Color, Antioxidant Capacity and Flavonoid Composition in Hibiscus rosa-sinensis Cultivars
Abstract
:1. Introduction
2. Results
2.1. Identification by UHPLC-ESI+-Orbitrap-MS of Flavonoids Present in the Extracts of H. rosa-sinensis Flowers
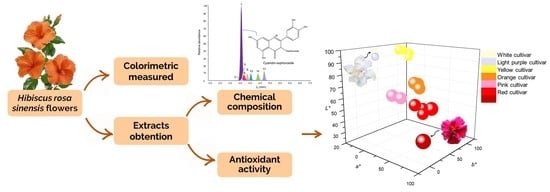
2.2. Relationship of Chemical Composition with Color and Antioxidant Capacity in the H. rosa-sinensis Cultivars under Study
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Vegetal Material
4.3. Color Measurement
4.4. Solvent Extraction
4.5. UHPLC-ESI+-Orbitrap-MS Analysis
4.6. Antioxidant Assay: Oxygen Radical Absorption Capacity
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Dwivedi, R.; Pandey, S.; Tripathi, V. Role of japapushpa (Hibiscus rosa-sinensis) in the treatment of arterial hypertension. A trial study. J. Res. Indian Med. 1977, 12, 13–36. [Google Scholar]
- Watson, R. Polyphenols in Plants: Isolation, Purification and Extract Preparation, 1st ed.; Elsevier Science Limited: Waltham, MA, USA, 2014; Volume 231, pp. 103–157. [Google Scholar]
- Gilani, A.; Bashir, S.; Janbaz, K.; Shah, A. Presence of cholinergic and calcium channel blocking activities explains the traditional use of Hibiscus rosa-sinensis in constipation and diarrhea. J. Ethnopharmacol. 2005, 102, 94–289. [Google Scholar] [CrossRef]
- Hammad, I. Genetic variation among Hibiscus rosa-sinensis (Malvaceae) of different flower colors using ISSR and isozymes. Aust. J. Basic Appl. Sci. 2009, 3, 113–125. [Google Scholar]
- Grotewold, E. The genetics and biochemistry of floral pigments. Annu. Rev. Plant Biol. 2006, 57, 761–780. [Google Scholar] [CrossRef]
- Tanaka, Y.; Sasaki, N.; Ohmiya, A. Biosynthesis of plant pigments: Anthocyanins, betalains and carotenoids. Plant J. 2008, 54, 733–749. [Google Scholar] [CrossRef] [PubMed]
- Koes, R.; Verweij, W.; Quattrocchio, F. Flavonoids: A colorful model for the regulation and evolution of biochemical pathways. Trends Plant Sci. 2005, 10, 236–242. [Google Scholar] [CrossRef]
- Speisky, H.; Shahidi, F.; Costa de Camargo, A.; Fuentes, J. Revisiting the oxidation of flavonoids: Loss, conservation or enhancement of their antioxidant properties. Antioxidants 2022, 11, 133. [Google Scholar] [CrossRef]
- Ponte, L.; Pavan, I.; Mancini, M.; da Silva, L.; Morelli, A.; Severino, M.; Simabuco, F. The hallmarks of flavonoids in cancer. Molecules 2021, 26, 2029. [Google Scholar] [CrossRef]
- Maleki, S.; Crespo, J.; Cabanillas, B. Anti-inflammatory effects of flavonoids. Food Chem. 2019, 299, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Faten, R.; Ghaffar, A.; El-Elaimy, I. In vitro, antioxidant and scavenging activities of Hibiscus rosa sinensis crude extract. J. Appl. Pharm. Sci. 2012, 2, 51–58. [Google Scholar]
- Garg, D.; Shaikh, A.; Muley, A.; Marar, T. In vitro antioxidant activity and phytochemical analysis in extracts of Hibiscus rosa-sinensis stem and leaves. Free Rad. Antiox. 2012, 2, 41–46. [Google Scholar] [CrossRef]
- Patel, R.; Patel, A.; Desai, A.; Nagee, A. Study of secondary metabolites and antioxidant properties of leaves, stem and root among Hibiscus rosa-sinensis cultivars. Asian J. Exp. Biol. Sci. 2012, 3, 719–725. [Google Scholar]
- Rao, K.; Geetha, K.; Raja, A.; Banji, D. Quality control study and standardization of Hibiscus rosa-sinensis L. flowers and leaves as per who guidelines. J. Pharm. Phytochem. 2014, 3, 29–37. [Google Scholar]
