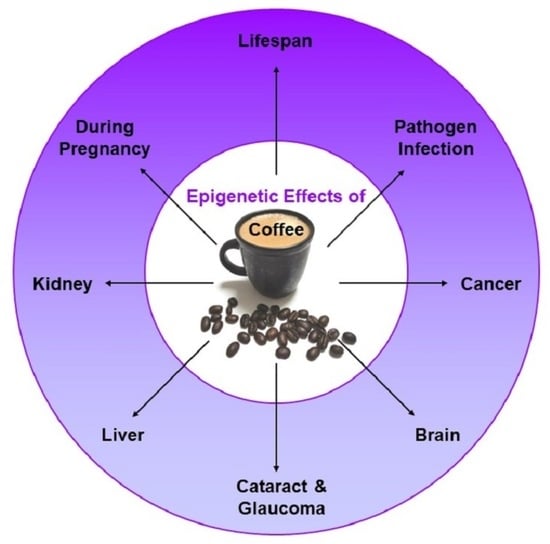
The Epigenetic Effects of Coffee
Abstract
:1. Introduction
2. Epigenetics and Diseases
3. The Impact of Coffee during Pregnancy and in Diseases
3.1. Impact during Pregnancy
3.1.1. Impact on Hypothalamic–Pituitary–Adrenal (HPA) Axis
3.1.2. Impact on Bone
3.1.3. Impact on Ovary
3.1.4. Impact on Cardiovascular Development
3.2. Impact on Cancer
3.2.1. Colon Carcinoma
3.2.2. Leukemia
3.2.3. Breast Cancer
3.2.4. Cancer Risk
3.3. Cataract and Glaucoma
3.4. Pathogen Infection
3.5. Impact on Brain
3.6. Impact on Liver
3.7. Impact on Kidney
3.8. Impact on Human Genome
3.9. Other Epigenetic Effects
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
| ACE | Angiotensin converting enzyme |
| ACF | Aberrant crypt foci |
| AD | Alzheimer’s disease |
| AKI | Acute kidney injury |
| ALI | Acute lung injury |
| ALL | Acute lymphoblastic leukemia |
| CA or CaA | Caffeic acid |
| CAF | Caffeine |
| CGA | Chlorogenic acid |
| CPP | Coffee polyphenols |
| CRH | Corticotrophin-releasing hormone |
| CSCs | Cancer stem cells |
| CUMS | Chronic unpredictable mild stress |
| DMBA | 7,12-dimethylbenz[a]anthracene |
| DMH/DCA | 1,2-dimethylhydrazine/deoxycholic acid |
| DMRs | Differentially methylated regions |
| DN | Diabetic nephropathy |
| GC | Glucocorticoid |
| GD | Gestational day |
| GR | Glucocorticoid receptor |
| HMGCR | 3-hydroxy-3-methylglutaryl-CoA reductase |
| HPA | Hypothalamic–pituitary–adrenal |
| IGF-1 | Insulin-like growth factor 1 |
| IUGR | Intrauterine growth retardation |
| KLF4 | Kruppel-like factor 4 |
| LINE-1 | Long interspersed element-1 |
| LPS | Lipopolysaccharide |
| MDD | Major depressive disorder |
| MEF | Myocyte enhancer factor |
| NAFLD | Nonalcoholic fatty liver disease |
| NF-κB | Nuclear factor-kappa B |
| NLRP3 | Nucleotide-binding oligomerization domain-like receptor protein 3 |
| Nrf2 | Nuclear factor erythroid-2 related factor 2 |
| NTs | Nuclear transfer embryos |
| OGD | Oxygen and glucose deprivation |
| PCE | Prenatal caffeine exposure |
| PD | Parkinson’s disease |
| RAS | Renin angiotensin system |
| RGC | Retinal ganglion cell |
| SF-1 | Steroidogenic factor-1 |
| SIRT1 | Sirtuin-1 |
| SR-BI | Scavenger receptor class B type I |
| SREBP | Sterol regulatory element-binding protein |
| ST | Salmonella typhimurium |
| StAR | Steroidogenic acute regulatory protein |
| STZ | Streptozotocin |
| TGFβR1 | Transforming growth factor beta receptor 1 |
| 11β-HSD-2 | 11β-hydroxysteroid dehydrogenase-2 |
References
- National Coffee Association of U.S.A. National Coffee Data Trends Fall 2022. Media Highlights. Available online: https://www.ncausa.org/Research-Trends/Market-Research/NCDT (accessed on 1 November 2022).
