KIT-5-Assisted Synthesis of Mesoporous SnO2 for High-Performance Humidity Sensors with a Swift Response/Recovery Speed
Abstract
:1. Introduction
2. Results and Discussion
2.1. Morphological/Structural Characterization of Materials Using TEM, SAXS/WAXS and BRT/BJH Methods
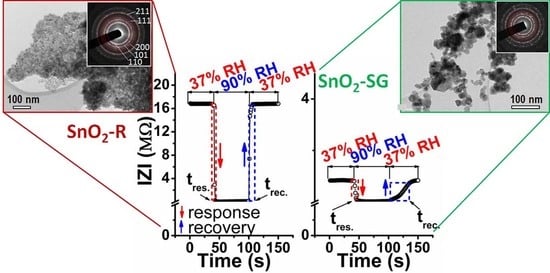
2.2. Humidity Sensing Performances of Sensors Based on Mesoporous and Conventionally Processed Tin Oxides
3. Materials and Methods
3.1. Materials
3.2. Syntheses of Mesoporous KIT-5 Template, SnO2-KIT-5 Hybrid and SnO2 Replica by Hard-Templating
3.3. Sol-Gel Synthesis of SnO2
3.4. Characterization Methods
3.5. Fabrication and Testing of SnO2 Humidity Sensors
4. Conclusions
- The sensor shows a relatively low hysteresis error (3.7%), significant change in impedance by 160 times and sensitivity of 406.8 kΩ/RH% over the 30–90% RH range, including a notably fast response (4s) and recovery (6s) time.
- Such improved humidity sensing properties of TF(SnO2-R) arise from the large mesoporous surface area with uniform pore-size, 3D interconnectivity of pore channels and well-crystalized and uniform-in-size SnO2 nanoparticles, as shown by TEM.
- This material provides a high concentration of active sites which creates an effective surface reaction with moisture and facilitates charge propagation across the active-layer surface and mesoporous channels of SnO2-R in accordance with the humidity sensing mechanism which facilitates the Grotthuss chain reaction above 60% RH.
- Contrary to TF(SnO2-SG), the presence of amorphous silica at the TF(SnO2-R) sensor surface (observed by XPS) contributes to its overall electrical characteristics and creation of additional oxygen related defects. By making the water-molecule dissociation easier, these defects enhance the charge carriers transport across the active-sensing layer and improve sensor performances, including the rapid transient response of the TF(SnO2-R) sensor.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mogera, U.; Sagade, A.A.; George, S.J.; Kulkarni, G.U. Ultrafast response humidity sensor using supramolecular nanofibre and its application in monitoring breath humidity and flow. Sci. Rep. 2014, 4, 4103. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, L.D. Assessment of sensors used in the food industry. Food Control 1991, 2, 87–98. [Google Scholar] [CrossRef]
- Chen, Z.; Lu, C. Humidity sensors: A Review of materials and mechanisms. Sens. Lett. 2005, 3, 274–295. [Google Scholar] [CrossRef]
- Farahani, H.; Wagiran, R.; Hamidon, M.N. Humidity sensors principle, mechanism, and fabrication technologies: A comprehensive review. Sensors 2014, 14, 7881–7939. [Google Scholar] [CrossRef] [PubMed]
- Tominc, S.; Rečnik, A.; Bernik, S.; Mazaj, M.; Spreitzer, M.; Daneu, N. Microstructure development in (Co,Ta)-doped SnO2-based ceramics with promising varistor and dielectric properties. J. Eur. Ceram. Soc. 2020, 40, 5518–5522. [Google Scholar] [CrossRef]
- Wu, P.; Wang, S.; Li, X.; Zhang, F. Advances in SnO2-based perovskite solar cells: From preparation to photovoltaic applications. J. Mater. Chem. A 2021, 9, 19554–19588. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, S.; Wang, G.; Tong, J.; Li, J.; Pan, D. Quantum-sized SnO2 nanoparticles with upshifted conduction band: A promising electron transportation material for quantum dot light-emitting diodes. Langmuir 2020, 36, 6605–6609. [Google Scholar] [CrossRef]
