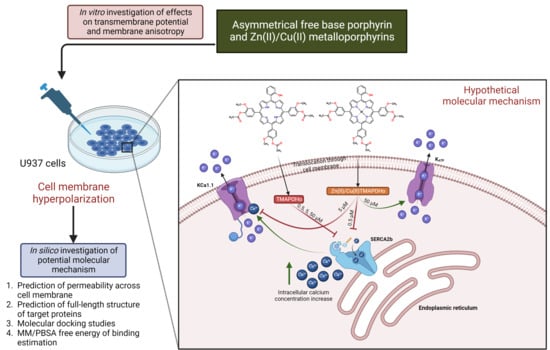
Interaction of Some Asymmetrical Porphyrins with U937 Cell Membranes–In Vitro and In Silico Studies
Abstract
:1. Introduction
2. Results
2.1. Asymmetric Porphyrins Induce Cell Membrane Hyperpolarization and Anysotropy
2.2. Prediction of Permeability across the Cell Membrane
2.3. Prediction of Full-Length Target Structures of SERCA2b, Slo1 and SUR2
2.4. Prediction of Interaction Models between Porphyrin Derivatives and SERCA2b, Slo1 and SUR2 through Molecular Docking
2.5. MM/PBSA Binding Free Energy
3. Materials and Methods
3.1. Evaluation of Transmembrane Potential and Membrane Anisotropy
3.2. The Prediction of Permeability across the Cell Membrane
3.3. Full-Length Protein Structure Modeling
3.4. Molecular Docking
3.5. MM/PBSA Free Energy of Binding Estimation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Socoteanu, R.; Boscencu, R.; Hirtopeanu, A.; Manda, G.; Sousa, A.; Ilie, M.; Vieira Ferreir, L.F. Trends in Interdisciplinary Studies Revealing Porphyrinic Compounds Multivalency Towards Biomedical Application. In Biomedical Engineering—From Theory to Applications; InTech: London, UK, 2011. [Google Scholar]
- Zhang, J.; Jiang, C.; Figueiró Longo, J.P.; Azevedo, R.B.; Zhang, H.; Muehlmann, L.A. An updated overview on the development of new photosensitizers for anticancer photodynamic therapy. Acta Pharm. Sin. B 2018, 8, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Roy Chowdhury, M.; Schumann, C.; Bhakta-Guha, D.; Guha, G. Cancer nanotheranostics: Strategies, promises and impediments. Biomed. Pharmacother. 2016, 84, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Ethirajan, M.; Chen, Y.; Joshi, P.; Pandey, R.K. The role of porphyrin chemistry in tumor imaging and photodynamic therapy. Chem. Soc. Rev. 2011, 40, 340–362. [Google Scholar] [CrossRef] [PubMed]
- Josefsen, L.B.; Boyle, R.W. Unique Diagnostic and Therapeutic Roles of Porphyrins and Phthalocyanines in Photodynamic Therapy, Imaging and Theranostics. Theranostics 2012, 2, 916–966. [Google Scholar] [CrossRef] [PubMed]
- Manda, G.; Hinescu, M.E.; Neagoe, I.V.; Ferreira, L.F.V.; Boscencu, R.; Vasos, P.; Basaga, S.H.; Cuadrado, A. Emerging Therapeutic Targets in Oncologic Photodynamic Therapy. Curr. Pharm. Des. 2019, 24, 5268–5295. [Google Scholar] [CrossRef] [PubMed]
- Razzaq, S.; Minhas, A.M.; Qazi, N.G.; Nadeem, H.; Khan, A.-u.; Ali, F.; Hassan, S.S.U.; Bungau, S. Novel Isoxazole Derivative Attenuates Ethanol-Induced Gastric Mucosal Injury through Inhibition of H+/K+-ATPase Pump. Oxidative Stress and Inflammatory Pathways. Molecules 2022, 27, 5065. [Google Scholar] [CrossRef]
- Das, S.S.; Bharadwaj, P.; Bilal, M.; Barani, M.; Rahdar, A.; Taboada, P.; Bungau, S.; Kyzas, G.Z. Stimuli-Responsive Polymeric Nanocarriers for Drug Delivery, Imaging, and Theragnosis. Polymers 2020, 12, 1397. [Google Scholar] [CrossRef]
- Yutaka, T.; Yoshiaki, O.; Yutaka, S.; Harutoshi, K. Benzodiazepine Receptor Agonists Modulate Thymocyte Apoptosis Through Reduction of the Mitochondrial Transmembrane Potential. Jpn. J. Pharmacol. 1999, 79, 177–183. [Google Scholar] [CrossRef]