- Saud, S.; Salamatullah, A.M. Relationship between the Chemical Composition and the Biological Functions of Coffee. Molecules 2021, 26, 7634. [Google Scholar] [CrossRef] [PubMed]
- Ciaramelli, C.; Palmioli, A.; Airoldi, C. Coffee variety, origin and extraction procedure: Implications for coffee beneficial effects on human health. Food Chem. 2019, 278, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Olechno, E.; Puścion-Jakubik, A.; Zujko, M.E.; Socha, K. Influence of Various Factors on Caffeine Content in Coffee Brews. Foods 2021, 10, 1208. [Google Scholar] [CrossRef] [PubMed]
- Fuller, M.; Rao, N.Z. The Effect of Time, Roasting Temperature, and Grind Size on Caffeine and Chlorogenic Acid Concentrations in Cold Brew Coffee. Sci. Rep. 2017, 7, 17979. [Google Scholar] [CrossRef]
- Kositamongkol, C.; Kanchanasurakit, S.; Auttamalang, C.; Inchai, N.; Kabkaew, T.; Kitpark, S.; Chaiyakunapruk, N.; Duangjai, A.; Saokaew, S.; Phisalprapa, P. Coffee Consumption and Non-alcoholic Fatty Liver Disease: An Umbrella Review and a Systematic Review and Meta-analysis. Front. Pharmacol. 2021, 12, 786596. [Google Scholar] [CrossRef]
- Chieng, D.; Kistler, P.M. Coffee and tea on cardiovascular disease (CVD) prevention. Trends Cardiovasc. Med. 2022, 32, 399–405. [Google Scholar] [CrossRef]
- Pauwels, E.K.J.; Volterrani, D. Coffee Consumption and Cancer Risk: An Assessment of the Health Implications Based on Recent Knowledge. Med. Princ. Pract. 2021, 30, 401–411. [Google Scholar] [CrossRef]
- Gökcen, B.B.; Şanlier, N. Coffee consumption and disease correlations. Crit. Rev. Food Sci. Nutr. 2019, 59, 336–348. [Google Scholar] [CrossRef]
- Yelanchezian, Y.M.M.; Waldvogel, H.J.; Faull, R.L.M.; Kwakowsky, A. Neuroprotective Effect of Caffeine in Alzheimer’s Disease. Molecules 2022, 27, 3737. [Google Scholar] [CrossRef]
- Jee, S.H.; He, J.; Whelton, P.K.; Suh, I.; Klag, M.J. The effect of chronic coffee drinking on blood pressure: A meta-analysis of controlled clinical trials. Hypertension 1999, 33, 647–652. [Google Scholar] [CrossRef] [Green Version]
- Mazeaud, S.; Castellana, F.; Coelho-Junior, H.J.; Panza, F.; Rondanelli, M.; Fassio, F.; De Pergola, G.; Zupo, R.; Sardone, R. Coffee Drinking and Adverse Physical Outcomes in the Aging Adult Population: A Systematic Review. Metabolites 2022, 12, 654. [Google Scholar] [CrossRef] [PubMed]
- Li, Y. Modern epigenetics methods in biological research. Methods 2021, 187, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Chen, Q.; Ward, S.M.; Duan, E.; Zhang, Y. Impacts of Caffeine during Pregnancy. Trends Endocrinol. Metab. 2020, 31, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Zhang, B.; Liang, G.; Ping, J.; Kou, H.; Li, X.; Xiong, J.; Hu, D.; Chen, L.; Magdalou, J.; et al. Caffeine-induced activated glucocorticoid metabolism in the hippocampus causes hypothalamic-pituitary-adrenal axis inhibition in fetal rats. PLoS ONE 2012, 7, e44497. [Google Scholar] [CrossRef] [PubMed]
- Ping, J.; Lei, Y.Y.; Liu, L.; Wang, T.T.; Feng, Y.H.; Wang, H. Inheritable stimulatory effects of caffeine on steroidogenic acute regulatory protein expression and cortisol production in human adrenocortical cells. Chem. Biol. Interact. 2012, 195, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Ping, J.; Wang, J.F.; Liu, L.; Yan, Y.E.; Liu, F.; Lei, Y.Y.; Wang, H. Prenatal caffeine ingestion induces aberrant DNA methylation and histone acetylation of steroidogenic factor 1 and inhibits fetal adrenal steroidogenesis. Toxicology 2014, 321, 53–61. [Google Scholar] [CrossRef]