- Leite, E.R.; Weber, I.T.; Longo, E.; Varela, J.A. A new method to control particle size and particle size distribution of SnO2 nanoparticles for gas sensor applications. Adv. Mater. 2000, 12, 965–968. [Google Scholar] [CrossRef]
- Wicker, S.; Guiltat, M.; Weimar, U.; Hémeryck, A.; Barsan, N. Ambient humidity influence on CO detection with SnO2 gas sensing materials. A combined DRIFTS/DFT investigation. J. Phys. Chem. 2017, 121, 25064–25073. [Google Scholar] [CrossRef]
- Li, H.; Liu, B.; Cai, D.; Wang, Y.; Liu, Y.; Mei, L.; Wang, L.; Wang, D.; Li, Q.; Wang, T. High-temperature humidity sensors based on WO3–SnO2 composite hollow nanospheres. J. Mater. Chem. A 2014, 2, 6854–6862. [Google Scholar] [CrossRef]
- Tang, Y.; Li, Z.; Ma, J.; Wang, L.; Yang, J.; Du, B.; Yu, Q.; Zu, X. Highly sensitive surface acoustic wave (SAW) humidity sensors based on sol-gel SiO2 films: Investigations on the sensing property and mechanism. Sens. Actuators B Chem. 2015, 215, 283–291. [Google Scholar] [CrossRef]
- Tawale, J.S.; Gupta, G.; Mohan, A.; Kumar, A.; Srivastava, A.K. Growth of thermally evaporated SnO2 nanostructures for optical and humidity sensing application. Sens. Actuators B Chem. 2014, 201, 369–377. [Google Scholar] [CrossRef]
- Ye, J.; Zhang, H.; Yang, R.; Li, X.; Qi, L. Morphology-controlled synthesis of SnO2 nanotubes by using 1D silica mesostructures as sacrificial templates and their applications in lithium-ion batteries. Small 2010, 6, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Hyodo, T.; Abe, S.; Shimizu, Y.; Egashira, M. Gas-sensing properties of ordered mesoporous SnO2 and effects of coatings thereof. Sens. Actuators B Chem. 2003, 93, 590–600. [Google Scholar] [CrossRef]
- Hyodo, T.; Nishida, N.; Shimizu, Y.; Egashira, M. Preparation and gas-sensing properties of thermally stable mesoporous SnO2. Actuators B Chem. 2002, 83, 209–215. [Google Scholar] [CrossRef]
- Malik, R.; Tomer, V.K.; Chaudhary, V.; Dahiya, M.S.; Sharma, A.; Nehra, S.P.; Duhan, S.; Lailasam, K. An excellent humidity sensor based on In–SnO2 loaded mesoporous graphitic carbon nitride. J. Mater. Chem. A 2017, 5, 14134–14143. [Google Scholar] [CrossRef]
- Tomer, V.K.; Duhan, S. In-situ synthesis of SnO2/SBA-15 hybrid nanocomposite as highly efficient humidity sensor. Sens. Actuators B Chem. 2015, 212, 517–525. [Google Scholar] [CrossRef]
- Huang, J.; Matsunaga, N.; Shimanoe, K.; Yamazoe, N.; Kunitake, T. Nanotubular SnO2 templated by cellulose fibers: Synthesis and gas sensing. Chem. Mater. 2005, 17, 3513–3518. [Google Scholar] [CrossRef]
- Zhang, Z.; Shao, C.; Li, X.; Zhang, L.; Xue, H.; Wang, C.; Liu, Y. Electrospun nanofibers of ZnO−SnO2 heterojunction with high photocatalytic activity. J. Phys. Chem. C 2010, 114, 7920–7925. [Google Scholar] [CrossRef]
- Sakai, G.; Baik, N.S.; Miura, N.; Yamazoe, N. Gas sensing properties of tin oxide thin films fabricated from hydrothermally treated nanoparticles. Sens. Actuators B Chem. 2001, 77, 116–121. [Google Scholar] [CrossRef]
- Mädler, L.; Roessler, A.; Pratsinis, S.E.; Sahm, T.; Gurlo, A.; Barsan, N.; Weimar, U. Direct formation of highly porous gas-sensing films by in situ thermophoretic deposition of flame-made Pt/SnO2 nanoparticles. Sens. Actuators B Chem. 2006, 114, 283–295. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, P.; Wang, Y.; Ma, Y.; Zhong, J.; Sun, X. Facile fabrication of a well-ordered porous Cu-doped SnO2 thin film for H2S sensing. ACS Appl. Mater. Interfaces 2014, 6, 14975–14980. [Google Scholar] [CrossRef] [PubMed]