- Wahid, S.; Tasleem, S.; Jahangir, S. Phytochemical profiling of ethanolic flower extract of Hibiscus rosa-sinensis and evaluation of its antioxidant potential. World J. Pharm. Res. 2019, 8, 161–168. [Google Scholar]
- Sri Raghavi, R.; Visalakshi, M.; Karthikeyan, S.; Amutha Selvi, G.; Thamaraiselvi, S.; Gurusamy, K. Standardisation of anthocyanin extraction techniques from hibiscus (Hibiscus rosa-sinensis) petals for biocolour utilisation. J. Pharm. Innov. 2022, 11, 303–309. [Google Scholar]
- Vankar, P.; Srivastava, J. Comparative study of total phenol, flavonoid contents and antioxidant activity in Canna indica and Hibiscus rosa sinensis: Prospective natural food dyes. Int. J. Food Eng. 2008, 4, 1–15. [Google Scholar] [CrossRef]
- dos Santos, N.; Gori, A.; Raffaelli, A.; Ferrini, F.; Brunetti, C. Phenolic compounds from leaves and flowers of Hibiscus roseus: Potential skin cosmetic applications of an underinvestigated species. Plants 2021, 10, 522. [Google Scholar] [CrossRef]
- Shen, H.; Zheng, Y.; Chen, R.; Huang, X.; Shi, G. Neuroprotective effects of quercetin 3-O-sophoroside from Hibiscus rosa-sinensis Linn. on scopolamine-induced amnesia in mice. J. Funct. Foods 2021, 76, 1–12. [Google Scholar] [CrossRef]
- Li, A.; Li, S.; Li, H.; Xu, D.; Xu, X.; Chen, F. Total phenolic contents and antioxidant capacities of 51 edible and wild flowers. J. Funct. Foods 2014, 6, 319–330. [Google Scholar] [CrossRef]
- Pascoal, A.; Quirantes, R.; Fernando, A.; Alexopoulou, E.; Segura, A. Phenolic composition and antioxidant activity of kenaf leaves. Ind. Crops Prod. 2015, 78, 116–123. [Google Scholar] [CrossRef]
- Nakamura, Y.; Hidaka, M.; Masaki, H.; Seto, H.; Uozumi, T. Major anthocyanin of the flowers of Hibiscus (Hibiscus rosa-sinensis L.). Agr. Biol. Chem. 1990, 54, 3345–3346. [Google Scholar] [CrossRef]
- Rengarajan, S.; Melanathuru, V.; Govindasamy, C.; Chinnadurai, V.; Elsadek, M. Antioxidant activity of flavonoid compounds isolated from the petals of Hibiscus rosa sinensis. J. King Saud Univ. Sci. 2020, 32, 2236–2242. [Google Scholar] [CrossRef]
- Zaki, L.; Mohamed, S.; Bashandy, S.; Morsy, F.; Tawfik, K.; Shahat, A. Hypoglycemic and antioxidant effects of Hibiscus rosa-sinensis L. leaves extract on liver and kidney damage in streptozotocin induced diabetic rats. Afr. J. Pharm. Pharmacol. 2017, 11, 161–169. [Google Scholar]
- Barnes, J.; Schug, K. Structural characterization of cyanidin-3,5-diglucoside and pelargonidin-3,5-diglucoside anthocyanins: Multidimensional fragmentation pathways using high-performance liquid chromatography-electrospray ionization-ion trap-time of flight mass spectrometry. Int. J. Mass Spectrom. 2011, 308, 71–80. [Google Scholar] [CrossRef]
- Roriz, C.; Barros, L.; Carvalho, A.; Santos, C.; Ferreira, I. Pterospartum tridentatum, Gomphrena globosa and Cymbopogon citratus: A phytochemical study focused on antioxidant compounds. Int. Food Res. J. 2014, 62, 684–693. [Google Scholar] [CrossRef]
- Pires, T.; Dias, M.; Barros, L.; Calhelha, R.; Alves, M.; Oliveira, M.; Ferreira, I. Edible flowers as sources of phenolic compounds with bioactive potential. Int. Food Res. J. 2018, 105, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.; Olivares, J.; Salem, A. Studies on biological activities and phytochemicals composition of Hibiscus species. A review. Life Sci. 2014, 11, 1–8. [Google Scholar]