- He, Z.; Zhang, J.; Chen, G.; Cao, J.; Chen, Y.; Ai, C.; Wang, H. H19/let-7 axis mediates caffeine exposure during pregnancy induced adrenal dysfunction and its multi-generation inheritance. Sci. Total Environ. 2021, 792, 148440. [Google Scholar] [CrossRef]
- Wu, D.M.; He, Z.; Ma, L.P.; Wang, L.L.; Ping, J.; Wang, H. Increased DNA methylation of scavenger receptor class B type I contributes to inhibitory effects of prenatal caffeine ingestion on cholesterol uptake and steroidogenesis in fetal adrenals. Toxicol. Appl. Pharmacol. 2015, 285, 89–97. [Google Scholar] [CrossRef]
- Tan, Y.; Liu, J.; Deng, Y.; Cao, H.; Xu, D.; Cu, F.; Lei, Y.; Magdalou, J.; Wu, M.; Chen, L.; et al. Caffeine-induced fetal rat over-exposure to maternal glucocorticoid and histone methylation of liver IGF-1 might cause skeletal growth retardation. Toxicol. Lett. 2012, 214, 279–287. [Google Scholar] [CrossRef]
- Tan, Y.; Lu, K.; Li, J.; Ni, Q.; Zhao, Z.; Magdalou, J.; Chen, L.; Wang, H. Prenatal caffeine exprosure increases adult female offspring rat’s susceptibility to osteoarthritis via low-functional programming of cartilage IGF-1 with histone acetylation. Toxicol. Lett. 2018, 295, 229–236. [Google Scholar] [CrossRef]
- Li, J.; Xiao, H.; Luo, H.; Tan, Y.; Ni, Q.; He, C.; Magdalou, J.; Chen, L.; Wang, H. GR/HDAC2/TGFβR1 pathway contributes to prenatal caffeine induced-osteoarthritis susceptibility in male adult offspring rats. Food Chem. Toxicol. 2020, 140, 111279. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Shangguan, Y.; Pan, Z.; Hu, H.; Magdalou, J.; Chen, L.; Wang, H. Activation of local bone RAS by maternal excessive glucocorticoid participated in the fetal programing of adult osteopenia induced by prenatal caffeine exposure. Toxicol. Appl. Pharmacol. 2019, 363, 1–10. [Google Scholar] [CrossRef]
- Xiao, H.; Wu, Z.; Li, B.; Shangguan, Y.; Stoltz, J.F.; Magdalou, J.; Chen, L.; Wang, H. The low-expression programming of 11β-HSD2 mediates osteoporosis susceptibility induced by prenatal caffeine exposure in male offspring rats. Br. J. Pharmacol. 2020, 177, 4683–4700. [Google Scholar] [CrossRef] [PubMed]
- Lv, F.; Fan, G.; Wan, Y.; Chen, Y.; Ni, Y.; Huang, J.; Xu, D.; Zhang, W.; Wang, H. Intrauterine endogenous high glucocorticoids program ovarian dysfunction in female offspring secondary to prenatal caffeine exposure. Sci. Total Environ. 2021, 789, 147691. [Google Scholar] [CrossRef] [PubMed]
- Buscariollo, D.L.; Fang, X.; Greenwood, V.; Xue, H.; Rivkees, S.A.; Wendler, C.C. Embryonic caffeine exposure acts via A1 adenosine receptors to alter adult cardiac function and DNA methylation in mice. PLoS ONE 2014, 9, e87547. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Mei, W.; Barbazuk, W.B.; Rivkees, S.A.; Wendler, C.C. Caffeine exposure alters cardiac gene expression in embryonic cardiomyocytes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 307, R1471–R1487. [Google Scholar] [CrossRef]
- Surma, S.; Witek, A. Coffee consumption during pregnancy—What the gynecologist should know? Review of the literature and clinical studies. Ginekol. Pol. 2022, 93, 591–600. [Google Scholar] [CrossRef]
- Ross, G.W.; Abbott, R.D.; Petrovitch, H.; Morens, D.M.; Grandinetti, A.; Tung, K.H.; Tanner, C.M.; Masaki, K.H.; Blanchette, P.L.; Curb, J.D.; et al. Association of coffee and caffeine intake with the risk of Parkinson disease. JAMA 2000, 283, 2674–2679. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, T.; Funakoshi-Tago, M.; Tamura, H. Coffee reduces KRAS expression in Caco-2 human colon carcinoma cells via regulation of miRNAs. Oncol. Lett. 2017, 14, 1109–1114. [Google Scholar] [CrossRef]