- Tomer, V.K.; Devi, S.; Malik, R.; Nehra, S.P.; Duhan, S. Fast response with high performance humidity sensing of Ag-SnO2/SBA-15 nanohybrid sensors. Microporous Mesoporous Mater. 2016, 219, 240–248. [Google Scholar] [CrossRef]
- Deng, X.; Chen, K.; Tüysüz, H. Protocol for the nanocasting method: Preparation of ordered mesoporous metal oxides. Chem. Mater. 2017, 29, 40–52. [Google Scholar] [CrossRef]
- Laskowski, Ł.; Laskowska, M.; Vila, N.; Schabikowski, M.; Walcarius, A. Mesoporous Silica-Based Materials for Electronics-Oriented Applications. Molecules 2019, 24, 2395. [Google Scholar] [CrossRef] [PubMed]
- Waitz, T.; Becker, B.; Wagner, T.; Sauerwald, T.; Kohl, C.-D.; Tiemann, M. Ordered nanoporous SnO2 gas sensors with high thermal stability. Sens. Actuators B Chem. 2010, 150, 788–793. [Google Scholar] [CrossRef]
- Juhari, J.; Bakar, M.Z.A.; Abdullah, A.Z. Nanocasting route for the synthesis of ordered mesoporous SnO2 with highly crystalline framework. J. Mater. Sci. Eng. B 2014, 4, 203–209. [Google Scholar]
- Fan, J.; Zhang, J.; Solsona, P.; Suriñach, S.; Baró, M.D.; Sort, J.; Pellicer, E. Nanocasting synthesis of mesoporous SnO2 with a tunable ferromagnetic response through Ni loading. RSC Adv. 2016, 6, 104799–104807. [Google Scholar] [CrossRef]
- Juhari, J.; Bakar, M.Z.A.; Abdullah, A.Z. Mesoporous SnO2 by nanocasting route using various silica templates for gas sensing application. Adv. Mater. Res. 2015, 1113, 140–146. [Google Scholar] [CrossRef]
- Kleitz, F.; Liu, D.; Anilkumar, G.M.; Park, I.-S.; Solovyov, L.A.; Shmakov, A.N.; Ryoo, R. Large cage face-centered-cubic Fm3m mesoporous silica: Synthesis and structure. J. Phys. Chem. B 2003, 107, 14296–14300. [Google Scholar] [CrossRef]
- Qu, Q.; Zhang, J.H.; Wang, J.; Li, Q.-Y.; Xu, C.-W.; Lu, X. Three-dimensional ordered mesoporous Co3O4 enhanced by Pd for oxygen evolution reaction. Sci. Rep. 2017, 7, 41542. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Zhang, Y.; Zhou, Y.; Zhang, Z.; Xue, J.; Xu, Y.; Zhang, C.; Sheng, X.; Kui, N. Nanocasting synthesis of an ordered mesoporous CeO2-supported Pt nanocatalyst with enhanced catalytic performance for the reduction of 4-nitrophenol. RSC Adv. 2016, 6, 730–739. [Google Scholar] [CrossRef]
- Rajalakshmi, R.; Vasudevan Srinivasan, V.; Pachamuthu, M.P.; Maheswari, R. Characterizations of tin (SnO2) doped KIT-5 by direct synthesis. Mater. Chem. Phys. 2015, 154, 164–169. [Google Scholar] [CrossRef]
- Li, L.; Lin, H.; Qu, F. Synthesis of mesoporous SnO2 nanomaterials with selective gas-sensing properties. J. Sol-Gel Sci. Technol. 2013, 67, 545–555. [Google Scholar] [CrossRef]
- Gulevich, G.; Rumyantseva, M.; Gerasimov, E.; Marikutsa, A.; Krivetskiy, V.K.; Shatalova, T.; Khmelevsky, N.; Gaskov, A. Nanocomposites SnO2/SiO2 for CO gas sensors: Microstructure and reactivity in the interaction with the gas phase. Materials 2019, 129, 1096. [Google Scholar] [CrossRef]
- Tao, B.; Fenf, L.; Miao, F.; Zang, Y. High sensitivity chipless RFID humidity sensor tags are based on SnO2/G nanomaterials. Vacuum 2022, 202, 111126. [Google Scholar] [CrossRef]
- Li, F.; Li, P.; Zhang, H. Preparation and research of a high-performance ZnO/SnO2 humidity sensor. Sensors 2022, 22, 293. [Google Scholar] [CrossRef] [PubMed]
