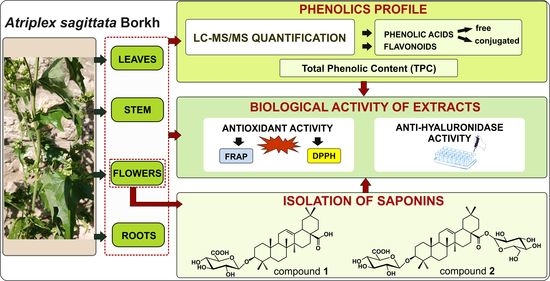
Antihyaluronidase and Antioxidant Potential of Atriplex sagittata Borkh. in Relation to Phenolic Compounds and Triterpene Saponins
Abstract
:1. Introduction
2. Results and Discussion
2.1. LC-ESI-MS/MS Profile of Phenolic Acids
2.2. LC-ESI-MS/MS Profile of Flavonoids
2.3. Antioxidant Activity of Extracts
2.4. Antihyaluronidase Activity of Extracts
2.5. Isolation of Saponins from A. sagittata Flower Extract
2.6. Antihyaluronidase Activity of Saponins from A. sagittata
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Plant Material
3.3. Extraction
3.4. Hydrolysis
3.5. Quantitative Determination of Phenolic Acids and Flavonoids
3.5.1. Sample Preparation
3.5.2. LC-ESI-MS/MS Analysis
3.6. Isolation of Saponins
3.7. Structure Elucidation of Isolated Compounds
3.7.1. Compound 1: Oleanolic acid-3-O-β-D-glucuronopyranoside (Calenduloside E) (Figure S1)
3.7.2. Compound 2: 3-O-β-D-glucuronopyranosyl Oleanolic acid 28-O-β-D-glucopyranosyl Ester (Chikusetsusaponin IVa) (Figure S1)
3.8. Determination of the Total Phenolic Content (TPC)
3.9. Determination of Antioxidant Activity
3.10. Determination of Antihyaluronidase Activity
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Kadereit, G.; Mavrodiev, E.V.; Zacharias, E.H.; Sukhorukov, A.P. Molecular phylogeny of Atripliceae (Chenopodioideae, Chenopodiaceae): Implications for systematics, biogeography, flower and fruit evolution, and the origin of C4 photosynthesis. Am. J. Bot. 2010, 97, 1664–1687. [Google Scholar] [CrossRef] [PubMed]
- Jman Redzic, S. Wild edible plants and their traditional use in the human nutrition in Bosnia-Herzegovina. Ecol. Food Nutr. 2006, 45, 189–232. [Google Scholar] [CrossRef]
- Rinchen, T.; Singh, N.; Maurya, S.B.; Soni, V.; Phour, M.; Kumar, B. Morphological characterization of indigenous vegetable (Atriplex hortensis L.) from trans-Himalayan region of Ladakh (Jammu and Kashmir), India. Aust. J. Crop Sci. 2017, 11, 258–263. [Google Scholar] [CrossRef]
- Wright, K.H.; Pike, O.A.; Fairbanks, D.J.; Huber, C.S. Composition of Atriplex hortensis, sweet and bitter Chenopodium quinoa seeds. J. Food Sci. 2002, 67, 1383–1385. [Google Scholar] [CrossRef]
- Zanella, L.; Vianello, F. Functional food from endangered ecosystems: Atriplex portulacoides as a case study. Foods 2020, 9, 1533. [Google Scholar] [CrossRef]
- Benarba, B. Use of medicinal plants by breast cancer patients in Algeria. EXCLI J. 2015, 14, 1164–1166. [Google Scholar] [CrossRef]
- Walker, D.J.; Lutts, S.; Sánchez-García, M.; Correal, E. Atriplex halimus L.: Its biology and uses. J. Arid Environ. 2014, 100, 111–121. [Google Scholar] [CrossRef]
- El Souda, S.S.E.D.; Matloub, A.A.; Nepveu, F.; Valentin, A.; Roques, C. Phenolic composition and prospective anti-infectious properties of Atriplex lindleyi. Asian Pac. J. Trop. Dis. 2015, 5, 786–791. [Google Scholar] [CrossRef]
- Wazir, S.M.; Dasti, A.A.; Shah, J. Common medicinal plants of chapursan valley, Gojal II, Gilgit-Pakistan. J. Res. Sci. Bahauddin Zakariya Univ. Multan Pak. 2004, 15, 41–43. [Google Scholar]
- Kadioğlu, S.; Mustafa, T.A.N.; Kadioğlu, B.; Sezer, K.K. Determination of the usability of some ethnobotanically used wild plant species as forage crops. Muş Alparslan Univ. J. Agric. Nat. 2022, 2, 30–37. [Google Scholar]
- Benhammou, N.; Bekkara, F.A.; Panovska, T.K. Antioxidant activity of methanolic extracts and some bioactive compounds of Atriplex halimus. Comptes Rendus Chim. 2009, 12, 1259–1266. [Google Scholar] [CrossRef]
