Application of Amino Acids for High-Dosage Measurements with Electron Paramagnetic Resonance Spectroscopy
Abstract
:1. Introduction
2. Results and Discussion
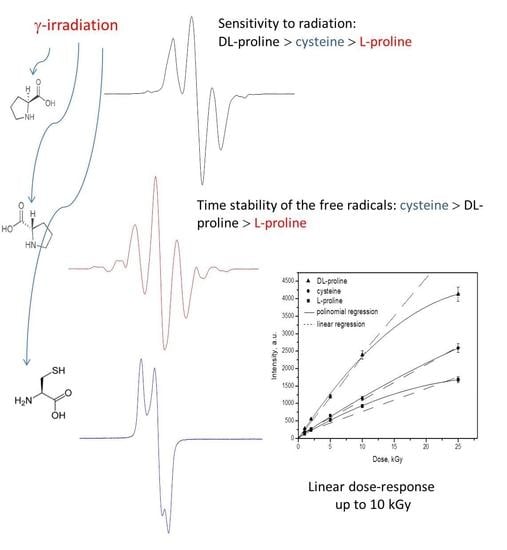
2.1. EPR Spectra
2.2. Effect of the Dose on the Shape of the EPR Spectra
2.3. Effect of the Microwave Power on the Shape and on the Saturation Degree of the EPR Signals
2.4. Time Dependence of the Free Radicals
2.5. Dose–Response Characteristics
3. Materials and Methods
3.1. Materials
3.2. Irradiation
3.3. Principles of the EPR Method
3.4. Instrument
3.5. Procedure of Measurement
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Zagorski, Z.P. Dosimetric applications of α-alanine. J. Radioanal. Nucl. Chem. 1994, 187, 73–78. [Google Scholar] [CrossRef]
- Gancheva, V.; Yordanov, N.D.; Callens, F.; Vanhaelewyn, G.; Raffi, J.; Bortolin, E.; Onori, S.; Malinen, E.; Sagstuen, E.; Fabisiak, S.; et al. An international intercomparison on “self-calibrated” alanine EPR dosimeters. Radiat. Phys. Chem. 2008, 77, 357–364. [Google Scholar] [CrossRef]
- Mehta, K.; Girzikowsky, R. Alanine-ESR dosimetry for radiotherapy IAEA experience. Appl. Radiat. Isot. 1996, 47, 1189–1191. [Google Scholar] [CrossRef]
- Desrosiers, M. Alanine dosimetry at the NIST. In Book of Abstracts, Proceeding of the International Conference on Biodosimetry and 5th International Symposium on ESR Dosimetry and Applications, Moscow/Obninsk, Russia, 22–26 June 1998; Publisher: Obninsk, Russia, 1998; p. 149. [Google Scholar]
- Sharpe, H.G.; Sephton, J.P. Alanine Dosimetry at NPL—The Development of a Mailed Reference Dosimetry Service at Radiotherapy Dose Level, in Techniques for High Dose Dosimetry in Industry, Agriculture and Medicine, Proceedings of the Symposium Held in Vienna, Austria, 2–5 November 1999; International Atomic Energy Agency: Vienna, Austria, 1999; p. 299. [Google Scholar]
- Ikeya, M.; Hassan, G.M.; Sasaoka, H.; Kinoshita, Y.; Takakiand, S.; Yamanaka, C. Strategy for finding new materials for ESR dosimeters. Appl. Radiat. Isot. 2000, 52, 1209–1215. [Google Scholar] [CrossRef] [PubMed]
- Lund, A.; Olsson, S.; Bonora, M.; Lund, E.; Gustafsson, H. New materials for ESR dosimetry. Spectrochem. Acta A 2002, 58, 1301–1311. [Google Scholar] [CrossRef] [PubMed]
- Hassan, G.M.; Ikeya, M. Metal ion-organic compound for high sensitive ESR dosimetry. Appl. Radiat. Isot. 2000, 52, 1247–1254. [Google Scholar] [CrossRef]
- Lund, E.; Gustafsson, H.; Danilczuk, M.; Sastry, M.D.; Lund, A.; Vestad, T.A.; Malinen, E.; Hole, E.O.; Sagstuen, E. Formates and dithionates: Sensitive EPR-dosimeter materials for radiation therapy. Appl. Radiat. Isot. 2005, 62, 317–324. [Google Scholar] [CrossRef]
- Olsson, S.; Sagstuen, E.; Bonora, M.; Lund, A. EPR dosimetric properties of 2-methylalanine: EPR, ENDOR and FT-EPR investigations. Radiat. Res. 2002, 157, 113–121. [Google Scholar] [CrossRef]
- Gancheva, V.; Sagstuen, E.; Yordanov, N.D. Study on the EPR/dosimetric properties of some substituted alanines. Radiat. Phys. Chem. 2006, 75, 329–335. [Google Scholar] [CrossRef]
- Soliman, Y.S.; Abdel-Fattah, A.A. Magnesium lactate mixed with EVA polymer/paraffin as an EPR dosimeter for radiation processing application. Radiat. Phys. Chem. 2012, 81, 1910–1916. [Google Scholar] [CrossRef]
- Lelie, S.; Hole, E.O.; Duchateau, M.; Schroeyers, W.; Schreurs, S.; Verellen, D. The investigation of lithium formate hydrate, sodium dithionate and N-methyl taurine as clinical EPR dosimeters. Radiat. Meas. 2013, 59, 218–224. [Google Scholar] [CrossRef]