- Yanamala, N.; Klein-Seetharaman, J. Allosteric Modulation of G Protein Coupled Receptors by Cytoplasmic, Transmembrane and Extracellular Ligands. Pharmaceuticals 2010, 3, 3324–3342. [Google Scholar] [CrossRef]
- Venugopal, J.; Blanco, G. Ouabain Enhances ADPKD Cell Apoptosis via the Intrinsic Pathway. Front. Physiol. 2016, 7, 107. [Google Scholar] [CrossRef]
- Klapperstück, T.; Glanz, D.; Klapperstück, M.; Wohlrab, J. Methodological aspects of measuring absolute values of membrane potential in human cells by flow cytometry. Cytom. Part A 2009, 75A, 593–608. [Google Scholar] [CrossRef]
- Boscencu, R. Microwave Synthesis Under Solvent-Free Conditions and Spectral Studies of Some Mesoporphyrinic Complexes. Molecules 2012, 17, 5592–5603. [Google Scholar] [CrossRef]
- Boscencu, R.; Oliveira, A.S.; Ferreira, D.P.; Ferreira, L.F.V. Synthesis and Spectral Evaluation of Some Unsymmetrical Mesoporphyrinic Complexes. Int. J. Mol. Sci. 2012, 13, 8112–8125. [Google Scholar] [CrossRef]
- Socoteanu, R.; Manda, G.; Boscencu, R.; Vasiliu, G.; Oliveira, A. Synthesis, Spectral Analysis and Preliminary in Vitro Evaluation of Some Tetrapyrrolic Complexes with 3d Metal Ions. Molecules 2015, 20, 15488–15499. [Google Scholar] [CrossRef]
- Boscencu, R.; Socoteanu, R.P.; Manda, G.; Radulea, N.; Anastasescu, M.; Gama, A.; Machado, I.F.; Ferreira, L.F.V. New A3B porphyrins as potential candidates for theranostic. Synthesis and photochemical behaviour. Dyes Pigments 2019, 160, 410–417. [Google Scholar] [CrossRef]
- Savitskiĭ, V.P.; Zorin, V.P. Selectivity of accumulation of chlorine e6 derivatives in blood leukocytes. Biofizika 2003, 48, 58–62. [Google Scholar] [PubMed]
- Krosl, G.; Korbelik, M.; Dougherty, G.J. Induction of immune cell infiltration into murine SCCVII tumour by photofrin-based photodynamic therapy. Br. J. Cancer 1995, 71, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Cecic, I.; Parkins, C.S.; Korbelik, M. Induction of systemic neutrophil response in mice by photodynamic therapy of solid tumors. Photochem. Photobiol. 2001, 74, 712–720. [Google Scholar] [CrossRef] [PubMed]
- Soyama, T.; Sakuragi, A.; Oishi, D.; Kimura, Y.; Aoki, H.; Nomoto, A.; Yano, S.; Nishie, H.; Kataoka, H.; Aoyama, M. Photodynamic therapy exploiting the anti-tumor activity of mannose-conjugated chlorin e6 reduced M2-like tumor-associated macrophages. Transl. Oncol. 2021, 14, 101005. [Google Scholar] [CrossRef]
- Tang, X.D.; Xu, R.; Reynolds, M.F.; Garcia, M.L.; Heinemann, S.H.; Hoshi, T. Haem can bind to and inhibit mammalian calcium-dependent Slo1 BK channels. Nature 2003, 425, 531–535. [Google Scholar] [CrossRef]
- Burton, M.J.; Kapetanaki, S.M.; Chernova, T.; Jamieson, A.G.; Dorlet, P.; Santolini, J.; Moody, P.C.E.; Mitcheson, J.S.; Davies, N.W.; Schmid, R.; et al. A heme-binding domain controls regulation of ATP-dependent potassium channels. Proc. Natl. Acad. Sci. USA 2016, 113, 3785–3790. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, N.; Goradia, N.; Ohlenschläger, O.; Schönherr, R.; Friedrich, M.; Plass, W.; Kappl, R.; Hoshi, T.; Heinemann, S.H. Heme impairs the ball-and-chain inactivation of potassium channels. Proc. Natl. Acad. Sci. USA 2013, 110, E4036–E4044. [Google Scholar] [CrossRef] [PubMed]