- Chikhi, I.; Allali, H.; Dib, M.E.A.; Medjdoub, H.; Tabti, B. Antidiabetic activity of aqueous leaf extract of Atriplex halimus L. (Chenopodiaceae) in streptozotocin-induced diabetic rats. Asian Pac. J. Trop. Dis. 2014, 4, 181–184. [Google Scholar] [CrossRef]
- Kamal, Z.; Ullah, F.; Ayaz, M.; Sadiq, A.; Ahmad, S.; Zeb, A.; Hussain, A.; Imran, M. Anticholinesterse and antioxidant investigations of crude extracts, subsequent fractions, saponins and flavonoids of Atriplex laciniata L.: Potential effectiveness in Alzheimer’s and other neurological disorders. Biol. Res. 2015, 48, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slama, K.; Boumendjel, M.; Taibi, F.; Boumendjel, A.; Messarah, M. Atriplex halimus aqueous extract abrogates carbon tetrachloride-induced hepatotoxicity by modulating biochemical and histological changes in rats. Arch. Physiol. Biochem. 2020, 126, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Zohra, T.; Ovais, M.; Khalil, A.T.; Qasim, M.; Ayaz, M.; Shinwari, Z.K.; Ahmad, S.; Zahoor, M. Bio-guided profiling and HPLC-DAD finger printing of Atriplex lasiantha Boiss. BMC Complement. Altern. Med. 2019, 19, 4. [Google Scholar] [CrossRef]
- Boughalleb, N.; Trabelsi, L.; Harzallah-Skhiri, F. Antifungal activity from polar and non-polar extracts of some Chenopodiaceae wild species growing in Tunisia. Nat. Prod. Res. 2009, 23, 988–997. [Google Scholar] [CrossRef]
- Bouaziz, S.; Amri, M.; Taibi, N.; Zeghir-Bouteldja, R.; Benkhaled, A.; Mezioug, D.; Touil-Boukoffa, C. Protoscolicidal activity of Atriplex halimus leaves extract against Echinococcus granulosus protoscoleces. Exp. Parasitol. 2021, 229, 108155. [Google Scholar] [CrossRef]
- Bounouar, E.; Missoun, F.; Amari, N.O.; Belabaci, F.Z.; Belabaci, S.; Sekkal, F.Z.; Djebli, N. Antidiabetic effect of Atriplex halimus long and short term treatment against Streptozotocin induced diabetes in rat. An. Biol. 2022, 44, 21–30. [Google Scholar] [CrossRef]
- Zeghib, K.; Boutlelis, D.A. Food additive (Sodium benzoate)-induced damage on renal function and glomerular cells in rats; modulating effect of aqueous extract of Atriplex halimus L. Iran. J. Pharm. Res. 2021, 20, 296–306. [Google Scholar] [CrossRef]
- Siddiqui, B.S.; Ahmed, S.; Khan, M.A.U. Triterpenoids of Atriplex stocksii. Phytochemistry 1994, 37, 1123–1125. [Google Scholar] [CrossRef]
- Ben Nejma, A.; Znati, M.; Nguir, A.; Daich, A.; Othman, M.; Lawson, A.M.; Ben Jannet, H. Phytochemical and biological studies of Atriplex inflata f. Muell.: Isolation of secondary bioactive metabolites. J. Pharm. Pharmacol. 2017, 69, 1064–1074. [Google Scholar] [CrossRef] [PubMed]
- Keckeis, K.; Sarker, S.D.; Dinan, L.N. Phytoecdysteroids from Atriplex nummularia. Fitoterapia 2000, 71, 456–458. [Google Scholar] [CrossRef] [PubMed]
- Rozentsvet, O.A.; Kotlova, E.R.; Bogdanova, E.S.; Nesterov, V.N.; Senik, S.V.; Shavarda, A.L. Balance of Δ5-and Δ7-sterols and stanols in halophytes in connection with salinity tolerance. Phytochemistry 2022, 198, 113156. [Google Scholar] [CrossRef]
- Ali, B.; Tabassum, R.; Riaz, N.; Yaqoob, A.; Khatoon, T.; Tareen, R.B.; Jabbar, A.; Nasim, F.U.; Saleem, M. Bioactive triterpenoids from Atriplex lasiantha. J. Asian Nat. Prod. Res. 2015, 17, 843–850. [Google Scholar] [CrossRef]
