Heterogeneous Photocatalysis as a Potent Tool for Organic Synthesis: Cross-Dehydrogenative C–C Coupling of N-Heterocycles with Ethers Employing TiO2/N-Hydroxyphthalimide System under Visible Light
Abstract
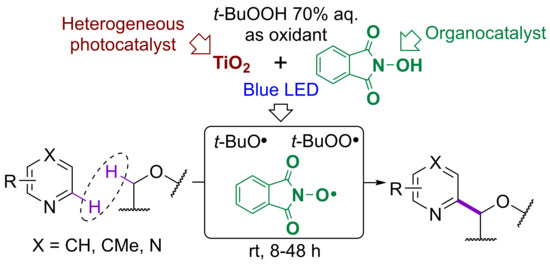
:1. Introduction
2. Results and Discussion
2.1. Optimization of Photocatalytic System Composition
2.2. Application of the Designed Photocatalytic NHPI/TiO2 System to the Minisci Reaction
3. Materials and Methods
3.1. General
- Experimental details for Table 1
- Experimental details for Table 2
- Experimental details for Scheme 4
3.2. Characterization Data of the Cross-Dehydrogenative C–C Coupling Products
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Friedmann, D.; Hakki, A.; Kim, H.; Choi, W.; Bahnemann, D. Heterogeneous Photocatalytic Organic Synthesis: State-of-the-Art and Future Perspectives. Green Chem. 2016, 18, 5391–5411. [Google Scholar] [CrossRef] [Green Version]
- Xiao, J.; Liu, X.; Pan, L.; Shi, C.; Zhang, X.; Zou, J.-J. Heterogeneous Photocatalytic Organic Transformation Reactions Using Conjugated Polymers-Based Materials. ACS Catal. 2020, 10, 12256–12283. [Google Scholar] [CrossRef]
- Kohtani, S.; Kawashima, A.; Miyabe, H. Stereoselective Organic Reactions in Heterogeneous Semiconductor Photocatalysis. Front. Chem. 2019, 7, 630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verma, S.K.; Verma, R.; Girish, Y.R.; Xue, F.; Yan, L.; Verma, S.; Singh, M.; Vaishnav, Y.; Shaik, A.B.; Bhandare, R.R.; et al. Heterogeneous Graphitic Carbon Nitrides in Visible-Light-Initiated Organic Transformations. Green Chem. 2022, 24, 438–479. [Google Scholar] [CrossRef]
- Chen, J.; Cen, J.; Xu, X.; Li, X. The Application of Heterogeneous Visible Light Photocatalysts in Organic Synthesis. Catal. Sci. Technol. 2016, 6, 349–362. [Google Scholar] [CrossRef]
- Li, F.; Cheng, L.; Fan, J.; Xiang, Q. Steering the Behavior of Photogenerated Carriers in Semiconductor Photocatalysts: A New Insight and Perspective. J. Mater. Chem. A 2021, 9, 23765–23782. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Chen, Z.; Hu, J.; Li, S.; Wang, Z.; Liu, J.; Wang, X. Semiconductor Heterojunction Photocatalysts: Design, Construction, and Photocatalytic Performances. Chem. Soc. Rev. 2014, 43, 5234. [Google Scholar] [CrossRef] [PubMed]
- Miyauchi, M.; Irie, H.; Liu, M.; Qiu, X.; Yu, H.; Sunada, K.; Hashimoto, K. Visible-Light-Sensitive Photocatalysts: Nanocluster-Grafted Titanium Dioxide for Indoor Environmental Remediation. J. Phys. Chem. Lett. 2016, 7, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.N.; Haider, W. Heterogeneous Photocatalysis and Its Potential Applications in Water and Wastewater Treatment: A Review. Nanotechnology 2018, 29, 342001. [Google Scholar] [CrossRef] [Green Version]
- Thambiliyagodage, C. Activity Enhanced TiO2 Nanomaterials for Photodegradation of Dyes—A Review. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100592. [Google Scholar] [CrossRef]
- Marchuk, M.V.; Asanov, I.P.; Panafidin, M.A.; Vorotnikov, Y.A.; Shestopalov, M.A. Nano TiO2 and Molybdenum/Tungsten Iodide Octahedral Clusters: Synergism in UV/Visible-Light Driven Degradation of Organic Pollutants. Nanomaterials 2022, 12, 4282. [Google Scholar] [CrossRef]