- Li, Z.; Lee, H.; Liang, X.; Liang, D.; Wang, Q.; Huang, D.; Ong, C. Profiling of phenolic compounds and antioxidant activity of 12 cruciferous vegetables. Molecules 2018, 23, 1139. [Google Scholar] [CrossRef] [PubMed]
- Vadivel, V. Distribution of flavonoids among Malvaceae family members. A review. Int. J Green Pharm. 2016, 10, 33–45. [Google Scholar]
- Zeng, X.; Su, W.; Zheng, Y.; Liu, H.; Li, P.; Zhang, W.; Yao, H. UFLC-Q-TOF-MS/MS-based screening and identification of flavonoids and derived metabolites in human urine after oral administration of exocarpium Citri grandis extract. Molecules 2018, 23, 895. [Google Scholar] [CrossRef] [Green Version]
- March, R.; Miao, X. A fragmentation study of kaempferol using electrospray quadrupole time-of-flight mass spectrometry at high mass resolution. Int. J. Mass Spectrom. 2004, 231, 157–167. [Google Scholar] [CrossRef]
- Tsimogiannis, D.; Samiotaki, M.; Panayotou, G.; Oreopoulou, V. Characterization of flavonoid subgroups and hydroxy substitution by HPLC-MS/MS. Molecules 2007, 12, 593–606. [Google Scholar] [CrossRef]
- Leitão, S.; Leitão, G.; Vicco, D.; Pereira, J.; de Morais, G.; Oliveira, D.; Rastrelli, L. Countercurrent chromatography with off-line detection by ultrahigh-performance liquid chromatography/high resolution mass spectrometry in the study of the phenolic profile of Lippia origanoides. J. Chromatogr. A 2017, 1520, 83–90. [Google Scholar] [CrossRef]
- Andre, C.; Ghislain, M.; Bertin, P.; Oufir, M.; Del Rosario Herrera, M.; Hoffmann, L.; Evers, D. Andean potato cultivars (Solanum tuberosum L.) as a source of antioxidant and mineral micronutrients. J. Agric. Food Chem. 2007, 55, 366–378. [Google Scholar] [CrossRef]
- Xiang, J.; Yang, C.; Beta, T.; Liu, S.; Yang, R. Phenolic profile and antioxidant activity of the edible tree peony flower and underlying mechanisms of preventive effect on H2O2-induced oxidative damage in Caco-2 cells. Foods 2019, 8, 471. [Google Scholar] [CrossRef]
- Prior, R.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Noda, Y.; Kaneyuki, T.; Mori, A.; Packer, L. Antioxidant activities of pomegranate fruit extract and its anthocyanidins: Delphinidin, cyanidin, and pelargonidin. J. Agric. Food Chem. 2002, 50, 166–171. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, S. Antioxidant activity and phenolic compounds in selected herbs. J. Agric. Food Chem. 2001, 49, 5165–5170. [Google Scholar] [CrossRef]
- Ben Elhadj, I.; Tajini, F.; Boulila, A.; Jebri, M.; Boussaid, M.; Messaoud, C.; Sebaï, H. Bioactive compounds from tunisian Pelargonium graveolens (L’Hér) essential oils and extracts: α-amylase and acethylcholinesterase inhibitory and antioxidant, antibacterial and phytotoxic activities. Ind. Crops Prod. 2020, 158, 1–11. [Google Scholar] [CrossRef]
- Yilmaz, Y.; Toledo, R. Major flavonoids in grape seeds and skins: Antioxidant capacity of catechin, epicatechin, and gallic acid. J. Agric. Food Chem. 2004, 52, 255–260. [Google Scholar] [CrossRef]
- Cortés-Chitala, M.; Flores-Martínez, H.; Orozco-Ávila, I.; León-Campos, C.; Suárez-Jacobo, Á.; Estarrón-Espinosa, M.; López-Muraira, I. Identification and quantification of phenolic compounds from Mexican oregano (Lippia graveolens HBK) hydroethanolic extracts and evaluation of its antioxidant capacity. Molecules 2021, 26, 702. [Google Scholar] [CrossRef]