- Panday, M.; Upadhyay, G.K.; Purohit, L.P. Sb incorporated SnO2 nanostructured thin films for CO2 gas sensing and humidity sensing applications. J. Alloys Compd. 2022, 904, 164053. [Google Scholar] [CrossRef]
- Padrón-Navarta, J.A.; Barou, F.; Daneu, N. Twinning in SnO2-based ceramics doped with CoO and Nb2O5: Morphology of multiple twins revealed by electron backscatter diffraction. Acta Cryst. B 2020, 76, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Tominc, S.; Rečnik, A.; Samardžija, Z.; Dražić, G.; Podlogar, M.; Bernik, S.; Daneu, N. Twinning and charge compensation in Nb2O5–doped SnO2–CoO ceramics exhibiting promising varistor characteristics. Ceram. Int. 2018, 44, 1603–1613. [Google Scholar] [CrossRef]
- Daneu, N.; Rečnik, A.; Bernik, S. Grain-growth phenomena in ZnO ceramics in the presence of inversion boundaries. J. Am. Ceram. Soc. 2011, 94, 1619–1626. [Google Scholar] [CrossRef]
- Zhao, D.; Wan, Y.; Zhouet, W. Ordered Mesoporous Materials; Wiley-VCH Verlag & Co., KGaA: Weinheim, Germany, 2013; p. 225. [Google Scholar]
- Hodzic, A.; Llusa, M.; Heigl, N.; Tritthart; Fraser, S.D.; Laggner, P.; Khinast, J.G. Effect of process variables on the Small and Wide Angle X-ray Scattering (SWAXS) patterns of powders, granules and pharmaceutical tablets. Powder Technol. 2012, 221, 447–452. [Google Scholar] [CrossRef]
- Hodzic, A.; Llusa, M.; Fraser, S.D.; Scheibelhofer, O.; Koller, D.M.; Reiter, F.; Laggner, P.; Khinast, J.G. Small- and wide-angle X-ray scattering (SWAXS) for quantification of aspirin content in a binary powder mixture. Int. J. Pharmaceut. 2012, 428, 91–95. [Google Scholar] [CrossRef]
- Reed, J.S. Principles of Ceramics Processing; John Wiley & Sons: New York, NY, USA, 1995. [Google Scholar]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Besenhard, M.O.; LaGrow, A.P.; Hodzic, A.; Kriechbaum, M.; Panariello, L.; Bais, G.; Loizou, K.; Damilos, S.; Cruz, M.M.; Thanh, N.T.K.; et al. Co-precipitation synthesis of stable iron oxide nanoparticles with NaOH: New insights and continuous production via flow chemistry. Chem. Eng. J. 2020, 399, 125740. [Google Scholar] [CrossRef]
- Bacani, R.; Martins, T.S.; Fantini, M.C.A.; Lamas, D.G. Structural studies of mesoporous ZrO2-CeO2 and ZrO2-CeO2/SiO2 mixed oxides for catalytical applications. J. Alloys Compd. 2016, 671, 396–402. [Google Scholar] [CrossRef]
- Jiao, F.; Hill, A.H.; Harrison, A.; Berko, A.; Chadwick, A.V.; Bruce, P.G. Synthesis of ordered mesoporous NiO with crystalline walls and a bimodal pore size distribution. J. Am. Chem. Soc. 2008, 130, 5262–5266. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, Y.; Yang, H.; Wang, W.; Dai, Y.-Z.; Niu, L.-G.; Lv, C.; Xia, H.; Liu, T. Fast-response humidity sensor based on laser printing for respiration monitoring. RSC Adv. 2020, 10, 8910–8916. [Google Scholar] [CrossRef]
- Lee, H.B.; Kumar, N.; Ovhal, M.M.; Kim, Y.J.; Song, Y.M.; Kang, J.-W. Dopant-free, amorphous-crystalline heterophase SnO2 electron transport bilayer enables >20% efficiency in triple-cation perovskite solar cells. Adv. Funct. Mater. 2020, 30, 2001559. [Google Scholar] [CrossRef]
- Lenshin, A.S.; Kashkarov, V.M.; Domashevskaya, E.P.; Bel’tyukov, A.N.; Gil’mutdinov, F.Z. Investigations of the composition of macro-, micro- and nanoporous silicon surface by ultrasoft X-ray spectroscopy and X-ray photoelectron spectroscopy. Appl. Surf. Sci. 2015, 359, 550–559. [Google Scholar] [CrossRef]