- Stavitskaya, A.; Glotov, A.; Pouresmaeil, F.; Potapenko, K.; Sitmukhanova, E.; Mazurova, K.; Ivanov, E.; Kozlova, E.; Vinokurov, V.; Lvov, Y. CdS Quantum Dots in Hierarchical Mesoporous Silica Templated on Clay Nanotubes: Implications for Photocatalytic Hydrogen Production. ACS Appl. Nano Mater. 2022, 5, 605–614. [Google Scholar] [CrossRef]
- Kurenkova, A.Y.; Medvedeva, T.B.; Gromov, N.V.; Bukhtiyarov, A.V.; Gerasimov, E.Y.; Cherepanova, S.V.; Kozlova, E.A. Sustainable Hydrogen Production from Starch Aqueous Suspensions over a Cd0.7Zn0.3S-Based Photocatalyst. Catalysts 2021, 11, 870. [Google Scholar] [CrossRef]
- Kozlova, E.A.; Lyulyukin, M.N.; Kozlov, D.V.; Parmon, V.N. Semiconductor Photocatalysts and Mechanisms of Carbon Dioxide Reduction and Nitrogen Fixation under UV and Visible Light. Russ. Chem. Rev. 2021, 90, 1520–1543. [Google Scholar] [CrossRef]
- Wang, J.; Guo, R.; Bi, Z.; Chen, X.; Hu, X.; Pan, W. A Review on TiO2−x-Based Materials for Photocatalytic CO2 Reduction. Nanoscale 2022, 14, 11512–11528. [Google Scholar] [CrossRef]
- Shtyka, O.; Shatsila, V.; Ciesielski, R.; Kedziora, A.; Maniukiewicz, W.; Dubkov, S.; Gromov, D.; Tarasov, A.; Rogowski, J.; Stadnichenko, A.; et al. Adsorption and Photocatalytic Reduction of Carbon Dioxide on TiO2. Catalysts 2020, 11, 47. [Google Scholar] [CrossRef]
- Eidsvåg, H.; Bentouba, S.; Vajeeston, P.; Yohi, S.; Velauthapillai, D. TiO2 as a Photocatalyst for Water Splitting—An Experimental and Theoretical Review. Molecules 2021, 26, 1687. [Google Scholar] [CrossRef]
- Bian, Y.; Gu, Y.; Zhang, X.; Chen, H.; Li, Z. Engineering BiOBrxI1−x Solid Solutions with Enhanced Singlet Oxygen Production for Photocatalytic Benzylic C-H Bond Activation Mediated by N-Hydroxyl Compounds. Chin. Chem. Lett. 2021, 32, 2837–2840. [Google Scholar] [CrossRef]
- Shi, G.; Xu, S.; Bao, Y.; Xu, J.; Liang, Y. Selective Aerobic Oxidation of Toluene to Benzaldehyde on Immobilized CoOx on SiO2 Catalyst in the Presence of N-Hydroxyphthalimide and Hexafluoropropan-2-Ol. Catal. Commun. 2019, 123, 73–78. [Google Scholar] [CrossRef]
- Krylov, I.B.; Lopat’eva, E.R.; Subbotina, I.R.; Nikishin, G.I.; Yu, B.; Terent’ev, A.O. Mixed Hetero-/Homogeneous TiO2/N-Hydroxyimide Photocatalysis in Visible-Light-Induced Controllable Benzylic Oxidation by Molecular Oxygen. Chin. J. Catal. 2021, 42, 1700–1711. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Antonietti, M. Polymeric Graphitic Carbon Nitride as a Heterogeneous Organocatalyst: From Photochemistry to Multipurpose Catalysis to Sustainable Chemistry. Angew. Chem. Int. Ed. 2012, 51, 68–89. [Google Scholar] [CrossRef] [PubMed]
- Hao, H.; Shi, J.-L.; Xu, H.; Li, X.; Lang, X. N-Hydroxyphthalimide-TiO2 Complex Visible Light Photocatalysis. Appl. Catal. B: Environ. 2019, 246, 149–155. [Google Scholar] [CrossRef]
- Li, H.; Li, X.; Lang, X. Extending the π-Conjugated Molecules on TiO2 for the Selective Photocatalytic Aerobic Oxidation of Sulfides Triggered by Visible Light. Sustain. Energy Fuels 2021, 5, 2127–2135. [Google Scholar] [CrossRef]
- Bhat, V.T.; Duspara, P.A.; Seo, S.; Abu Bakar, N.S.B.; Greaney, M.F. Visible Light Promoted Thiol-Ene Reactions Using Titanium Dioxide. Chem. Commun. 2015, 51, 4383–4385. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; Jeong, J.; Fujita, K.; Yamamoto, A.; Yoshida, H. Anti-Markovnikov Hydroamination of Alkenes with Aqueous Ammonia by Metal-Loaded Titanium Oxide Photocatalyst. J. Am. Chem. Soc. 2020, 142, 12708–12714. [Google Scholar] [CrossRef]