- Daly, D.; Al-Sabi, A.; Kinsella, G.K.; Nolan, K.; Dolly, J.O. Porphyrin derivatives as potent and selective blockers of neuronal Kv1 channels. Chem. Commun. 2015, 51, 1066–1069. [Google Scholar] [CrossRef] [PubMed]
- Obara, K.; Miyashita, N.; Xu, C.; Toyoshima, I.; Sugita, Y.; Inesi, G.; Toyoshima, C. Structural role of countertransport revealed in Ca 2+ pump crystal structure in the absence of Ca 2+. Proc. Natl. Acad. Sci. USA 2005, 102, 14489–14496. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Bonkovsky, H.L.; Guo, J. Structural analysis of heme proteins: Implications for design and prediction. BMC Struct. Biol. 2011, 11, 13. [Google Scholar] [CrossRef] [PubMed]
- Vasiliu, G.; Boscencu, R.; Socoteanu, R.; Nacea, V. Complex combinations of some transition metals with new unsymmetrical porphirins. Rev. Chim. 2014, 65, 998–1001. [Google Scholar]
- Boscencu, R.; Socoteanu, R.; Vasiliu, G.; Nacea, V. Synthesis under solvent free conditions of some unsymmetrically substituted porphyrinic compounds. Rev. Chim. 2014, 65, 888–891. [Google Scholar]
- Baudin, B.; Bruneel, A.; Bosselut, N.; Vaubourdolle, M. A protocol for isolation and culture of human umbilical vein endothelial cells. Nat. Protoc. 2007, 2, 481–485. [Google Scholar] [CrossRef]
- Instrumentation for Fluorescence Spectroscopy. Principles of Fluorescence Spectroscopy; Springer: Boston, MA, USA, 2006; pp. 27–61. [Google Scholar]
- Lomize, A.L.; Hage, J.M.; Schnitzer, K.; Golobokov, K.; LaFaive, M.B.; Forsyth, A.C.; Pogozheva, I.D. PerMM: A Web Tool and Database for Analysis of Passive Membrane Permeability and Translocation Pathways of Bioactive Molecules. J. Chem. Inf. Model. 2019, 59, 3094–3099. [Google Scholar] [CrossRef]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An Open chemical toolbox. J. Cheminform. 2011, 3, 33. [Google Scholar] [CrossRef]
- Land, H.; Humble, M.S. YASARA: A Tool to Obtain Structural Guidance in Biocatalytic Investigations. Methods Mol. Biol. 2018, 1685, 43–67. [Google Scholar]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef] [PubMed]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Bateman, A.; Martin, M.-J.; Orchard, S.; Magrane, M.; Agivetova, R.; Ahmad, S.; Alpi, E.; Bowler-Barnett, E.H.; Britto, R.; Bursteinas, B.; et al. UniProt: The universal protein knowledgebase in 2021. Nucleic Acids Res. 2021, 49, D480–D489. [Google Scholar]
- Zhang, Y.; Inoue, M.; Tsutsumi, A.; Watanabe, S.; Nishizawa, T.; Nagata, K.; Kikkawa, M.; Inaba, K. Cryo-EM structures of SERCA2b reveal the mechanism of regulation by the luminal extension tail. Sci. Adv. 2020, 6, eabb0147. [Google Scholar] [CrossRef]
- Tao, X.; Mackinnon, R. Molecular structures of the human slo1 k+ channel in complex with b4. Elife 2019, 8, e51409. [Google Scholar] [CrossRef] [PubMed]
- Sung, M.W.; Yang, Z.; Driggers, C.M.; Patton, B.L.; Mostofian, B.; Russo, J.D.; Zuckerman, D.M.; Shyng, S.-L. Vascular K ATP channel structural dynamics reveal regulatory mechanism by Mg-nucleotides. Proc. Natl. Acad. Sci. USA 2021, 118, e2109441118. [Google Scholar] [CrossRef]