- Luque-Badillo, A.C.; Hernandez-Tapia, G.; Ramirez-Castillo, D.A.; Espinoza-Serrano, D.; Cortes-Limon, A.M.; Cortes-Gallardo, J.P.; Jacobo-Velázquez, D.A.; Martinez-Fierro, M.L.; Rios-Ibarra, C.P. Gold nanoparticles enhance microRNA 31 detection in colon cancer cells after inhibition with chlorogenic acid. Oncol. Lett. 2021, 22, 742. [Google Scholar] [CrossRef] [PubMed]
- Bartolomeu, A.R.; Romualdo, G.R.; Lisón, C.G.; Besharat, Z.M.; Corrales, J.A.M.; Chaves MÁ, G.; Barbisan, L.F. Caffeine and Chlorogenic Acid Combination Attenuate Early-Stage Chemically Induced Colon Carcinogenesis in Mice: Involvement of oncomiR miR-21a-5p. Int. J. Mol. Sci. 2022, 23, 6292. [Google Scholar] [CrossRef] [PubMed]
- Timms, J.A.; Relton, C.L.; Sharp, G.C.; Rankin, J.; Strathdee, G.; McKay, J.A. Exploring a potential mechanistic role of DNA methylation in the relationship between in utero and post-natal environmental exposures and risk of childhood acute lymphoblastic leukaemia. Int. J. Cancer 2019, 145, 2933–2943. [Google Scholar] [CrossRef] [PubMed]
- Hernandes, L.C.; Machado, A.R.T.; Tuttis, K.; Ribeiro, D.L.; Aissa, A.F.; Dévoz, P.P.; Antunes, L.M.G. Caffeic acid and chlorogenic acid cytotoxicity, genotoxicity and impact on global DNA methylation in human leukemic cell lines. Genet. Mol. Biol. 2020, 43, e20190347. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J.; Zhu, B.T. Inhibition of DNA methylation by caffeic acid and chlorogenic acid, two common catechol-containing coffee polyphenols. Carcinogenesis 2006, 27, 269–277. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, F.; Chen, L.; Yang, Y.; Cao, S.; Ye, Y.; Wang, X.; Mu, J.; Li, Z.; Li, L. Blockage of TGFβ-SMAD2 by demethylation-activated miR-148a is involved in caffeic acid-induced inhibition of cancer stem cell-like properties in vitro and in vivo. FEBS Open Bio. 2015, 5, 466–475. [Google Scholar] [CrossRef]
- Molnar, R.; Szabo, L.; Tomesz, A.; Deutsch, A.; Darago, R.; Raposa, B.L.; Ghodratollah, N.; Varjas, T.; Nemeth, B.; Orsos, Z.; et al. The Chemopreventive Effects of Polyphenols and Coffee, Based upon a DMBA Mouse Model with microRNA and mTOR Gene Expression Biomarkers. Cells 2022, 11, 1300. [Google Scholar] [CrossRef]
- Yuan, S.; Wolk, A.; Larsson, S.C. Metabolic and lifestyle factors in relation to senile cataract: A Mendelian randomization study. Sci. Rep. 2022, 12, 409. [Google Scholar] [CrossRef]
- Bae, J.H.; Kim, J.M.; Lee, J.M.; Song, J.E.; Lee, M.Y.; Chung, P.W.; Park, K.H. Effects of consumption of coffee, tea, or soft drinks on open-angle glaucoma: Korea National Health and Nutrition Examination Survey 2010 to 2011. PLoS ONE 2020, 15, e0236152. [Google Scholar] [CrossRef]
- Li, X.; Cheng, S.; Cheng, J.; Wang, M.; Zhong, Y.; Yu, A.Y. Habitual Coffee Consumption Increases Risk of Primary Open-Angle Glaucoma: A Mendelian Randomization Study. Ophthalmology 2022, 129, 1014–1021. [Google Scholar] [CrossRef]
- Pasquale, L.R.; Wiggs, J.L.; Willett, W.C.; Kang, J.H. The Relationship between caffeine and coffee consumption and exfoliation glaucoma or glaucoma suspect: A prospective study in two cohorts. Investig. Ophthalmol. Vis. Sci. 2012, 53, 6427–6433. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Aschard, H.; Kang, J.H.; Lentjes, M.A.H.; Do, R.; Wiggs, J.L.; Khawaja, A.P.; Pasquale, L.R. Intraocular Pressure, Glaucoma, and Dietary Caffeine Consumption: A Gene-Diet Interaction Study from the UK Biobank. Ophthalmology 2021, 128, 866–876. [Google Scholar] [CrossRef] [PubMed]