- Manley, D.W.; Walton, J.C. A Clean and Selective Radical Homocoupling Employing Carboxylic Acids with Titania Photoredox Catalysis. Org. Lett. 2014, 16, 5394–5397. [Google Scholar] [CrossRef] [Green Version]
- Manley, D.W.; McBurney, R.T.; Miller, P.; Walton, J.C.; Mills, A.; O’Rourke, C. Titania-Promoted Carboxylic Acid Alkylations of Alkenes and Cascade Addition–Cyclizations. J. Org. Chem. 2014, 79, 1386–1398. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Q.; Nocera, D.G. Photocatalytic Hydromethylation and Hydroalkylation of Olefins Enabled by Titanium Dioxide Mediated Decarboxylation. J. Am. Chem. Soc. 2020, 142, 17913–17918. [Google Scholar] [CrossRef]
- Savateev, A.; Ghosh, I.; König, B.; Antonietti, M. Photoredox Catalytic Organic Transformations Using Heterogeneous Carbon Nitrides. Angew. Chem. Int. Ed. 2018, 57, 15936–15947. [Google Scholar] [CrossRef]
- Bianchi, P.; Williams, J.D.; Kappe, C.O. Continuous Flow Processing of Bismuth-Photocatalyzed Atom Transfer Radical Addition Reactions Using an Oscillatory Flow Reactor. Green Chem. 2021, 23, 2685–2693. [Google Scholar] [CrossRef]
- Ghosh, I.; Khamrai, J.; Savateev, A.; Shlapakov, N.; Antonietti, M.; König, B. Organic Semiconductor Photocatalyst Can Bifunctionalize Arenes and Heteroarenes. Science 2019, 365, 360–366. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Su, P.; Abdolmohammadi, S.; Vessally, E. A Walk around the Application of Nanocatalysts for Cross-Dehydrogenative Coupling of C–H Bonds. RSC Adv. 2019, 9, 41684–41702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buglioni, L.; Riente, P.; Palomares, E.; Pericàs, M.A. Visible-Light-Promoted Arylation Reactions Photocatalyzed by Bismuth(III) Oxide: Visible-Light-Promoted Arylation Reactions Photocatalyzed by Bismuth(III) Oxide. Eur. J. Org. Chem. 2017, 2017, 6986–6990. [Google Scholar] [CrossRef]
- Franchi, D.; Amara, Z. Applications of Sensitized Semiconductors as Heterogeneous Visible-Light Photocatalysts in Organic Synthesis. ACS Sustain. Chem. Eng. 2020, 8, 15405–15429. [Google Scholar] [CrossRef]
- Peiris, S.; Silva, H.B.; Ranasinghe, K.N.; Bandara, S.V.; Perera, I.R. Recent Development and Future Prospects of TiO2 Photocatalysis. J. Chin. Chem. Soc. 2021, 68, 738–769. [Google Scholar] [CrossRef]
- Arora, I.; Chawla, H.; Chandra, A.; Sagadevan, S.; Garg, S. Advances in the Strategies for Enhancing the Photocatalytic Activity of TiO2: Conversion from UV-Light Active to Visible-Light Active Photocatalyst. Inorg. Chem. Commun. 2022, 143, 109700. [Google Scholar] [CrossRef]
- Basavarajappa, P.S.; Patil, S.B.; Ganganagappa, N.; Reddy, K.R.; Raghu, A.V.; Reddy, C.V. Recent Progress in Metal-Doped TiO2, Non-Metal Doped/Codoped TiO2 and TiO2 Nanostructured Hybrids for Enhanced Photocatalysis. Int. J. Hydrogen Energy 2020, 45, 7764–7778. [Google Scholar] [CrossRef]
- Zani, L.; Melchionna, M.; Montini, T.; Fornasiero, P. Design of Dye-Sensitized TiO2 Materials for Photocatalytic Hydrogen Production: Light and Shadow. J. Phys. Energy 2021, 3, 031001. [Google Scholar] [CrossRef]
- Choi, S.K.; Yang, H.S.; Kim, J.H.; Park, H. Organic Dye-Sensitized TiO2 as a Versatile Photocatalyst for Solar Hydrogen and Environmental Remediation. Appl. Catal. B Environ. 2012, 121–122, 206–213. [Google Scholar] [CrossRef]