- Jabrane, A.; Ben Jannet, H.; Miyamoto, T.; Tanaka, C.; Mirjolet, J.F.; Duchamp, O.; Féthia, H.S.; Lacaille-Dubois, M.A. Glaucasides A-C, three saikosaponins from Atriplex glauca L. var. ifiniensis (Caball) Maire. Magn. Reason. Chem. 2011, 49, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Shaker, K.H.; Dockendorff, K.; Seifert, K. Triterpenoid saponins from Atriplex semibaccata. Z. Naturforsch. 2003, 58, 485–489. [Google Scholar] [CrossRef] [PubMed]
- El-Aasr, M.; Kabbash, A.; El-Seoud, K.A.A.; Al-Madboly, L.A.; Ikeda, T. Antimicrobial and immunomodulatory activities of flavonol glycosides isolated from Atriplex halimus L. herb. J. Pharm. Sci. Res. 2016, 8, 1159–1168. [Google Scholar]
- Stanković, J.; Gođevac, D.; Tešević, V.; Dajić-Stevanović, Z.; Ćirić, A.; Soković, M.; Novaković, M. Antibacterial and antibiofilm activity of flavonoid and saponin derivatives from Atriplex tatarica against Pseudomonas aeruginosa. J. Nat. Prod. 2019, 82, 1487–1495. [Google Scholar] [CrossRef]
- Bacr, A.F.; Shao, P.; Farag, M.A. Recent advances in glycyrrhizin metabolism, health benefits, clinical effects and drug delivery systems for efficacy improvement; a comprehensive review. Phytomedicine 2022, 99, 153999. [Google Scholar] [CrossRef]
- Biswas, T.; Dwivedi, U.N. Plant triterpenoid saponins: Biosynthesis, In Vitro production, and pharmacological relevance. Protoplasma 2019, 256, 1463–1486. [Google Scholar] [CrossRef]
- Gallelli, L.; Cione, E.; Wang, T.; Zhang, L. Glucocorticoid-like activity of escin: A new mechanism for an old drug. Drug Des. Dev. Ther. 2021, 15, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Christensen, S.B.; Omar, A.A. Atriplex nummularia, a source for the two molluscicide saponins: Hederagenin-3-O-β-D-glucuronopyranoside and calenduloside E. J. Nat. Prod. 1985, 48, 161. [Google Scholar] [CrossRef]
- El-Sayed, M. Molluscicidal saponins from Atriplex leucoclada. Zagazig J. Pharm. Sci. 1995, 4, 143–146. [Google Scholar] [CrossRef]
- El-Sayed, M.M. Study of the saponin content of Atriplex stylosa Viv. and its molluscicidal effect. Bull. Pharm. Sci. 1998, 21, 237–243. [Google Scholar] [CrossRef]
- Shetty, K. Biotechnology to harness the benefits of dietary phenolics; focus on Lamiaceae. Asia Pac. J. Clin. Nutr. 1997, 6, 162–171. [Google Scholar] [PubMed]
- Andreasen, M.F.; Kroon, P.A.; Williamson, G.; Garcia-Conesa, M.T. Intestinal release and uptake of phenolic antioxidant diferulic acids. Free Radic. Biol. Med. 2001, 31, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Kampa, M.; Alexaki, V.I.; Notas, G.; Nifli, A.P.; Nistikaki, A.; Hatzoglou, A.; Bakogeorgou, E.; Kouimtzoglou, E.; Blekas, G.; Boskou, D.; et al. Antiproliferative and apoptotic effects of selective phenolic acids on T47D human breast cancer cells: Potential mechanisms of action. Breast Cancer Res. 2004, 6, R63–R74. [Google Scholar] [CrossRef] [Green Version]
- Wróbel-Biedrawa, D.; Grabowska, K.; Galanty, A.; Sobolewska, D.; Podolak, I. A flavonoid on the brain: Quercetin as a potential therapeutic agent in central nervous system disorders. Life 2022, 12, 591. [Google Scholar] [CrossRef]
- Yu, Y.S.; Hsu, C.L.; Yen, G.C. Anti-inflammatory effects of the roots of Alpinia pricei Hayata and its phenolic compounds. J. Agric. Food Chem. 2009, 57, 7673–7680. [Google Scholar] [CrossRef]
- Awaad, A.S.; Maitland, D.J.; Donia, A.E.R.M.; Alqasoumi, S.I.; Soliman, G.A. Novel flavonoids with antioxidant activity from a Chenopodiaceous plant. Pharm. Biol. 2012, 50, 99–104. [Google Scholar] [CrossRef]
- Bylka, W.; Stobiecki, M.; Frański, R. Sulphated flavonoid glycosides from leaves of Atriplex hortensis. Acta Physiol. Plant. 2001, 23, 285–290. [Google Scholar] [CrossRef]