- Nakano, E.; Miyake, M.; Hosoda, Y.; Mori, Y.; Suda, K.; Kameda, T.; Ikeda-Ohashi, H.; Tabara, Y.; Yamashiro, K.; Tamura, H.; et al. Relationship between Intraocular Pressure and Coffee Consumption in a Japanese Population without Glaucoma: The Nagahama Study. Ophthalmol. Glaucoma 2021, 4, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.M.; Wu, A.M.; Tseng, V.L.; Yu, F.; Coleman, A.L. Frequency of a diagnosis of glaucoma in individuals who consume coffee, tea and/or soft drinks. Br. J. Ophthalmol. 2018, 102, 1127–1133. [Google Scholar] [CrossRef]
- Varma, S.D.; Kovtun, S. Protective effect of caffeine against high sugar-induced transcription of microRNAs and consequent gene silencing: A study using lenses of galactosemic mice. Mol. Vis. 2013, 19, 493–500. [Google Scholar]
- Gong, W.; Li, J.; Zhu, G.; Wang, Y.; Zheng, G.; Kan, Q. Chlorogenic acid relieved oxidative stress injury in retinal ganglion cells through IncRNA-TUG1/Nrf2. Cell Cycle 2019, 18, 1549–1559. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; He, S.; Li, R.; Zhou, X.; Zhang, S.; Yu, M.; Ye, Y.; Wang, Y.; Huang, C.; Wu, M. Pseudomonas aeruginosa infection augments inflammation through miR-301b repression of c-Myb-mediated immune activation and infiltration. Nat. Microbiol. 2016, 1, 16132. [Google Scholar] [CrossRef]
- Liu, C.; Cheng, X.; Sun, J.; Zhang, S.; Zhang, Q. Mechanism of chlorogenic acid reducing lipopolysaccharide-induced acute lung injury in mice by regulating miR-223/NLRP3 axis. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2022, 47, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, F.; Xue, J.; Zhou, X.; Luo, L.; Ma, Q.; Chen, Y.F.; Zhang, J.; Zhang, S.L.; Zhao, L. Antischistosomiasis Liver Fibrosis Effects of Chlorogenic Acid through IL-13/miR-21/Smad7 Signaling Interactions in vivo and in vitro. Antimicrob. Agents Chemother. 2017, 61, e01347-16. [Google Scholar] [CrossRef]
- Tan, S.; Yan, F.; Li, Q.; Liang, Y.; Yu, J.; Li, Z.; He, F.; Li, R.; Li, M. Chlorogenic Acid Promotes Autophagy and Alleviates Salmonella Typhimurium Infection Through the lncRNAGAS5/miR-23a/PTEN Axis and the p38 MAPK Pathway. Front. Cell Dev. Biol. 2020, 8, 552020. [Google Scholar] [CrossRef]
- Yenisetti, S.C.; Muralidhara. Beneficial Role of Coffee and Caffeine in Neurodegenerative Diseases: A Minireview. AIMS Public Health 2016, 3, 407–422. [Google Scholar] [CrossRef]
- Kim, J.W.; Byun, M.S.; Yi, D.; Lee, J.H.; Jeon, S.Y.; Jung, G.; Lee, H.N.; Sohn, B.K.; Lee, J.Y.; Kim, Y.K.; et al. Coffee intake and decreased amyloid pathology in human brain. Transl. Psychiatry 2019, 9, 270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossetto, I.M.U.; Cagnon, V.H.A.; Lizarte, F.S.N.; Tirapelli, L.F.; Tirapelli, D.P.C.; Arantes, R.M.S.; Chuffa, L.G.A.; Martinez, F.E.; Martinez, M. Ethanol and caffeine consumption modulates the expression of miRNAs in the cerebellum and plasma of UChB rats. Life Sci. 2019, 229, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Li, Y.; Yang, Y.; Lin, K.; Lin, Q.; Luo, S.; Zhou, X.; Lin, Q.; Zhang, F. Chlorogenic Acid Prevents Microglia-Induced Neuronal Apoptosis and Oxidative Stress under Hypoxia-Ischemia Environment by Regulating the MIR497HG/miR-29b-3p/SIRT1 Axis. Dis. Mrk. 2022, 2022, 1194742. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Cao, S.; Zhang, Z.; Wang, L.; Wang, D.; Wu, Q.; Li, L. Effects of caffeic acid on epigenetics in the brain of rats with chronic unpredictable mild stress. Mol. Med. Rep. 2020, 22, 5358–5368. [Google Scholar] [CrossRef] [PubMed]