- Heiland, G.; Kohl., D. Physical and Chemical Aspects of Oxidic Semiconductor Gas Sensors; Elsevier: Amsterdam, The Netherlands, 1988; pp. 15–38. [Google Scholar]
- Faia, P.M.; Furtado, C.S.; Ferreira, A.J. AC impedance spectroscopy: A new equivalent circuit for titania thick film humidity sensors. Sens. Actuators B Chem. 2005, 107, 353–359. [Google Scholar] [CrossRef] [Green Version]
- Kulwicky, B.M. Humidity sensors. J. Am. Ceram. Soc. 1991, 74, 697–708. [Google Scholar] [CrossRef]
- Li, W.; Liu, J.; Ding, C.; Bai, G.; Xu, J.; Ren, Q.; Li, J. Fabrication of ordered SnO2 nanostructures with enhanced humidity sensing performance. Sensors 2017, 17, 2392. [Google Scholar] [CrossRef]
- Pan, S.; Gayathri, G.; Reshma, T.S.; Mangamma, G.; Prasad, A.K.; Das, A. A sensitive humidity sensor at low pressure with SnO2 QDs. Sens. Actuators A Phys. 2022, 346, 113835. [Google Scholar] [CrossRef]
- Simović, B.; Dapčević, A.; Zdravković, J.; Tasić, N.; Kovač, S.; Krstić, J.; Branković, G. From titania to titanates: Phase and morphological transition in less alkaline medium under mild conditions. J. Alloys Compd. 2019, 781, 810–819. [Google Scholar] [CrossRef]
- Nikolic, M.V.; Vasiljevic, Z.Z.; Lukovic, M.D.; Pavlovic, V.P.; Vujancevic, J.; Radovanovic, M.; Krstic, J.B.; Vlahovic, B.; Pavlovic, V.B. Humidity sensing properties of nanocrystalline pseudobrookite (Fe2TiO5) based thick films. Sens. Actuators B Chem. 2018, 277, 654–664. [Google Scholar] [CrossRef] [Green Version]









| Sample | Rg/nm | Q/nm−3 | K/nm−3 | S/V/m2·cm−3 | /nm | m2·g−1 |
|---|---|---|---|---|---|---|
| KIT-5 | 6.6 ± 0.8 | 53.9·10−3 | 14.9 ·10−3 | 277 | 6.6 | 760 |
| SnO2-KIT-5 | 13.6 ± 0.7 | 2.4·10−3 | 1.7 ·10−4 | 72 | 6.7 | 442 |
| SnO2-R | 14.4 ± 0.6 | 6.2·10−3 | 9.8 ·10−4 | 158 | 8.6 | 66.7 |
| Sample | RH/% | Order of Impedance Change | /s | /s | /% | Refs |
|---|---|---|---|---|---|---|
| ordered-SnO2 | 11–96 | - | 32 | 42 | <5 | [56] |
| SnO2-SBA15WI | 11–98 | 4.5 | 33 | 50 | 2.9 | [17] |
| In-SnO2/mesoCN | 11–96 | 5 | 3.5 | 1.5 | 0.7 | [16] |
| SnO2 QDs | 48–70 | - | - | 35 * | - | [57] |
| SnO2-graphene | 11–98 | - | 15 ** | 13 ** | 5.37 | [36] |
| ZnO-SnO2 | 11–95 | 4 | 35 | 8 | 6.6 | [37] |
| TF(SnO2-SG) | 30–90 | 0.5 | 16tw | 40tw | 13.2 | this work |
| TF(SnO2-R) | 30–90 | 2.3 | 4tw | 6tw | 3.7 | this work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vojisavljević, K.; Savić, S.M.; Počuča-Nešić, M.; Hodžić, A.; Kriechbaum, M.; Ribić, V.; Rečnik, A.; Vukašinović, J.; Branković, G.; Djokić, V. KIT-5-Assisted Synthesis of Mesoporous SnO2 for High-Performance Humidity Sensors with a Swift Response/Recovery Speed. Molecules 2023, 28, 1754. https://doi.org/10.3390/molecules28041754
Vojisavljević K, Savić SM, Počuča-Nešić M, Hodžić A, Kriechbaum M, Ribić V, Rečnik A, Vukašinović J, Branković G, Djokić V. KIT-5-Assisted Synthesis of Mesoporous SnO2 for High-Performance Humidity Sensors with a Swift Response/Recovery Speed. Molecules. 2023; 28(4):1754. https://doi.org/10.3390/molecules28041754
Chicago/Turabian StyleVojisavljević, Katarina, Slavica M. Savić, Milica Počuča-Nešić, Aden Hodžić, Manfred Kriechbaum, Vesna Ribić, Aleksander Rečnik, Jelena Vukašinović, Goran Branković, and Veljko Djokić. 2023. "KIT-5-Assisted Synthesis of Mesoporous SnO2 for High-Performance Humidity Sensors with a Swift Response/Recovery Speed" Molecules 28, no. 4: 1754. https://doi.org/10.3390/molecules28041754