- Gorduk, S.; Avciata, O.; Avciata, U. Hydrothermal in Situ Preparation of Phthalocyanine–TiO2 Nanocomposites for Photocatalytic Activity under Visible Light Irradiation. Res. Chem. Intermed. 2021, 47, 615–635. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, X.; Li, J.; Zhang, X. Enhanced Photocatalytic Activity in Metal Phthalocyanine-Sensitized TiO2 Nanorods. Res. Chem. Intermed. 2021, 47, 1519–1533. [Google Scholar] [CrossRef]
- Yang, L.; Li, L.; Li, L.; Liu, C.; Li, J.; Lai, B.; Li, N. N/Fe/Zn Co-Doped TiO2 Loaded on Basalt Fiber with Enhanced Photocatalytic Activity for Organic Pollutant Degradation. RSC Adv. 2021, 11, 4942–4951. [Google Scholar] [CrossRef] [PubMed]
- Wafi, A.; Szabó-Bárdos, E.; Horváth, O.; Makó, É.; Jakab, M.; Zsirka, B. Coumarin-Based Quantification of Hydroxyl Radicals and Other Reactive Species Generated on Excited Nitrogen-Doped TiO2. J. Photochem. Photobiol. A Chem. 2021, 404, 112913. [Google Scholar] [CrossRef]
- Gui, Q.-W.; Teng, F.; Yu, P.; Wu, Y.-F.; Nong, Z.-B.; Yang, L.-X.; Chen, X.; Yang, T.-B.; He, W.-M. Visible Light-Induced Z-Scheme V2O5/g-C3N4 Heterojunction Catalyzed Cascade Reaction of Unactivated Alkenes. Chin. J. Catal. 2023, 44, 111–116. [Google Scholar] [CrossRef]
- Khan, S.; Sadiq, M.; Kim, D.; Ullah, M.; Muhammad, N. TiO2 and Its Binary ZnTiO2 and Ternary CdZnTiO2 Nanocomposites as Efficient Photocatalysts for the Organic Dyes Degradation. Appl. Water Sci. 2022, 12, 118. [Google Scholar] [CrossRef]
- Wu, J.; Ou, P.; Lin, Y.; Tan, X.; Wei, F.; Mi, Y.; Liu, S.; Huang, K. Oxygen Vacancies and Bi2S3 Nanoparticles Co-Sensitized TiO2 Nanotube Arrays for Enhanced Photoelectrochemical Sensing of Chlorpyrifos. J. Electroanal. Chem. 2022, 911, 116220. [Google Scholar] [CrossRef]
- Liu, S.; Zou, Q.; Ma, Y.; Chi, D.; Chen, R.; Fang, H.; Hu, W.; Zhang, K.; Chen, L.-F. Metal-Organic Frameworks Derived TiO2/Carbon Nitride Heterojunction Photocatalyst with Efficient Catalytic Performance under Visible Light. Inorg. Chim. Acta 2022, 536, 120918. [Google Scholar] [CrossRef]
- Shawky, A.; Alahmadi, N.; Mohamed, R.M.; Zaki, Z.I. Bi2S3-Sensitized TiO2 Nanostructures Prepared by Solution Process for Highly Efficient Photoreduction of Hexavalent Chromium Ions in Water under Visible Light. Opt. Mater. 2022, 124, 111964. [Google Scholar] [CrossRef]
- Cipagauta-Díaz, S.; Estrella-González, A.; Navarrete-Magaña, M.; Gómez, R. N Doped -TiO2 Coupled to BiVO4 with High Performance in Photodegradation of Ofloxacin Antibiotic and Rhodamine B Dye under Visible Light. Catal. Today 2022, 394–396, 445–457. [Google Scholar] [CrossRef]
- Rajh, T.; Nedeljkovic, J.M.; Chen, L.X.; Poluektov, O.; Thurnauer, M.C. Improving Optical and Charge Separation Properties of Nanocrystalline TiO2 by Surface Modification with Vitamin C. J. Phys. Chem. B 1999, 103, 3515–3519. [Google Scholar] [CrossRef]
- Sredojević, D.N.; Kovač, T.; Džunuzović, E.; Ðorđević, V.; Grgur, B.N.; Nedeljković, J.M. Surface-Modified TiO2 Powders with Phenol Derivatives: A Comparative DFT and Experimental Study. Chem. Phys. Lett. 2017, 686, 167–172. [Google Scholar] [CrossRef]
- Zhang, T.; Wojtal, P.; Rubel, O.; Zhitomirsky, I. Density Functional Theory and Experimental Studies of Caffeic Acid Adsorption on Zinc Oxide and Titanium Dioxide Nanoparticles. RSC Adv. 2015, 5, 106877–106885. [Google Scholar] [CrossRef]