- Skowyra, M.; Gallego, M.; Segovia, F.; Almajano, M. Antioxidant properties of Artemisia annua extracts in model food emulsions. Antioxidants 2014, 3, 116–128. [Google Scholar] [CrossRef]
- Wan, H.; Yu, C.; Han, Y.; Guo, X.; Luo, L.; Pan, H.; Zheng, T.; Wang, J.; Cheng, T.; Zhang, Q. Determination of flavonoids and carotenoids and their contributions to various colors of rose cultivars (Rosa spp.). Front. Plant Sci. 2019, 10, 1–14. [Google Scholar] [CrossRef]
- Yoshida, K.; Mori, M.; Kondo, T. Blue flower color development by anthocyanins: From chemical structure to cell physiology. Nat. Prod. Rep. 2009, 26, 884–915. [Google Scholar] [CrossRef]
- Olivas, F.; García, J.; Martínez, N.; Cárdenas, A.; Mendoza, S.; Álvarez, E.; Wall, A. Cyanidin-3-O-glucoside: Physical-chemistry, foodomics and health effects. Molecules 2016, 21, 1–30. [Google Scholar]
- Gordillo, B.; Rodríguez, F.; González, M.; Quijada, N.; Rivas, J.; García, I.; Escribano, M. Application of differential colorimetry to evaluate anthocyanin–flavonol–flavanol ternary copigmentation interactions in model solutions. J. Agric. Food Chem. 2015, 63, 7645–7653. [Google Scholar] [CrossRef]
- Bakowska, A.; Kucharska, A.; Oszmiański, J. The effects of heating, UV irradiation, and storage on stability of the anthocyanin–polyphenol copigment complex. Food Chem. 2003, 81, 349–355. [Google Scholar] [CrossRef]
- Zhu, M.; Zheng, X.; Shu, Q.; Li, H.; Zhong, P.; Zhang, H.; Wang, L. Relationship between the composition of flavonoids and flower colors variation in tropical water lily (Nymphaea) cultivars. PLoS ONE 2012, 7, e34335. [Google Scholar] [CrossRef]
- Li, X.; Lu, M.; Tang, D.; Shi, Y. Composition of carotenoids and flavonoids in narcissus cultivars and their relationship with flower color. PLoS ONE 2015, 10, e0142074. [Google Scholar] [CrossRef]
- Tai, D.; Tian, J.; Zhang, J.; Song, T.; Yao, Y. A Malus crabapple chalcone synthase gene, McCHS, regulates red petal color and flavonoid biosynthesis. PLoS ONE 2014, 9, e110570. [Google Scholar] [CrossRef]
- Yin, N.; Wang, S.; Jia, L.; Zhu, M.; Yang, J.; Zhou, B.; Qu, C. Identification and characterization of major constituents in different-colored rapeseed petals by UPLC–HESI-MS/MS. J. Agric. Food Chem. 2019, 67, 11053–11065. [Google Scholar] [CrossRef] [PubMed]
- Sheth, F.; De, S. Evaluation of comparative antioxidant potential of four cultivars of Hibiscus rosa-sinensis L. by HPLC-DPPH method. Free Radic. Antioxid. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Hammerbacher, A.; Kandasamy, D.; Ullah, C.; Schmidt, A.; Wright, L.; Gershenzon, J. Flavanone-3-hydroxylase plays an important role in the biosynthesis of spruce phenolic defenses against bark beetles and their fungal associates. Front. Plant Sci. 2019, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Gerats, T.; Strommer, J. Petunia: Evolutionary, Developmental and Physiological Genetics, 2nd ed.; Springer Science & Business Media: New York, NY, USA, 2008; pp. 271–274. [Google Scholar]
- Hong, G.; Wang, J.; Zhang, Y.; Hochstetter, D.; Zhang, S.; Pan, Y.; Wang, Y. Biosynthesis of catechin components is differentially regulated in dark-treated tea (Camellia sinensis L.). Plant Physiol. Biochem. 2014, 78, 49–52. [Google Scholar] [CrossRef]