- Gođevac, D.; Stanković, J.; Novaković, M.; Anđelkovicć, B.; Dajić-Stevanović, Z.; Petrović, M.; Stanković, M. Phenolic compounds from Atriplex littoralis and their radiation-mitigating activity. J. Nat. Prod. 2015, 78, 2198–2204. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, W.A.; Abdel-Mohsen, M.M.; Radwan, H.M.; Habib, A.A.; Yeramian, M.A. Phytochemical and biological investigations of Atriplix semibacata Br. growing in Egypt. Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz, P.K.; Kolak, U. Determination of phenolic acids in Atriplex hortensis L. by novel solid-phase extraction and high-performance liquid chromatography. Anal. Lett. 2016, 49, 2157–2164. [Google Scholar] [CrossRef]
- Clauser, M.; Dall’Acqua, S.; Loi, M.C.; Innocenti, G. Phytochemical investigation on Atriplex halimus L. from Sardinia. Nat. Prod. Res. 2013, 27, 1940–1944. [Google Scholar] [CrossRef]
- Emam, S.S. Bioactive constituents of Atriplex halimus plant. J. Nat. Prod. 2011, 4, 25–41. [Google Scholar]
- Boutaoui, N.; Zaiter, L.; Benayache, F.; Benayache, S.; Cacciagrano, F.; Cesa, S.; Secci, D.; Carradori, S.; Giusti, A.M.; Campestre, C.; et al. Atriplex mollis Desf. aerial parts: Extraction procedures, secondary metabolites and color analysis. Molecules 2018, 23, 1962. [Google Scholar] [CrossRef] [Green Version]
- Zengin, G.; Aumeeruddy-Elalfi, Z.; Mollica, A.; Yilmaz, M.A.; Mahomoodally, M.F. In vitro and in silico perspectives on biological and phytochemical profile of three halophyte species-A source of innovative phytopharmaceuticals from nature. Phytomedicine 2018, 38, 35–44. [Google Scholar] [CrossRef]
- Nicoletti, I.; Martini, D.; De Rossi, A.; Taddei, F.; D’Egidio, M.G.; Corradini, D. Identification and quantification of soluble free, soluble conjugated, and insoluble bound phenolic acids in durum wheat (Triticum turgidum L. var. durum) and derived products by RP-HPLC on a semimicro separation scale. J. Agric. Food Chem. 2013, 61, 11800–11807. [Google Scholar] [CrossRef]
- Pyrzynska, K.; Biesaga, M. Analysis of phenolic acids and flavonoids in honey. TrAC-Trends Anal. Chem. 2009, 28, 893–902. [Google Scholar] [CrossRef]
- Ross, K.A.; Beta, T.; Arntfield, S.D. A comparative study on the phenolic acids identified and quantified in dry beans using HPLC as affected by different extraction and hydrolysis methods. Food Chem. 2009, 113, 336–344. [Google Scholar] [CrossRef]
- Karadeniz, F.; Oh, J.H.; Im Lee, J.; Seo, Y.; Kong, C.S. 3,5-dicaffeoyl epi-quinic acid from Atriplex gmelinii enhances the osteoblast differentiation of bone marrow-derived human mesenchymal stromal cells via WnT/BMP signaling and suppresses adipocyte differentiation via AMPK activation. Phytomedicine 2020, 71, 153225. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.H.; Lee, J.I.; Karadeniz, F.; Seo, Y.; Kong, C.S. 3, 5-Dicaffeoyl-Epi-Quinic acid isolated from edible halophyte Atriplex gmelinii inhibits adipogenesis via AMPK/MAPK pathway in 3T3-L1 adipocytes. Evid.-Based Complement. Alternat. Med. 2018, 21, 8572571. [Google Scholar] [CrossRef] [PubMed]
- Gębalski, J.; Graczyk, F.; Załuski, D. Paving the way towards effective plant-based inhibitors of hyaluronidase and tyrosinase: A critical review on a structure–activity relationship. J. Enzyme Inhib. Med. Chem. 2022, 37, 1120–1195. [Google Scholar] [CrossRef]
- Krygier, K.; Sosulski, F.; Hogge, L. Free, esterified, and insoluble-bound phenolic acids. 1. Extraction and purification procedure. J. Agric. Food Chem. 1982, 30, 330–334. [Google Scholar] [CrossRef]