- Rushdi, M.A.H.; Abdel-Fattah, A.A.; Sherif, M.M.; Soliman, Y.S.; Mansour, A. Strontium sulfate as an EPR dosimeter for radiation technology application. Radiat. Phys. Chem. 2015, 106, 130–135. [Google Scholar] [CrossRef]
- Rushdi, M.A.H.; Abdel-Fattah, A.A.; Soliman, Y. Radiation-induced defects in strontium carbonate rod for EPR dosimetry applications. Radiat. Phys. Chem. 2017, 131, 1–6. [Google Scholar] [CrossRef]
- Gallo, S.; Iacoviello, G.; Bartolotta, A.; Dondi, D.; Panzeca, S.; Marrale, M. ESR dosimeter material properties of phenols compound exposed to radiotherapeutic electron beams. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2017, 407, 110–11715. [Google Scholar] [CrossRef]
- Karakirova, Y.; Yordanov, N.D.; De Cooman, H.; Vrielinck, H.; Callens, F. Dosimetric characteristics of different types of saccharides: An EPR and UVspectrometric study. Radiat. Phys. Chem. 2010, 79, 654–659. [Google Scholar] [CrossRef]
- Gustafsson, H.; Danilczuk, M.; Sastry, M.D.; Lund, A.; Lund, E. Enhanced sensitivity of lithium dithionates doped with rhodium and nickel for EPR dosimetry. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2005, 62, 614–620. [Google Scholar] [CrossRef]
- Belahmara, A.; Mikou, M.; El Ghalmi, M. Analysis by EPR measurements and spectral deconvolution of the dosimetric properties of lithium formate monohydrate. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2018, 431, 19–24. [Google Scholar] [CrossRef]
- Olsson, S.K.; Lund, E.; Lund, A. Development of ammonium tartrate as an ESR dosimeter material for clinical purposes. Appl. Radiat. Isot. 2000, 52, 1235–1241. [Google Scholar] [CrossRef]
- Danilczuk, M.; Gustafsson, H.; Sastry, M.D.; Lund, E.; Lund, A. Ammonium dithionate—A new material for highly sensitive EPR dosimetry. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2008, 69, 18–21. [Google Scholar] [CrossRef]
- Tuner, H.; Korkmaz, M. ESR study of ascorbic acid irradiated with gamma-rays. J. Radioanal. Nucl. Chem. 2007, 273, 609–614. [Google Scholar] [CrossRef]
- Olsson, S.K.; Bagherian, S.; Lund, E.; Carlsson, G.A.; Lund, A. Ammonium tartrate as an ESR dosimeter material. Appl. Radiat. Isot. 1999, 50, 955–965. [Google Scholar] [CrossRef]
- Olsson, S.; Lund, E.; Erickson, R. Dose response and fading characteristics of an alanine-agarose gel. Appl. Radiat. Isot. 1996, 47, 1211–1217. [Google Scholar] [CrossRef]
- Flores, C.; Cabrera, E.; Calderon, T.; Munoz, E.; Adem, E.; Hernandez, J.; Boldu, J.; Ovalle, P.; Murrieta, H. ESR and optical absorption studies of gamma and electron-irradiation sugar crystals. Appl. Radiat. Isot. 2000, 52, 1229–1234. [Google Scholar] [CrossRef] [PubMed]
- Karakirova, Y.; Lund, E.; Yordanov, N.D. EPR and UV investigation of sucrose irradiated with nitrogen ions and gamma rays. Radiat. Meas. 2008, 43, 1337–1342. [Google Scholar] [CrossRef]
- Mikou, M.; Ghosne, N.; El Baydaoui, R.; Zirari, Z.; Kuntz, F. Performance characteristics of the EPR dosimetry system with table sugar in radiotherapy applications. Appl. Radiat. Isot. 2015, 99, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Karakirova, Y.; Yordanov, N.D. EPR and UV spectrometry investigation of sucrose irradiated with carbon particles. Radiat. Meas. 2010, 45, 831–835. [Google Scholar] [CrossRef]
- Karakirova, Y.; Nakagawa, K.; Yordanov, N.D. EPR and UV spectroscopic investigations of sucrose irradiated with heavy-ion particles. Radiat. Meas. 2010, 45, 10–14. [Google Scholar] [CrossRef]