- Nitulescu, G.; Nitulescu, G.M.; Zanfirescu, A.; Mihai, D.P.; Gradinaru, D. Candidates for Repurposing as Anti-Virulence Agents Based on the Structural Profile Analysis of Microbial Collagenase Inhibitors. Pharmaceutics 2021, 14, 62. [Google Scholar] [CrossRef]
- Williams, C.J.; Headd, J.J.; Moriarty, N.W.; Prisant, M.G.; Videau, L.L.; Deis, L.N.; Verma, V.; Keedy, D.A.; Hintze, B.J.; Chen, V.B.; et al. MolProbity: More and better reference data for improved all-atom structure validation. Protein Sci. 2018, 27, 293–315. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Eriksson, E.S.E.; Eriksson, L.A. Identifying the sarco(endo)plasmic reticulum Ca2+ ATPase (SERCA) as a potential target for hypericin – a theoretical study. Phys. Chem. Chem. Phys. 2012, 14, 12637. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.S.u.; Abbas, S.Q.; Ali, F.; Ishaq, M.; Bano, I.; Hassan, M.; Jin, H.-Z.; Bungau, S.G. A Comprehensive In Silico Exploration of Pharmacological Properties, Bioactivities, Molecular Docking, and Anticancer Potential of Vieloplain F from Xylopia vielana Targeting B-Raf Kinase. Molecules 2022, 27, 917. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Meng, E.C.; Couch, G.S.; Croll, T.I.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Structure visualization for researchers, educators, and developers. Protein Sci. 2021, 30, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Maier, J.A.; Martinez, C.; Kasavajhala, K.; Wickstrom, L.; Hauser, K.E.; Simmerling, C. ff14SB: Improving the Accuracy of Protein Side Chain and Backbone Parameters from ff99SB. J. Chem. Theory Comput. 2015, 11, 3696–3713. [Google Scholar] [CrossRef]
- Dickson, C.J.; Madej, B.D.; Skjevik, A.A.; Betz, R.M.; Teigen, K.; Gould, I.R.; Walker, R.C. Lipid14: The Amber Lipid Force Field. J. Chem. Theory Comput. 2014, 10, 865–879. [Google Scholar] [CrossRef]
- Wang, J.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and testing of a general amber force field. J. Comput. Chem. 2004, 25, 1157–1174. [Google Scholar] [CrossRef]
- Jakalian, A.; Jack, D.B.; Bayly, C.I. Fast, efficient generation of high-quality atomic charges. AM1-BCC model: II. Parameterization and validation. J. Comput. Chem. 2002, 23, 1623–1641. [Google Scholar] [CrossRef]
- Krieger, E.; Nielsen, J.E.; Spronk, C.A.E.M.; Vriend, G. Fast empirical pKa prediction by Ewald summation. J. Mol. Graph. Model. 2006, 25, 481–486. [Google Scholar] [CrossRef]
- Krieger, E.; Darden, T.; Nabuurs, S.B.; Finkelstein, A.; Vriend, G. Making optimal use of empirical energy functions: Force-field parameterization in crystal space. Proteins 2004, 57, 678–683. [Google Scholar] [CrossRef]
- Krieger, E.; Vriend, G. New ways to boost molecular dynamics simulations. J. Comput. Chem. 2015, 36, 996–1007. [Google Scholar] [CrossRef]
- Kato, H.; Komagoe, K.; Inoue, T.; Masuda, K.; Katsu, T. Structure—Activity relationship of porphyrin-induced photoinactivation with membrane function in bacteria and erythrocytes. Photochem. Photobiol. Sci. 2018, 17, 954–963. [Google Scholar] [CrossRef]