- Wadhawan, M.; Anand, A.C. Coffee and Liver Disease. J. Clin. Exp. Hepatol. 2016, 6, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Murase, T.; Misawa, K.; Minegishi, Y.; Aoki, M.; Ominami, H.; Suzuki, Y.; Shibuya, Y.; Hase, T. Coffee polyphenols suppress diet-induced body fat accumulation by downregulating SREBP-1c and related molecules in C57BL/6J mice. Am. J. Physiol. Endocrinol. Metab. 2011, 300, E122–E133. [Google Scholar] [CrossRef]
- Hu, S.; Xia, L.; Luo, H.; Xu, Y.; Yu, H.; Xu, D.; Wang, H. Prenatal caffeine exposure increases the susceptibility to non-alcoholic fatty liver disease in female offspring rats via activation of GR-C/EBPα-SIRT1 pathway. Toxicology 2019, 417, 23–34. [Google Scholar] [CrossRef]
- Xu, D.; Luo, H.W.; Hu, W.; Hu, S.W.; Yuan, C.; Wang, G.H.; Zhang, L.; Yu, H.; Magdalou, J.; Chen, L.B.; et al. Intrauterine programming mechanism for hypercholesterolemia in prenatal caffeine-exposed female adult rat offspring. FASEB J. 2018, 32, 5563–5576. [Google Scholar] [CrossRef]
- Hu, S.; Liu, K.; Luo, H.; Xu, D.; Chen, L.; Zhang, L.; Wang, H. Caffeine programs hepatic SIRT1-related cholesterol synthesis and hypercholesterolemia via A2AR/cAMP/PKA pathway in adult male offspring rats. Toxicology 2019, 418, 11–21. [Google Scholar] [CrossRef]
- Yang, F.; Luo, L.; Zhu, Z.D.; Zhou, X.; Wang, Y.; Xue, J.; Zhang, J.; Cai, X.; Chen, Z.L.; Ma, Q.; et al. Chlorogenic Acid Inhibits Liver Fibrosis by Blocking the miR-21-Regulated TGF-β1/Smad7 Signaling Pathway in vitro and in vivo. Front. Pharmacol. 2017, 8, 929. [Google Scholar] [CrossRef]
- Zhao, J.; Huang, Y.; Yu, X. Caffeine intake and the risk of incident kidney stones: A systematic review and meta-analysis. Int. Urol. Nephrol. 2022, 54, 2457–2466. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, W.; Lu, Y.; Zhang, J. Coffee consumption is associated with a decreased risk of incident chronic kidney disease: A protocol for systematic review and meta-analysis. Medicine 2021, 100, e27149. [Google Scholar] [CrossRef] [PubMed]
- Rhee, J.; Lim, R.K.; Purdue, M.P. Coffee consumption and risk of renal cancer: A meta-analysis of cohort evidence. Cancer Causes Control 2022, 33, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Tommerdahl, K.L.; Hu, E.A.; Selvin, E.; Steffen, L.M.; Coresh, J.; Grams, M.E.; Bjornstad, P.; Rebholz, C.M.; Parikh, C.R. Coffee Consumption May Mitigate the Risk for Acute Kidney Injury: Results from the Atherosclerosis Risk in Communities Study. Kidney Int. Rep. 2022, 7, 1665–1672. [Google Scholar] [CrossRef]
- Matboli, M.; Eissa, S.; Ibrahim, D.; Hegazy, M.G.A.; Imam, S.S.; Habib, E.K. Caffeic Acid Attenuates Diabetic Kidney Disease via Modulation of Autophagy in a High-Fat Diet/Streptozotocin- Induced Diabetic Rat. Sci. Rep. 2017, 7, 2263. [Google Scholar] [CrossRef]
- Salem, A.M.; Ragheb, A.S.; Hegazy, M.G.A.; Matboli, M.; Eissa, S. Caffeic Acid Modulates miR-636 Expression in Diabetic Nephropathy Rats. Indian J. Clin. Biochem. 2019, 34, 296–303. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, H.; Zhao, X.; Li, B.; He, H.; Cheng, H.; Wang, H.; Ao, Y. Decreased H3K9ac level of KLF4 mediates podocyte developmental toxicity induced by prenatal caffeine exposure in male offspring rats. Toxicol. Lett. 2019, 314, 63–74. [Google Scholar] [CrossRef]
- Chuang, Y.H.; Quach, A.; Absher, D.; Assimes, T.; Horvath, S.; Ritz, B. Coffee consumption is associated with DNA methylation levels of human blood. Eur. J. Hum. Genet. 2017, 25, 608–616. [Google Scholar] [CrossRef]
- Karabegović, I.; Portilla-Fernandez, E.; Li, Y.; Ma, J.; Maas, S.C.E.; Sun, D.; Hu, E.A.; Kühnel, B.; Zhang, Y.; Ambatipudi, S.; et al. Epigenome-wide association meta-analysis of DNA methylation with coffee and tea consumption. Nat. Commun. 2021, 12, 2830. [Google Scholar] [CrossRef]