- Sierra, L.; Córdoba, Y.; Mejía, J.; Rueda, E.; Ocazionez, R.; Avila, J.; Stashenko, E. Photoprotective activity of Ipomoea horsfalliae flower extract. Rev. Bras. Farmacogn. 2020, 30, 69–79. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch, M.; Prior, R. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Ou, B.; Prior, R. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]






| Fig 2 N° | Type | Compound | Formula | Calculated Mass | Exp. Mass | ∆ ppm | HCD *, eV | Product Ions | Identification Criteria | Refs. | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [M]+ | [M+H]+ | Fragment | Formula | m/z (I,%) | |||||||||
| 1 | Anthocyanin | Cyanidin-sophoroside | C27H31O16 | 611.16066 | - | 611.16132 | 1.07 | 20 | [M-2C6H10O5]+ | C15H11O6 | 287.05493 (100%) | a,b | [22,25] |
| [M-2C6H10O5-C6H4O4]+ | C9H7O2 | 147.04404 (1%) | |||||||||||
| [M-2C6H10O5-C8H6O3]+ | C7H5O3 | 137.02324 (1%) | |||||||||||
| [M-2C6H10O5-C8H6O4]+ | C7H5O2 | 121.02866 (1%) | |||||||||||
| 2 | Flavonol | Quercetin-hexuronide-hexoside | C27H28O18 | - | 641.13484 | 641.13501 | 0.26 | 20 | [(M+H)-C6H10O5]+ | C21H19O13 | 479.08209 (100%) | a,b | [26] |
| [(M+H)-C6H10O5-C6H8O6]+ | C15H11O7 | 303.04999 (43%) | |||||||||||
| [(M+H)-C6H10O5-C6H8O6-H2O]+ | C15H9O6 | 285.03949 (2%) | |||||||||||
| [(M+H)-C6H10O5-C6H8O6-C8H6O3]+ | C7H5O4 | 153.01836 (1%) | |||||||||||
| 3 | Flavanonol | Taxifolin-dihexoside | C27H32O17 | - | 629.17122 | 629.17078 | 0.71 | 10 | [(M+H)-C6H10O5]+ | C21H23O12 | 467.11832 (15%) | a,b | [27] |
| [(M+H)-2C6H10O5]+ | C15H13O7 | 305.06561 (100%) | |||||||||||
| [(M+H)-2C6H10O5-H2O]+ | C15H11O6 | 287.05496 (40%) | |||||||||||
| [(M+H)-2C6H10O5-C8H8O3]+ | C7H5O4 | 153.01832 (2%) | |||||||||||
| 4 | Anthocyanin | Cyanidin-sambubioside | C26H29O15 | 581.15009 | - | 581.15039 | 0.50 | 20 | [M-C6H10O5-C5H8O4]+ | C15H11O6 | 287.05508 (100%) | a,b | [25,28] |
| [M-C6H10O5-C5H8O4-C6H4O4]+ | C9H7O2 | 147.04404 (1%) | |||||||||||
| [M-C6H10O5-C5H8O4-C8H6O3]+ | C7H5O3 | 137.02339 (66%) | |||||||||||
| [M-C6H10O5-C5H8O4-C8H6O4]+ | C7H5O2 | 121.02853 (1%) | |||||||||||
| 5 | Flavan-3-ol | Catechin | C15H14O6 | - | 291.08631 | 291.08636 | 0.17 | 10 | [(M+H)-C6H6O3]+ | C9H9O3 | 165.05470 (25%) | a,b,c | [18] |
| [(M+H)-C8H8O3]+ | C7H7O3 | 139.03903 (100%) | |||||||||||
| [(M+H)-C8H8O4]+ | C7H7O2 | 123.04424 (47%) | |||||||||||
| 6 | Anthocyanin | Cyanidin-3-glucoside | C21H21O11 | 449.10784 | - | 449.10818 | 0.77 | 20 | [M-C6H10O5]+ | C15H11O6 | 287.05515 (100%) | a,b,c | [20,25] |
| [M-C6H10O5-C8H6O3]+ | C7H5O3 | 137.02344 (25%) | |||||||||||
| [M-C6H10O5-C8H6O4]+ | C7H5O2 | 121.02854 (1%) | |||||||||||
| 7 | Flavonol | Kaempferol-hexuronide-hexoside | C27H28O17 | - | 625.13992 | 625.14044 | 0.82 | 20 | [(M+H)-C6H10O5]+ | C21H19O12 | 463.08743 (100%) | a,b | [26] |
| [(M+H)-C6H10O5-C6H8O6]+ | C15H11O6 | 287.05490 (45%) | |||||||||||
| [(M+H)-C6H10O5-C6H8O6-C8H6O2]+ | C7H5O4 | 153.01833(1%) | |||||||||||
| 8 | Flavonol | Quercetin-trihexoside | C33H40O22 | - | 789.20840 | 789.20880 | 0.50 | 20 | [(M+H)-C6H10O5]+ | C27H31O17 | 627.15594 (3%) | a,b | [29] |
| [(M+H)-2C6H10O5]+ | C21H21O12 | 465.10339 (60%) | |||||||||||
| [(M+H)-3C6H10O5]+ | C15H11O7 | 303.05011 (100%) | |||||||||||
| [(M+H)-3C6H10O5-C8H6O3]+ | C7H5O4 | 153.01836 (15%) | |||||||||||
| 9 | Flavan-3-ol | Epicatechin | C15H14O6 | - | 291.08631 | 291.08626 | 0.14 | 10 | [(M+H)-C6H6O3]+ | C9H9O3 | 165.05473 (25%) | a,b,c | [18] |