- Rakhmankulova, Z.F.; Shuyskaya, E.V.; Shcherbakov, A.V.; Fedyaev, V.V.; Biktimerova, G.Y.; Khafisova, R.R.; Usmanov, I.Y. Content of proline and flavonoids in the shoots of halophytes inhabiting the South Urals. Russ. J. Plant Physiol. 2015, 62, 71–79. [Google Scholar] [CrossRef]
- Mohammed, R.; El-Hawary, S.S.; Abo-youssef, A.M. Biological investigation of some wild Aizoaceae and Chenopediaceae species growing in Egypt. J. Nat. Prod. 2012, 5, 193–206. [Google Scholar]
- Al-Jaber, A.A.; Hujahid, T.G.; Al-Hazmi, H.M.G. Flavonoids from Atriplex farinosa. J. King Saud Univ. Sci. 1991, 3, 163–167. [Google Scholar]
- Tran, T.M.T.; Nguyen, T.B.; Winterhalter, P.; Jerz, G. Off-line ESI-MS/MS profiling of betalains and flavonoid glycosides isolated from (fruit) Opuntia stricta var. dillenii and (vegetable) Atriplex hortensis var. rubra by countercurrent chromatography. Vietnam J. Sci. Technol. Eng. 2022, 64, 20–26. [Google Scholar] [CrossRef]
- Jeong, H.; Kim, H.; Ju, E.; Kong, C.S.; Seo, Y. Antioxidant effect of the halophyte Atriplex gmelinii. KSBB J. 2016, 31, 200–207. [Google Scholar] [CrossRef] [Green Version]
- Zine, H.; Ibrahimi, M.; Loqman, S.; Papazoglou, E.G.; Ouhaddou, S.; Elgadi, S.; Ouhdouch, Y.; Hakkou, R.; Adnani, M.E.; Ouhammou, A. Chemical composition, antioxidant, and antibacterial activities of essential oil of Atriplex semibaccata R.Br. aerial parts: First assessment against multidrug-resistant bacteria. Agronomy 2021, 11, 362. [Google Scholar] [CrossRef]
- Pulido, R.; Bravo, L.; Saura-Calixto, F. Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J. Agric. Food Chem. 2000, 48, 3396–3402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozgen, M.; Reese, R.N.; Tulio, A.Z.; Scheerens, J.C.; Miller, A.R. Modified 2, 2-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) method to measure antioxidant capacity of selected small fruits and comparison to ferric reducing antioxidant power (FRAP) and 2, 2 ‘-diphenyl-1-picrylhydrazyl (DPPH) methods. J. Agric. Food Chem. 2006, 54, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Chłopicka, J.; Paśko, P.; Gorinstein, S.; Jedryas, A.; Zagrodzki, P. Total phenolic and total flavonoid content, antioxidant activity and sensory evaluation of pseudocereal breads. LWT-Food Sci. Technol. 2012, 46, 548–555. [Google Scholar] [CrossRef]
- Kachout, S.S.; Mansoura, A.; Leclerc, J.C.; Mechergui, R.; Rejeb, M.N.; Ouerghi, Z. Effects of heavy metals on antioxidant activities of Atriplex hortensis and A. rosea. J. Food Agric. Environ. 2009, 7, 938–945. [Google Scholar]
- Chen, J.; Yang, J.; Ma, L.; Li, J.; Shahzad, N.; Kim, C.K. Structure-antioxidant activity relationship of methoxy, phenolic hydroxyl, and carboxylic acid groups of phenolic acids. Sci. Rep. 2020, 10, 2611. [Google Scholar] [CrossRef] [Green Version]
- Dias, M.C.; Pinto, D.C.; Silva, A.M. Plant flavonoids: Chemical characteristics and biological activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef]
- Kiokias, S.; Proestos, C.; Oreopoulou, V. Phenolic acids of plant origin—A review on their antioxidant activity In Vitro (o/w emulsion systems) along with their In Vivo health biochemical properties. Foods 2020, 9, 534. [Google Scholar] [CrossRef] [Green Version]
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef]
- Van Acker, S.A.; Tromp, M.N.; Griffioen, D.H.; Van Bennekom, W.P.; Van Der Vijgh, W.J.; Bast, A. Structural aspects of antioxidant activity of flavonoids. Free Radic. Biol. Med. 1996, 20, 331–342. [Google Scholar] [CrossRef]
