H)-3,5-bis(1-triazole)benzene for sensing Fe3+, Cr2O72−, and CrO42− in water. J. Solid State Chem. 2020, 287, 121342. [Google Scholar] [CrossRef]
- Xu, T.-Y.; Wang, H.; Li, J.-M.; Zhao, Y.-L.; Han, Y.-H.; Wang, X.-L.; He, K.-H.; Wang, A.-R.; Shi, Z.-F. A water-stable luminescent Zn(II) coordination polymer based on 5-sulfosalicylic acid and 1,4-bis(1H-imidazol-1-yl)benzene for highly sensitive and selective sensing of Fe3+ ion. Inorg. Chim. Acta 2019, 493, 72–80. [Google Scholar] [CrossRef]
- Li, J.-M.; Xu, T.-Y.; Zhao, Y.-L.; Hu, X.-L.; He, K.-H. Two 6/10-connected Cu12S6 cluster-based organic frameworks: Crystal structure and proton conduction. Dalton Trans. 2021, 50, 7484–7495. [Google Scholar] [CrossRef]
- Blatov, V.A.; Shevchenko, A.P.; Proserpio, D.M. Applied Topological Analysis of Crystal Structures with the Program Package ToposPro. Cryst. Growth Des. 2014, 14, 3576–3586. [Google Scholar] [CrossRef]
- Spek, A.L. Structure validation in chemical crystallography. Acta Crystallogr. Sect. D Biol. Crystallogr. 2009, 65, 148–155. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Yin, C.; Chen, P.; Zhang, M.; Parkin, S.; Zhou, P.; Li, T.; Yu, F.; Long, S. sp2 C-H⋯Cl hydrogen bond in the conformational polymorphism of 4-chloro-phenylanthranilic acid. CrystEngComm 2017, 19, 4345–4354. [Google Scholar] [CrossRef]
- Tresca, B.W.; Zakharov, L.N.; Carroll, C.N.; Johnson, D.W.; Haley, M.M. Aryl C-H⋯Cl− hydrogen bonding in a fluorescent anion sensor. Chem. Commun. 2013, 49, 7240–7242. [Google Scholar] [CrossRef] [Green Version]
- Feng, G.; Gou, Q.; Evangelisti, L.; Vallejo-López, M.; Lesarri, A.; Cocineroc, E.J.; Caminati, W. Competition between weak hydrogen bonds: C-H⋯Cl is preferred to C-H⋯F in CH2ClF-H2CO, as revealed by rotational spectroscopy. Phys. Chem. Chem. Phys. 2014, 16, 12261–12265. [Google Scholar] [CrossRef]
- Chen, B.; Wang, L.; Zapata, F.; Qian, G.; Lobkovsky, B. A Luminescent Microporous Metal-Organic Framework for the Recognition and Sensing of Anions. J. Am. Chem. Soc. 2008, 130, 6718–6719. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.Y.; Chen, Y.H.; Chen, Y.T.; Hsu, Y.H.; Shen, J.Y.; Chen, C.L.; Chen, Y.A.; Chou, P.T. The excited-state triple proton transfer reaction of 2,6-diazaindoles and 2,6-diazatryptophan in aqueous solution. J. Am. Chem. Soc. 2017, 139, 6396–6402. [Google Scholar] [CrossRef] [PubMed]
- Padalkar, V.S.; Seki, S. Excited-state intramolecular proton-transfer (ESIPT)-inspired solid state emitters. Chem. Soc. Rev. 2016, 45, 169–202. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. Acta Cryst. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.-Q.; Feng, D.-D.; Tang, J.; Zhao, Y.-D.; Li, J.; Yang, J.; Kim, C.K.; Su, F. A Water-Stable Zinc(II)-Organic Framework as a Multi-responsive Luminescent Sensor for Toxic Heavy Metal Cations, Oxyanions and Organochlorine Pesticides in Aqueous Solution. Dalton Trans. 2019, 48, 16776–16785. [Google Scholar] [CrossRef]
- Sun, J.; Zhou, T.; Pan, D.; Zhang, X.; Wang, Y.; Shi, Y.-C.; Yu, H. Synthesis, structure, and photoluminescence properties of coordination polymers of 4,4′,4″,4′′′′-tetrakiscarboxyphenylsilane and 3,5-bis(1′,2′,4′-triazol-1′-yl)pyridine. CrystEngComm 2020, 22, 534–545. [Google Scholar] [CrossRef]















Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Wang, A.; Qiu, S.; Wang, X.; Li, J. A 12-Connected [Y4((μ3-OH)4]8+ Cluster-Based Luminescent Metal-Organic Framework for Selective Turn-on Detection of F− in H2O. Molecules 2023, 28, 1893. https://doi.org/10.3390/molecules28041893
Li J, Wang A, Qiu S, Wang X, Li J. A 12-Connected [Y4((μ3-OH)4]8+ Cluster-Based Luminescent Metal-Organic Framework for Selective Turn-on Detection of F− in H2O. Molecules. 2023; 28(4):1893. https://doi.org/10.3390/molecules28041893
Chicago/Turabian StyleLi, Juan, Airong Wang, Shiming Qiu, Xiaoli Wang, and Jiaming Li. 2023. "A 12-Connected [Y4((μ3-OH)4]8+ Cluster-Based Luminescent Metal-Organic Framework for Selective Turn-on Detection of F− in H2O" Molecules 28, no. 4: 1893. https://doi.org/10.3390/molecules28041893