| [(M+H)-C8H8O3]+ | C7H7O3 | 139.03902 (100%) | |||||||||||
| [(M+H)-C8H8O4]+ | C7H7O2 | 123.04424 (47%) | |||||||||||
| 10 | Flavonol | Quercetin-dihexoside | C27H30O17 | - | 627.15557 | 627.15613 | 0.88 | 10 | [(M+H)-C6H10O5]+ | C21H21O12 | 465.10281 (16%) | a,b | [19,29] |
| [(M+H)-2C6H10O5]+ | C15H11O7 | 303.04990 (100%) | |||||||||||
| [(M+H)-2C6H10O5-C8H6O3]+ | C7H5O4 | 153.01819 (1%) | |||||||||||
| 11 | Flavonol | Kaempferol-deoxyhexoside-hexoside | C27H30O15 | - | 595.16574 | 595.16632 | 0.96 | 10 | [(M+H)-C6H10O5]+ | C21H21O10 | 433.11310 (20%) | a,b | [21] |
| [(M+H)-C6H10O5-C6H10O4]+ | C15H11O6 | 287.05502 (100%) | |||||||||||
| [(M+H)-C6H10O5-C6H10O4-C8H6O2]+ | C7H5O4 | 153.01822(1%) | |||||||||||
| 12 | Flavonol | Kaempferol-dihexoside | C27H30O16 | - | 611.16066 | 611.16132 | 1.01 | 10 | [(M+H)-C6H10O5]+ | C21H21O11 | 449.10754 (20%) | a,b | [21] |
| [(M+H)-2C6H10O5]+ | C15H11O6 | 287.05508 (100%) | |||||||||||
| [(M+H)-2C6H10O5-H2O]+ | C15H9O5 | 269.04465 (1%) | |||||||||||
| [(M+H)-2C6H10O5-C8H6O2]+ | C7H5O4 | 153.01814 (1%) | |||||||||||
| 13 | Flavonol | Kaempferol-deoxyhexoside | C21H20O10 | - | 433.11292 | 433.11292 | 0.68 | 20 | [(M+H)-C6H10O4]+ | C15H11O6 | 287.05508 (100%) | a,b | [21] |
| [(M+H)-C6H10O4-C8H6O2]+ | C7H5O4 | 153.01820 (1%) | |||||||||||
| 14 | Flavonol | Quercetin-hexuronide | C21H18O13 | - | 479.08201 | 479.08203 | 0.03 | 20 | [(M+H)-C6H8O6]+ | C15H11O7 | 303.04968 (100%) | a,b | [30] |
| [(M+H)-C6H8O6-H2O]+ | C15H9O6 | 285.03899 (1%) | |||||||||||
| [(M+H)-C6H8O6-C8H6O3]+ | C7H5O4 | 153.01825 (1%) | |||||||||||
| 15 | Flavanonol | Didhydrokaempferol-hexuronide | C21H20O12 | - | 465.10275 | 465.10297 | 0.45 | 20 | [(M+H)-C6H8O6]+ | C15H13O6 | 289.07037 (100%) | a,b | [29] |
| [(M+H)-C6H8O6-C8H8O2]+ | C7H5O4 | 153.01805 (10%) | |||||||||||
| 16 | Flavonol | Quercetin-3-glucoside | C21H20O12 | - | 465.10275 | 465.10300 | 0.52 | 10 | [(M+H)-C6H10O5]+ | C15H11O7 | 303.05005 (100%) | a,b,c | [18] |
| [(M+H)-C6H10O5-H2O]+ | C15H9O6 | 285.03960 (1%) | |||||||||||
| [(M+H)-C6H10O5-C8H6O3]+ | C7H5O4 | 153.01799 (1%) | |||||||||||
| 17 | Flavanone | Naringenin-hexuronide | C21H20O11 | - | 449.10784 | 449.10770 | 0.31 | 10 | [(M+H)-C6H8O6]+ | C15H13O5 | 273.07559 (100%) | a,b | [31] |
| [(M+H)-C6H8O6-C8H8O]+ | C7H5O4 | 153.01829(8%) | |||||||||||
| 18 | Flavonol | Kaempferol-hexuronide | C21H18O12 | - | 463.08670 | 463.08710 | 0.86 | 20 | [(M+H)-C6H8O6]+ | C15H11O6 | 287.05490 (45%) | a,b | [21] |
| [(M+H)-C6H8O6-C8H6O2]+ | C7H5O4 | 153.01833(1%) | |||||||||||
| 19 | Flavonol | Kaempferol-3-glucoside | C21H20O11 | - | 449.10784 | 449.10784 | 0.56 | 10 | [(M+H)-C6H10O5]+ | C15H11O6 | 287.04718 (100%) | a,b,c | [18,32] |
| [(M+H)-C6H10O5-C8H6O2]+ | C7H5O4 | 153.01855 (4%) | |||||||||||
| 20 | Flavone | Apigenin-hexuronide | C21H18O11 | - | 447.09218 | 447.09216 | 0.05 | 20 | [(M+H)-H2O]+ | C21H17O10 | 429.08162 (1%) | a,b | [31] |
| [(M+H)-C6H8O6]+ | C15H11O5 | 271.05997(45%) | |||||||||||
| [(M+H)-C6H8O6-C8H6O]+ | C7H5O4 | 153.01824 (17%) | |||||||||||
| [(M+H)-C6H8O6-C8H6O3]+ | C7H5O2 | 121.02857 (1%) | |||||||||||
| Cultivar | CIELAB Parameters (Value ± S, n = 5) | Total Flavonoids (mg Flavonoid/g Extract) (Value ± S, n = 3) | Antioxidant Activity | Color * | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | Code Figure 1 | L* | a* | b* | C*ab | hab | Anthocyanins | Flavonols | Flavan-3-ols | Flavanonols | Flavones | μmol Trolox®/gExtract, ±S, n = 3 | |