- Nakagawa, K.; Hara, H.; Matsumoto, K. C-ion and X-ray-induced sucrose radicals investigated by CW EPR and 9 GHz EPR Imaging. Bull. Chem. Soc. Jpn. 2017, 90, 30–33. [Google Scholar] [CrossRef]
- Ayscough, P.R. Electron Spin Resonance in Chemistry; Methuen & Co., Ltd.: London, UK, 1967; pp. 349–350. [Google Scholar]
- Sagstuen, E.; Hole, E.O.; Haugedal, S.R.; Nelson, W.H. Alanine radicals: Structure determination by EPR and ENDOR of single crystals X-irradiated at 295 K. J. Phys. Chem. A 1997, 101, 9763–9772. [Google Scholar] [CrossRef]
- Heydari, M.Z.; Malinen, E.; Hole, E.O.; Sagstuen, E. Alanine radicals. 2. The composite polycrystalline alanine EPR spectrum studied by ENDOR, thermal annealing, and spectrum simulations. J. Phys. Chem. A 2002, 106, 8971–8977. [Google Scholar] [CrossRef]
- Ban, F.; Wetmore, S.D.; Boyd, R.J. A Density-Functional Theory Investigation of the Radiation Products of L-α-Alanine. J. Phys. Chem. A 1999, 103, 4303–4308. [Google Scholar] [CrossRef]
- Pauwels, E.; Van Speybroeck, V.; Lahorte, P.; Waroquier, M. Density functional calculations on alanine-derived radicals: Influence of molecular environment on EPR hyperfine coupling constants. J. Phys. Chem. A 2001, 105, 8794–8804. [Google Scholar] [CrossRef]
- Malinen, E.; Heydari, M.Z.; Sagstuen, E.; Hole, E.O. Alanine radicals, Part 3: Properties of the components contributing to the EPR spectrum of X-irradiated alanine dosimeters. Radiat. Res. 2003, 159, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Malinen, E.; Hult, E.A.; Hole, E.O.; Sagstuen, E. Alanine radicals, part 4: Relative amounts of radical species in alanine dosimeters after exposure to 6–19 MeV electrons and 10 kV–15 MV photons. Radiat. Res. 2003, 159, 149–153. [Google Scholar] [CrossRef]
- Jåstad, E.O.; Torheim, T.; Villeneuve, K.M.; Kvaal, K.; Hole, E.O.; Sagstuen, E.; Malinen, E.; Futsaether, C.M. In Quest of the Alanine R3 Radical: Multivariate EPR Spectral Analyses of X-Irradiated Alanine in the Solid State. J. Phys. Chem. A 2017, 121, 7139–7147. [Google Scholar] [CrossRef] [PubMed]
- Regulla, D.F.; Deffner, U. Dosimetry by ESR spectroscopy of alanine. Appl. Rad. Isot. 1982, 33, 1101–1114. [Google Scholar] [CrossRef]
- Hansen, J.W.; Olsen, K.J. Theoretical and experimental radiation effectiveness of the free radical dosimeter alanine to irradiation with heavy charged particles. Radiat. Res. 1985, 104, 15–27. [Google Scholar] [CrossRef]









| Sample | Linear Regression I = A + BD | Polynomial Regression I = A + B1 + B2D2 | |||||
|---|---|---|---|---|---|---|---|
| A | B | R | A | B1 | B2 | R | |
| DL-proline | 251.45 | 163.81 | 0.9872 | 17.93 | 279.50 | −4.60 | 0.9984 |
| L-proline | 110.58 | 65.94 | 0.9865 | 14.32 | 109.46 | −1.71 | 0.9997 |
| Cysteine | 77.41 | 101.76 | 0.9984 | 33.32 | 121.70 | −0.78 | 0.9992 |
| Alanine | 360.99 | 290.95 | 0.9944 | 97.55 | 410.05 | −4.69 | 0.9991 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karakirova, Y. Application of Amino Acids for High-Dosage Measurements with Electron Paramagnetic Resonance Spectroscopy. Molecules 2023, 28, 1745. https://doi.org/10.3390/molecules28041745
Karakirova Y. Application of Amino Acids for High-Dosage Measurements with Electron Paramagnetic Resonance Spectroscopy. Molecules. 2023; 28(4):1745. https://doi.org/10.3390/molecules28041745
Chicago/Turabian StyleKarakirova, Yordanka. 2023. "Application of Amino Acids for High-Dosage Measurements with Electron Paramagnetic Resonance Spectroscopy" Molecules 28, no. 4: 1745. https://doi.org/10.3390/molecules28041745