- Kuppusamy, U.R.; Khoo, H.E.; Das, N.P. Structure-activity studies of flavonoids as inhibitors of hyaluronidase. Biochem. Pharmacol. 1990, 40, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Ma, J.; Yang, R.; Jing, Y.; Qu, L. Molecular interactions of flavonoids to hyaluronidase: Insights from spectroscopic and molecular modeling studies. J. Fluoresc. 2015, 25, 941–959. [Google Scholar] [CrossRef] [PubMed]
- Edelmann, M.; Dawid, C.; Hochreiter, K.; Ralla, T.; Stark, T.D.; Salminen, H.; Hofmann, T. Molecularization of foam-active saponins from sugar beet side streams (Beta vulgaris ssp. vulgaris var altissima). J. Agric. Food Chem. 2020, 68, 10962–10974. [Google Scholar] [CrossRef] [PubMed]
- Lavaud, C.; Voutquenne, L.; Bal, P.; Pouny, I. Saponins from Chenopodium album. Fitoterapia 2000, 71, 338–340. [Google Scholar] [CrossRef]
- Yin, M.; Wang, X.; Wang, M.; Chem, Y.; Dong, Y.; Zhao, Y.; Feng, X. A new triterpenoid saponin and other saponins from Salicornia europea. Chem. Nat. Compd. 2012, 48, 258–261. [Google Scholar] [CrossRef]
- Mroczek, A. Phytochemistry and bioactivity of triterpene saponins from Amaranthaceae family. Phytochem. Rev. 2015, 14, 577–605. [Google Scholar] [CrossRef]
- Grabowska, K.; Wróbel, D.; Żmudzki, P.; Podolak, I. Anti-inflammatory activity of saponins from roots of Impatiens parviflora DC. Nat. Prod. Res. 2020, 34, 1581–1585. [Google Scholar] [CrossRef]
- Murata, T.; Suzuki, A.; Mafune, N.; Sato, E.; Miyase, T.; Yoshizaki, F. Triterpene saponins from Clethra barbinervis and their hyaluronidase inhibitory activities. Chem. Pharm. Bull. 2013, 61, 134–143. [Google Scholar] [CrossRef] [Green Version]
- Mynarski, A.; Pietrzak, W.; Galanty, A.; Dawiec, E.; Nowak, R.; Podolak, I. Phenolic Acid LC/MS Profile of Chenopodium rubrum and Evaluation of Cytotoxic Activity. Nat. Prod. Commun. 2018, 13, 855–857. [Google Scholar] [CrossRef] [Green Version]
- Grabowska, K.; Pecio, Ł.; Galanty, A.; Żmudzki, P.; Oleszek, W.; Podolak, I. Serjanic acid glycosides from Chenopodium hybridum L. with good cytotoxicity and selectivity profile against several panels of human cancer cell lines. Molecules 2021, 26, 4915. [Google Scholar] [CrossRef]
- Grabowska, K.; Podolak, I.; Galanty, A.; Żmudzki, P.; Koczurkiewicz, P.; Piska, K.; Pękala, E.; Janeczko, Z. Two new triterpenoid saponins from the leaves of Impatiens parviflora DC. and their cytotoxic activity. Ind. Crops Prod. 2017, 96, 71–79. [Google Scholar] [CrossRef]
- Paśko, P.; Gdula-Argasinska, J.; Podporska-Carroll, J.; Quilty, B.; Wietecha-Posluszny, R.; Tyszka-Czochara, M.; Zagrodzki, P. Influence of selenium supplementation on fatty acids profile and biological activity of four edible amaranth sprouts as new kind of functional food. J. Food Sci. Technol. 2015, 52, 4724–4736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barton, H.J. A “zero sample concentration approach”: Standardization of methods for the estimation of total antioxidant activity by the use of extrapolation to zero sample concentration. A novel standard. 1. ABTS cation radical scavenging. J. Agric. Food. Chem. 2010, 58, 8918–8926. [Google Scholar] [CrossRef] [PubMed]
- ICH Harmonized Tripartite Guideline Q3c (R6) on Impurities: Guideline for Residual Solvents. In Proceedings of the International Conference for Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH), Basel, Switzerland, 2016.