- Mukwevho, E.; Kohn, T.A.; Lang, D.; Nyatia, E.; Smith, J.; Ojuka, E.O. Caffeine induces hyperacetylation of histones at the MEF2 site on the Glut4 promoter and increases MEF2A binding to the site via a CaMK-dependent mechanism. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E582–E588. [Google Scholar] [CrossRef]
- Shi, J.; Pabon, K.; Scotto, K.W. Methylxanthines Increase Expression of the Splicing Factor SRSF2 by Regulating Multiple Post-transcriptional Mechanisms. J. Biol. Chem. 2015, 290, 14986–15003. [Google Scholar] [CrossRef] [Green Version]
- Martinez, M.; Rossetto, I.M.U.; Arantes, R.M.S.; Lizarte, F.S.N.; Tirapelli, L.F.; Tirapelli, D.P.C.; Chuffa, L.G.A.; Martinez, F.E. Serum miRNAs are differentially altered by ethanol and caffeine consumption in rats. Toxicol. Res. 2019, 8, 842–849. [Google Scholar] [CrossRef]
- Szabo, L.; Molnar, R.; Tomesz, A.; Deutsch, A.; Darago, R.; Nowrasteh, G.; Varjas, T.; Nemeth, B.; Budan, F.; Kiss, I. The effects of flavonoids, green tea polyphenols and coffee on DMBA induced LINE-1 DNA hypomethylation. PLoS ONE 2021, 16, e0250157. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.J.; Park, C.K.; Yang, B.K.; Cheong, H.T. Control of nuclear remodelling and subsequent in vitro development and methylation status of porcine nuclear transfer embryos. Reproduction 2008, 135, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Strachecka, A.; Krauze, M.; Olszewski, K.; Borsuk, G.; Paleolog, J.; Merska, M.; Chobotow, J.; Bajda, M.; Grzywnowicz, K. Unexpectedly strong effect of caffeine on the vitality of western honeybees (Apis mellifera). Biochemistry 2014, 79, 1192–1201. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.H.; Park, J.H.; Im, S.S.; Song, D.K. Coffee and health. Integr. Med. Res. 2014, 3, 189–191. [Google Scholar] [CrossRef] [PubMed]
- Machado-Fragua, M.D.; Struijk, E.A.; Yévenes-Briones, H.; Caballero, F.F.; Rodríguez-Artalejo, F.; Lopez-Garcia, E. Coffee consumption and risk of hearing impairment in men and women. Clin. Nutr. 2021, 40, 3429–3435. [Google Scholar] [CrossRef]
- Pham, K.; Mulugeta, A.; Zhou, A.; O’Brien, J.T.; Llewellyn, D.J.; Hyppönen, E. High coffee consumption, brain volume and risk of dementia and stroke. Nutr. Neurosci. 2022, 25, 2111–2122. [Google Scholar] [CrossRef] [PubMed]
- Bagdas, D.; Gul, Z.; Meade, J.A.; Cam, B.; Cinkilic, N.; Gurun, M.S. Pharmacologic Overview of Chlorogenic Acid and its Metabolites in Chronic Pain and Inflammation. Curr. Neuropharmacol. 2020, 18, 216–228. [Google Scholar] [CrossRef] [PubMed]


| Coffee Ingredients | DNA Modification | Histone Modification | ncRNA Expression |
|---|---|---|---|
| Coffee extract | Yes | No data | Yes |
| Caffeine | Yes | Yes | Yes |
| Chlorogenic acid | Yes | No data | Yes |
| Caffeic acid | Yes | No data | Yes |
| Focus of Study | Model | Impact | Gene Modified/Epigenetic Alterations | Disease Involved/Consequence | Ref |
|---|---|---|---|---|---|
| HPA axis |
| DNA |
| Inhibited the functional development of the fetal HPA axis | [15] |
|
| ||||
| DNA |
| Increased the cortisol production in cells | [16] | |
| DNA and Histone |
| Led to the occurrence of IUGR | [17] | |
| DNA and ncRNA |
| Resulted in adrenal dysfunction | [18] | |
| DNA |
| Suppressed the cholesterol uptake followed by the decreased production of steroid hormones and increased IUGR rates | [19] | |
| Bone |
| DNA |
| Contributed to the retardation of both chondrogenesis and skeletal growth | [20] |