| Red | A | 28 ± 2 | 65 ± 4 | 35 ± 4 | 73 ± 2 | 0.5 ± 0.07 | 19.9 ± 0.9 | 14.9 ± 0.9 | 0.91 ± 0.01 | 1.48 ± 0.05 | 0.07 ± 0.001 | 3840 ± 74 |  |
| Red | B | 54 ± 1 | 65 ± 4 | 53 ± 2 | 84 ± 3 | 0.7 ± 0.04 | 23.6 ± 0.6 | 5.7 ± 0.3 | 2.5 ± 0.1 | 2.0 ± 0.2 | 0.31 ± 0.02 | 3480 ± 64 |  |
| Red | C | 61 ± 1 | 64 ± 2 | 24 ± 2 | 68 ± 2 | 0.4 ± 0.01 | 21.8 ± 0.9 | 8.0 ± 0.4 | 2.3 ± 0.1 | 2.0 ± 0.1 | 0.53 ± 0.04 | 3200 ± 100 |  |
| Red | D | 56 ± 3 | 77 ± 3 | 15 ± 0.5 | 78 ± 3 | 0.2 ± 0.01 | 19.8 ± 0.9 | 6.2 ± 0.5 | 1.3 ± 0.4 | 1.9 ± 0.1 | 0.5 ± 0.2 | 2900 ± 133 |  |
| Pink | E | 58 ± 2 | 69 ± 0.3 | 26 ± 1 | 74 ± 0.6 | 0.4 ± 0.02 | 6.6 ± 0.1 | 15.8 ± 0.7 | 0.23 ± 0.01 | 0.59 ± 0.03 | 0.18 ± 0.03 | 2900 ± 126 |  |
| Pink | F | 69 ± 3 | 41 ± 3 | −6 ± 0.8 | 42 ± 3 | −0.2 ± 0.03 | 3.4 ± 0.2 | 19 ± 1 | 0.31 ± 0.05 | 0.32 ± 0.01 | 0.41 ± 0.01 | 3200 ± 132 |  |
| Pink | G | 69 ± 2 | 57 ± 0.4 | −7 ± 2 | 57 ± 0.2 | −0.1 ± 0.03 | 9.8 ± 0.5 | 17.8 ± 0.6 | 1.9 ± 0.1 | 0.95 ± 0.05 | 0.17 ± 0.04 | 3130 ± 33 |  |
| Orange | H | 73 ± 1 | 35 ± 3 | 51 ± 2 | 62 ± 2 | 1.0 ± 0.03 | 1.6 ± 0.1 | 34 ± 1 | 0.14 ± 0.01 | 0.11 ± 0.01 | 0.53 ± 0.03 | 3250 ± 40 |  |
| Orange | I | 69 ± 3 | 44 ± 3 | 51 ± 3 | 68 ± 3 | 0.9 ± 0.04 | 1.7 ± 0.1 | 28 ± 1 | 0.23 ± 0.04 | 0.12 ± 0.02 | 0.16 ± 0.01 | 3200 ± 135 |  |
| Orange | J | 72 ± 2 | 37 ± 3 | 58 ± 1 | 68 ± 2 | 1.0 ± 0.03 | 3.0 ± 0.1 | 32 ± 1 | 0.34 ± 0.03 | 0.09 ± 0.001 | 0.13 ± 0.01 | 3000 ± 77 |  |
| Yellow | K | 93 ± 1 | −8.1 ± 0.1 | 93 ± 0.3 | 93 ± 0.3 | −1.5 ± 0.01 | - | 29 ± 1 | 0.22 ± 0.02 | - | 0.17 ± 0.03 | 2580 ± 53 |  |
| Yellow | L | 96 ± 2 | −16 ± 0.8 | 77 ± 2 | 79 ± 2 | −1.4 ± 0.01 | - | 23.9 ± 0.5 | 0.050 ± 0.002 | - | 0.25 ± 0.01 | 2440 ± 90 |  |
| Yellow | M | 95 ± 3 | −18 ± 2 | 91 ± 0.8 | 93 ± 1 | −1.4 ± 0.01 | - | 19.2 ± 0.8 | 0.92 ± 0.02 | - | 0.56 ± 0.02 | 2100 ± 99 |  |
| Yellow | N | 90 ± 1 | −7 ± 0.5 | 89 ± 2 | 89 ± 2 | −1.5 ± 0.01 | 0.30 ± 0.01 | 16.0 ± 0.5 | 0.20 ± 0.03 | - | 0.22 ± 0.01 | 1930 ± 72 |  |
| Light purple | O | 96 ± 2 | 5 ± 1 | 6 ± 1 | −0.1 ± 0.03 | 1.1 ± 0.1 | 12.3 ± 0.6 | 0.35 ± 0.04 | 0.18 ± 0.01 | 0.21 ± 0.01 | 1850 ± 87 |  | |
| White | P | 99 ± 5 | −4 ± 1 | 9 ± 2 | 10 ± 2 | −1.1 ± 0.09 | - | 12.6 ± 0.6 | 0.021 ± 0.003 | - | 0.28 ± 0.02 | 1580 ± 41 |  |
| Figure 2 N° | Compound | Cultivar, mg/g of Extract, (Value ± S, n = 3) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Red | Orange | Pink | Yellow | ||||||||||
| 1 | Cyanidin-sophoroside 1 | 21 | ± | 1 | 2.0 | ± | 0.1 | 2.4 | ± | 0.2 | - | ||
| 2 | Quercetin-hexuronide-hexoside 2 | 0.1 | ± | 0.01 | 1.0 | ± | 0.1 | 0.40 | ± | 0.04 | 0.50 | ± | 0.06 |
| 3 | Taxifolin-dihexoside 2 | 1.5 | ± | 0.2 | - | 0.20 | ± | 0.01 | - | ||||
| 4 | Cyanidin-sambubioside1 | 1.1 | ± | 0.1 | - | - | - | ||||||
| 5 | Catechin | 1.5 | ± | 0.1 | 0.20 | ± | 0.02 | 0.20 | ± | 0.04 | 0.20 | ± | 0.02 |
| 6 | Cyanidin-3-glucoside | 1.5 | ± | 0.2 | 1.1 | ± | 0.1 | 1.0 | ± | 0.1 | - | ||
| 7 | Kaempferol-hexuronide-hexoside 3 | - | 1.70 | ± | 0.02 | 0.20 | ± | 0.02 | 0.60 | ± | 0.06 | ||
| 8 | Quercetin-trihexoside | - | - | 0.20 | ± | 0.01 | 0.10 | ± | 0.01 | ||||
| 9 | Epicatechin | 1.1 | ± | 0.1 | 0.20 | ± | 0.01 | 0.20 | ± | 0.01 | - | ||