| Compound | Sample | |||||||
|---|---|---|---|---|---|---|---|---|
| Flower | Leaves | Stem | Root | |||||
| Extract | Hydrolyzed Extr. | Extract | Hydrolyzed Extr. | Extract | Hydrolyzed Extr. | Extract | Hydrolyzed Extr. | |
| ferulic acid | 18.57 ± 3.05 a | 2.99 ± 1.23 | 22.59 ± 1.16 a | 36.53 ± 7.27 | <LOD | <LOQ | <LOD | <LOQ |
| protocatechuic acid | <LOQ | 26.04 ± 3.32 | Nd | 14.13 ± 2.21 | <LOQ | <LOQ | <LOD | <LOQ |
| gentisic acid | <LOQ | 2.05 ± 0.76 | Nd | 26.59 ± 5.57 | <LOQ | <LOQ | Nd | 0.64 ± 0.21 |
| 4-hydroxybenzoic acid | 12.80 ± 4.42 | 52.12 ± 7.66 | 21.25 ± 1.30 | 84.29 ± 5.84 | <LOQ | 1.46 ± 0.82 | <LOQ | 4.03 ± 1.16 |
| salicylic acid | 13.83 ± 2.93 a | 11.07 ± 1.9 a | <LOQ | 1.82 ± 0.62 | <LOQ | 0.27 ± 0.10 | <LOQ | 0.59 ± 0.15 |
| 4-hydroxycynamic acid | <LOQ | <LOQ | <LOQ | 3.28 ± 1.19 | <LOQ | <LOQ | <LOQ | <LOQ |
| vanillic acid | Nd | 21.26 ± 7.21 a | Nd | 20.25 ± 6.24 a | Nd | <LOQ | Nd | 4.33 ± 0.65 |
| caffeic acid | Nd | Nd | Nd | 1.25 ± 0.23 | Nd | Nd | Nd | Nd |
| syringic acid | <LOQ | <LOQ | <LOQ | 33.26 ± 9.03 | <LOQ | 5.58 ± 2.39 a | <LOQ | 7.10 ± 2.60 a |
| sinapic acid | Nd | Nd | Nd | 3.84 ± 2.04 | <LOQ | <LOQ | Nd | <LOQ |
| 3-hydroxycynamic acid | <LOQ | <LOQ | <LOQ | Nd | Nd | Nd | Nd | Nd |
| Other # | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd |
| Sum * | 45.20 ± 10.4 a | 115.53 ± 22.15 | 43.84 ± 2.46 a | 225.24 ± 40.29 | 0.00 | 6.45 ± 3.32 | 0.00 | 16.68 ± 4.78 |
| Compound | Plant Part | |||
|---|---|---|---|---|
| Flowers | Leaves | Stem | Root | |
| Astragalin | <LOQ | 77.38 ± 2.25 | <LOQ | <LOQ |
| Kaempferol-3-rutinoside | 4.97 ± 0.12 | 9.54 ± 0.40 | 0.24 ± 0.01 | <LOQ |
| Kaempferol-3-glucoside-7-Rhamnoside | 73.76 ± 2.72 | 97.13 ± 2.82 | 8.61 ± 0.37 | <LOQ |
| Vitexin/isovitexin | 29.55 ± 0.75 | Nd | Nd | Nd |
| Rutin | <LOQ | 2.82 ± 0.09 | <LOQ | <LOQ |
| Isoquercetin | 100.84 ± 2.75 | <LOQ | <LOQ | <LOQ |
| Narcisoside | 33.59 ± 0.57 | 15.99 ± 0.03 | 4.19 ± 0.79 | Nd |
| Isorhamnetin-3-glucoside | <LOQ | <LOQ | <LOQ | <LOQ |
| Naringin | <LOQ | Nd | Nd | Nd |
| Other flavonoids # | Nd | Nd | Nd | Nd |
| Sum * | 242.71 ± 6.91 | 202.86 ± 5.59 | 13.05 ± 1.17 | 0.00 |
| Plant Part | TPC [mg GAE/100 g dw.] | Flav. SUM [μg/g dw.] | PA SUM [μg/g dw.] | Antioxidant Potential | |
|---|---|---|---|---|---|