- Erdogan, A.; Schaefer, C.A.; Most, A.K.; Schaefer, M.B.; Mayer, K.; Tillmanns, H.; Kuhlmann, C.R.W. Lipopolysaccharide-induced proliferation and adhesion of U937 cells to endothelial cells involves barium chloride sensitive hyperpolarization. J. Endotoxin Res. 2006, 12, 224–230. [Google Scholar] [CrossRef]
- Prasad, A.; Sedlářová, M.; Balukova, A.; Ovsii, A.; Rác, M.; Křupka, M.; Kasai, S.; Pospíšil, P. Reactive Oxygen Species Imaging in U937 Cells. Front. Physiol. 2020, 11, 552569. [Google Scholar] [CrossRef] [PubMed]
- Femling, J.K.; Cherny, V.V.; Morgan, D.; Rada, B.; Davis, A.P.; Czirják, G.; Enyedi, P.; England, S.K.; Moreland, J.G.; Ligeti, E.; et al. The Antibacterial Activity of Human Neutrophils and Eosinophils Requires Proton Channels but Not BK Channels. J. Gen. Physiol. 2006, 127, 659–672. [Google Scholar] [CrossRef] [PubMed]
- Ling, M.-Y.; Ma, Z.-Y.; Wang, Y.-Y.; Qi, J.; Liu, L.; Li, L.; Zhang, Y. Up-regulated ATP-sensitive potassium channels play a role in increased inflammation and plaque vulnerability in macrophages. Atherosclerosis 2013, 226, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Park, S.W.; Zhou, Y.; Lee, J.; Lee, J.; Ozcan, U. Sarco(endo)plasmic reticulum Ca2+-ATPase 2b is a major regulator of endoplasmic reticulum stress and glucose homeostasis in obesity. Proc. Natl. Acad. Sci. USA 2010, 107, 19320–19325. [Google Scholar] [CrossRef] [PubMed]

 control without probe,
control without probe,  control with probe,
control with probe,  50 μM ZnTMAPOHo without probe,
50 μM ZnTMAPOHo without probe,  50 μM ZnTMAPOHo with probe); (b)—Hyperpolarizing effect for TMAPOHo; (c)—Hyperpolarizing effect for Zn(II)TMAPOHo; (d)—Hyperpolarizing effect for Cu(II)TMAPOHo; (e)—Comparative effect of porphyrinic compounds (24 h incubation time and 0.5 µM, 5 µM, 50 µM concentrations) on the transmembrane potential of U937 cells. In Figure 2b,c,d results are presented as mean hyperpolarizing effect (%) ± standard deviation (SD) for triplicate samples.
50 μM ZnTMAPOHo with probe); (b)—Hyperpolarizing effect for TMAPOHo; (c)—Hyperpolarizing effect for Zn(II)TMAPOHo; (d)—Hyperpolarizing effect for Cu(II)TMAPOHo; (e)—Comparative effect of porphyrinic compounds (24 h incubation time and 0.5 µM, 5 µM, 50 µM concentrations) on the transmembrane potential of U937 cells. In Figure 2b,c,d results are presented as mean hyperpolarizing effect (%) ± standard deviation (SD) for triplicate samples.
 control without probe,
control without probe,  control with probe,
control with probe,  50 μM ZnTMAPOHo without probe,
50 μM ZnTMAPOHo without probe,  50 μM ZnTMAPOHo with probe); (b)—Hyperpolarizing effect for TMAPOHo; (c)—Hyperpolarizing effect for Zn(II)TMAPOHo; (d)—Hyperpolarizing effect for Cu(II)TMAPOHo; (e)—Comparative effect of porphyrinic compounds (24 h incubation time and 0.5 µM, 5 µM, 50 µM concentrations) on the transmembrane potential of U937 cells. In Figure 2b,c,d results are presented as mean hyperpolarizing effect (%) ± standard deviation (SD) for triplicate samples.