| Histone |
| Induced cartilage dysplasia and increased osteoarthritis susceptibility in male adult offspring | [22] | |
| DNA |
| PCE caused fetal growth retardation and adult osteopenia, as well as increased susceptibility to osteoporosis-related fractures | [23] | |
| Histone |
| Associated with bone development dysplasia and increased osteoporosis susceptibility in male adult offspring | [24] | |
| Ovary |
| Histone |
| Induced ovarian dysfunction in adulthood | [25] |
| Cardiovascular system |
| DNA |
| Related to the development of the heart | [26] |
|
| ||||
|
| ||||
| DNA and ncRNA |
| Related to cardiovascular development and related diseases | [27] | |
|
| ||||
|
| ||||
|
| ||||
| Colon carcinoma |
| ncRNA |
| Prevented the malignant growth of colon carcinoma cells | [30] |
| ncRNA |
| Related to the decreased colon cancer cell proliferation | [31] | |
| ncRNA |
| Attenuated preneoplastic ACF development | [32] | |
| Leukemia |
| DNA |
| Showed benefits against hematological malignances whose pathogenic processes involve impairment of DNA methylation | [34] |
| Breast cancer |
| DNA |
| ---- | [35] |
| Cancer risk |
| DNA and ncRNA |
| Attenuated the CSC-like properties | [36] |
| ncRNA |
| Revealed the chemopreventive effects of coffee in cancer risk | [37] | |
| Cataract |
| ncRNA |
| Attenuated the cataract formation | [45] |
| Glaucoma |
| ncRNA |
| Relieved retinal injury in glaucoma mouse model | [46] |
| Pathogen infection |
| ncRNA |
| Alleviated lung injury and decreased the susceptibility to bacterial infection | [47] |
|
| ||||
|
| ||||
| ncRNA |
| Alleviated pathological damage of lung tissues | [48] | |
| ncRNA |
| Reduced the degree of liver fibrosis in pathological manifestations | [49] | |
| ncRNA |
| Reduced intestinal damage caused by ST | [50] | |
| Brain |
| ncRNA |
| CAF had a neuroprotective effect against ethanol on the cerebellar tissue | [53] |
| ncRNA |
| Reduced the hypoxic ischemia reperfusion damage in cells | [54] | |
| DNA |
| Exerted a slight antidepressant-like effect | [55] | |
| Liver |
| ncRNA |
| Related to suppression of body fat accumulation | [57] |
| Histone |
| Induced hepatic lipid accumulation and increased the susceptibility to NAFLD in PCE adult female rats | [58] | |
| Histone |
| Induced hypercholesterolemia in adult female offspring | [59] | |
| Histone |
| Induced the susceptibility to hypercholesterolemia and related cardiovascular and cerebrovascular diseases in male adult offspring | [60] | |
| ncRNA |
| Lessened the degree of liver fibrosis in the pathological manifestation | [61] | |
| |||||
| Kidney |
| ncRNA |
| Improved DN | [66] |
| ncRNA |
| CaA effectively ameliorated renal damage in diabetic rats | [67] | |
| Histone |
| Induced podocyte dysplasia in utero and podocyte phenotypic damage in adulthood in male offspring that contributes to glomerular diseases such as glomerulosclerosis | [68] | |
| Nuclear reprogramming |
| DNA |
| Resulted in nuclear reprogramming | [75] |
| Lifespan |
| DNA |
| Extended the lifespan and increased resistance to Nosema | [76] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, Q.; Xu, Y.-M.; Lau, A.T.Y. The Epigenetic Effects of Coffee. Molecules 2023, 28, 1770. https://doi.org/10.3390/molecules28041770
Ding Q, Xu Y-M, Lau ATY. The Epigenetic Effects of Coffee. Molecules. 2023; 28(4):1770. https://doi.org/10.3390/molecules28041770
Chicago/Turabian StyleDing, Qi, Yan-Ming Xu, and Andy T. Y. Lau. 2023. "The Epigenetic Effects of Coffee" Molecules 28, no. 4: 1770. https://doi.org/10.3390/molecules28041770