| FRAP[mmolFe2+/ 100 g dw.] | DPP HmmolTrolox/100 g dw.) | ||||
| Flowers | 85.36 ± 3.00 | 242.71 ± 6.91 | 45.20 ± 10.4 | 0.44 ± 0.05 a | 0.16 ± 0.009 a |
| Leaves | 169.91 ± 1.4 | 202.86 ± 5.59 | 43.84 ± 2.46 | 0.70 ± 0.03 b | 0.32 ± 0.04 |
| Stem | 611.86 ± 10.42 | 13.05 ± 1.17 | 0.00 | 5.46 ± 0.21 | 2.99 ± 0.26 |
| Root | 59.16 ± 1.16 | 0.00 | 0.00 | 0.59 ± 0.04 a,b | 0.13 ± 0.02 a |
| Hyaluronidase Inhibition [%] | |||||
|---|---|---|---|---|---|
| Concentration [µg/mL] | Control | Flowers | Leaves | Stem | Root |
| 1000 | 91.11 ± 1.50 | 100.00 ± 0.01 a | 100.00 ± 0.001 a | 100.00 ± 0.001 a | 99.79 ± 0.36 a |
| 700 | 80.56 ± 0.49 | 100.00 ± 0.01 a | 99.12 ± 0.38 a | 97.34 ± 0.57 a | 98.11 ± 0.63 a |
| 500 | 38.84 ± 1.33 | 98.33 ± 1.89 a | 93.90 ± 1.37 b | 96.83 ± 0.54 a,b | 87.16 ± 2.18 |
| 300 | 21.86 ± 4.04 | 95.93 ± 1.70 | 87.47 ± 0.74 | 66.67 ± 0.34 | 59.38 ± 1.88 |
| 200 | 13.86 ± 2.70 | 94.45 ± 2.19 | 37.39 ± 1.96 | 30.88 ± 2.48 | 24.05 ± 0.63 |
| 100 | 4.39 ± 0.88 | 61.61 ± 4.67 | 13.20 ± 2.28 a | 11.31 ± 1.19 a | 6.94 ± 0.41 |
| 50 | 1.24 ± 0.99 | 17.72 ± 1.52 | 5.29 ± 1.21 a | 5.26 ± 1.39 a | NA |
| 20 | 0.62 ± 0.54 a | 0.76 ± 0.32 a | NA | NA | NA |
| 10 | 0.61 ± 0.53 | NA | NA | NA | NA |
| 0 | NA | NA | NA | NA | NA |
| IC50 | 514.28 | 84.67 | 216.2 | 244.5 | 272.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grabowska, K.; Pietrzak, W.; Paśko, P.; Sołtys, A.; Galanty, A.; Żmudzki, P.; Nowak, R.; Podolak, I. Antihyaluronidase and Antioxidant Potential of Atriplex sagittata Borkh. in Relation to Phenolic Compounds and Triterpene Saponins. Molecules 2023, 28, 982. https://doi.org/10.3390/molecules28030982
Grabowska K, Pietrzak W, Paśko P, Sołtys A, Galanty A, Żmudzki P, Nowak R, Podolak I. Antihyaluronidase and Antioxidant Potential of Atriplex sagittata Borkh. in Relation to Phenolic Compounds and Triterpene Saponins. Molecules. 2023; 28(3):982. https://doi.org/10.3390/molecules28030982
Chicago/Turabian StyleGrabowska, Karolina, Wioleta Pietrzak, Paweł Paśko, Agnieszka Sołtys, Agnieszka Galanty, Paweł Żmudzki, Renata Nowak, and Irma Podolak. 2023. "Antihyaluronidase and Antioxidant Potential of Atriplex sagittata Borkh. in Relation to Phenolic Compounds and Triterpene Saponins" Molecules 28, no. 3: 982. https://doi.org/10.3390/molecules28030982