50 μM ZnTMAPOHo with probe); (b)—Hyperpolarizing effect for TMAPOHo; (c)—Hyperpolarizing effect for Zn(II)TMAPOHo; (d)—Hyperpolarizing effect for Cu(II)TMAPOHo; (e)—Comparative effect of porphyrinic compounds (24 h incubation time and 0.5 µM, 5 µM, 50 µM concentrations) on the transmembrane potential of U937 cells. In Figure 2b,c,d results are presented as mean hyperpolarizing effect (%) ± standard deviation (SD) for triplicate samples.











| Target | Method | MolProbity Score | Ramachandran Distribution Z-Score | Residues in Most Favored Regions (%) | Residues in Disallowed Regions (%) | Favored Rotamers (%) | Poor Rotamers (%) |
|---|---|---|---|---|---|---|---|
| SERCA2b | SWISS-MODEL | 1.35 | −1.61 ± 0.24 | 96.70 | 0.19 | 91.37 | 2.95 |
| YASARA | 0.94 | −1.06 ± 0.24 | 97.40 | 0.19 | 96.75 | 1.57 | |
| AlphaFold | 1.02 | 0.36 ± 0.25 | 97.98 | 0.48 | 95.74 | 1.57 | |
| Slo1 | SWISS-MODEL | 1.40 | −1.59 ± 0.23 | 94.06 | 0.73 | 93.05 | 2.70 |
| YASARA | 1.65 | −1.94 ± 0.11 | 94.48 | 0.36 | 97.68 | 1.15 | |
| AlphaFold | 1.77 | −1.75 ± 0.22 | 84.93 | 8.27 | 94.17 | 2.59 | |
| SUR2 | SWISS-MODEL | 1.27 | −1.14 ± 0.19 | 94.42 | 0.65 | 93.49 | 2.07 |
| (KATP) | YASARA | 1.24 | −0.70 ± 0.19 | 96.19 | 0.32 | 95.58 | 2.43 |
| AlphaFold | 0.71 | 0.15 ± 0.19 | 97.87 | 0.45 | 97.42 | 0.88 |
| Free Energy of Binding (kcal/mol) | |||
|---|---|---|---|
| Ligand | SERCA2b | Slo1 | SUR2 |
| BHQ | −48.892 | - | - |
| Heme | - | −72.996 | −42.346 |
| TMAPOHo | −103.677 | 37.164 | 31.457 |
| Zn(II)TMAPOHo | −150.943 | −68.181 | −64.116 |
| Cu(II)TMAPOHo | −157.041 | −90.028 | −30.083 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mihai, D.P.; Boscencu, R.; Manda, G.; Burloiu, A.M.; Vasiliu, G.; Neagoe, I.V.; Socoteanu, R.P.; Lupuliasa, D. Interaction of Some Asymmetrical Porphyrins with U937 Cell Membranes–In Vitro and In Silico Studies. Molecules 2023, 28, 1640. https://doi.org/10.3390/molecules28041640
Mihai DP, Boscencu R, Manda G, Burloiu AM, Vasiliu G, Neagoe IV, Socoteanu RP, Lupuliasa D. Interaction of Some Asymmetrical Porphyrins with U937 Cell Membranes–In Vitro and In Silico Studies. Molecules. 2023; 28(4):1640. https://doi.org/10.3390/molecules28041640
Chicago/Turabian StyleMihai, Dragos Paul, Rica Boscencu, Gina Manda, Andreea Mihaela Burloiu, Georgiana Vasiliu, Ionela Victoria Neagoe, Radu Petre Socoteanu, and Dumitru Lupuliasa. 2023. "Interaction of Some Asymmetrical Porphyrins with U937 Cell Membranes–In Vitro and In Silico Studies" Molecules 28, no. 4: 1640. https://doi.org/10.3390/molecules28041640