| 10 | Quercetin-dihexoside 2 | 1.5 | ± | 0.2 | 8.2 | ± | 0.9 | 6.8 | ± | 0.2 | 8.20 | ± | 0.08 |
| 11 | Kaempferol-deoxyhexoside-hexoside 3 | 0.20 | ± | 0.01 | 0.20 | ± | 0.01 | 0.50 | ± | 0.01 | 0.30 | ± | 0.04 |
| 12 | Kaempferol-dihexoside 3 | - | 3.1 | ± | 0.3 | 0.60 | ± | 0.01 | 1.30 | ± | 0.01 | ||
| 13 | Kaempferol-deoxyhexoside 3 | 0.30 | ± | 0.01 | 0.50 | ± | 0.01 | 1.50 | ± | 0.01 | 2.60 | ± | 0.06 |
| 14 | Quercetin-hexuronide 2 | 0.20 | ± | 0.01 | 0.70 | ± | 0.01 | 0.70 | ± | 0.03 | 1.20 | ± | 0.09 |
| 15 | Didhydrokaempferol-hexuronide 3 | 0.10 | 0.01 | - | 0.30 | 0.02 | - | ||||||
| 16 | Quercetin-3-glucoside | 2.0 | ± | 0.03 | 11 | ± | 1 | 7 | ± | 1 | 11.0 | ± | 0.5 |
| 17 | Naringenin-hexuronide 2 | 2.40 | ± | 0.15 | - | 7.0 | ± | 0.6 | - | ||||
| 18 | Kaempferol-hexuronide 3 | - | 0.80 | ± | 0.04 | 0.20 | ± | 0.01 | 0.9 | ± | 0.1 | ||
| 19 | Kaempferol-3-glucoside | - | 5.1 | ± | 0.3 | - | 2.7 | ± | 0.3 | ||||
| 20 | Apigenin-hexuronide 2 | 0.30 | ± | 0.02 | 0.10 | ± | 0.01 | 0.40 | ± | 0.01 | 0.20 | ± | 0.01 |
| Species | Family | Components | Assay | Result | |||
|---|---|---|---|---|---|---|---|
| Values * | Units | ||||||
| Punica granatum [38] | Lythraceae | Anthocyanins (delphinidin, cyanidin, and pelargonidin) | SOD | 17 | ± | 1 | SOD-equivalent units/mg of freezedried extract |
| Solanum tuberosum [35] | Solanaceae | Anthocyanins, flavonoids and phenolic acids | ORAC | 28.25 | - | 250.67 | µmol Trolox®/g of extract |
| Ginkgo biloba [39] | Lamiaceae | Flavonols (quercetin-3-rutinoside, quercetin-3-O-rhamnosyl-rhamnosyl-glucoside, and kaempferol-3-O-rhamnosyl-rhamnosyl-glucoside) | ORAC | 13.18 | ± | 0.24 | µmol Trolox®/g of extract |
| Pelargonium graveolens [40] | Geraniaceae | Flavonols and tannins | ABTS+• | 131.54 | - | 241.83 | μg Trolox equivalents/mg of extract |
| Vitis vinifera [41] | Vitaceae | Flavan-3-ols (catechin and epicatechin), flavonol (quercetin), phenolic acids (gallic and ellagic acids), stilbenoid (resveratrol) and ascorbic acid | ORAC | 450.51 | ± | 74.02 | µmol Trolox®/g of extract |
| Lippia graveolens [42] | Verbenaceae | Flavanonol (taxifolin), flavones (apigenin-7-O-glucoside and hispidulin), dihydrochalcone (phlorizin), flavanones (eriodictyol, naringenin and pinocembrin), O-methylated flavone (genkwanin) and flavonols (quercetin and galangin) | ORAC | 1.66 | ± | 0.284 | mg Trolox®/mL of extract |
| Artemisia annua [43] | Asteraceae | Flavone (apigenin), flavonol (rutin) and phenolic acid (caffeic acid) | ORAC | 736.26 | ± | 17.55 | µM Trolox®/g of plant |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mejía, J.J.; Sierra, L.J.; Ceballos, J.G.; Martínez, J.R.; Stashenko, E.E. Color, Antioxidant Capacity and Flavonoid Composition in Hibiscus rosa-sinensis Cultivars. Molecules 2023, 28, 1779. https://doi.org/10.3390/molecules28041779
Mejía JJ, Sierra LJ, Ceballos JG, Martínez JR, Stashenko EE. Color, Antioxidant Capacity and Flavonoid Composition in Hibiscus rosa-sinensis Cultivars. Molecules. 2023; 28(4):1779. https://doi.org/10.3390/molecules28041779
Chicago/Turabian StyleMejía, Jesica J., Lady J. Sierra, José G. Ceballos, Jairo R. Martínez, and Elena E. Stashenko. 2023. "Color, Antioxidant Capacity and Flavonoid Composition in Hibiscus rosa-sinensis Cultivars" Molecules 28, no. 4: 1779. https://doi.org/10.3390/molecules28041779