- Higashimoto, S.; Nishi, T.; Yasukawa, M.; Azuma, M.; Sakata, Y.; Kobayashi, H. Photocatalysis of Titanium Dioxide Modified by Catechol-Type Interfacial Surface Complexes (ISC) with Different Substituted Groups. J. Catal. 2015, 329, 286–290. [Google Scholar] [CrossRef]
- Moongraksathum, B.; Hsu, P.-T.; Chen, Y.-W. Photocatalytic Activity of Ascorbic Acid-Modified TiO2 Sol Prepared by the Peroxo Sol–Gel Method. J. Sol-Gel Sci. Technol. 2016, 78, 647–659. [Google Scholar] [CrossRef]
- Fujisawa, J.; Matsumura, S.; Hanaya, M. A Single Ti-O-C Linkage Induces Interfacial Charge-Transfer Transitions between TiO2 and a π-Conjugated Molecule. Chem. Phys. Lett. 2016, 657, 172–176. [Google Scholar] [CrossRef]
- Bui, H.T.; Park, H.Y.; Alvarez, P.J.J.; Lee, J.; Kim, W.; Kim, E.-J. Visible-Light Activation of a Dissolved Organic Matter–TiO2 Complex Mediated via Ligand-to-Metal Charge Transfer. Environ. Sci. Technol. 2022, 56, 10829–10837. [Google Scholar] [CrossRef] [PubMed]
- Terent’ev, A.O.; Krylov, I.B.; Sharipov, M.Y.; Kazanskaya, Z.M.; Nikishin, G.I. Generation and Cross-Coupling of Benzyl and Phthalimide-N-Oxyl Radicals in a Cerium(IV) Ammonium Nitrate/N-Hydroxyphthalimide/ArCH2R System. Tetrahedron 2012, 68, 10263–10271. [Google Scholar] [CrossRef]
- Terent’ev, A.O.; Krylov, I.B.; Timofeev, V.P.; Starikova, Z.A.; Merkulova, V.M.; Ilovaisky, A.I.; Nikishin, G.I. Oxidative C-O Cross-Coupling of 1,3-Dicarbonyl Compounds and Their Heteroanalogues with N -Substituted Hydroxamic Acids and N -Hydroxyimides. Adv. Synth. Catal. 2013, 355, 2375–2390. [Google Scholar] [CrossRef]
- Krylov, I.B.; Lopat’eva, E.R.; Budnikov, A.S.; Nikishin, G.I.; Terent’ev, A.O. Metal-Free Cross-Dehydrogenative C–O Coupling of Carbonyl Compounds with N -Hydroxyimides: Unexpected Selective Behavior of Highly Reactive Free Radicals at an Elevated Temperature. J. Org. Chem. 2020, 85, 1935–1947. [Google Scholar] [CrossRef] [PubMed]
- Kammer, L.; Rahman, A.; Opatz, T. A Visible Light-Driven Minisci-Type Reaction with N-Hydroxyphthalimide Esters. Molecules 2018, 23, 764. [Google Scholar] [CrossRef]
- Fiorati, A.; Gambarotti, C.; Melone, L.; Pastori, N.; Punta, C.; Raffaini, G.; Truscello, A. Recent Advances in Photocatalytic Minisci Reaction: An Eco-Friendly Functionalization of Biologically Relevant Heteroarenes. In Green Synthetic Approaches for Biologically Relevant Heterocycles; Elsevier: Amsterdam, The Netherlands, 2021; pp. 189–206. ISBN 978-0-12-820586-0. [Google Scholar]
- Proctor, R.S.J.; Phipps, R.J. Recent Advances in Minisci-Type Reactions. Angew. Chem. Int. Ed. 2019, 58, 13666–13699. [Google Scholar] [CrossRef] [PubMed]
- Minisci, F.; Bernardi, R.; Bertini, F.; Galli, R.; Perchinummo, M. Nucleophilic Character of Alkyl Radicals—VI. Tetrahedron 1971, 27, 3575–3579. [Google Scholar] [CrossRef]
- Duncton, M.A.J. Minisci Reactions: Versatile CH-Functionalizations for Medicinal Chemists. Med. Chem. Commun. 2011, 2, 1135. [Google Scholar] [CrossRef]
- Fontana, F.; Minisci, F.; Yong, M.Y.; Lihua, Z. A Novel and Mild Source of Carbon-Centered Radicals by Iodosobenzene Diacetate (IBDA) and Sodium Azide from Alcohols, Ethers, Aldehydes, Amides and Alkyl Iodides. Tetrahedron Lett. 1993, 34, 2517–2520. [Google Scholar] [CrossRef]
- Devari, S.; Shah, B.A. Visible Light-Promoted C–H Functionalization of Ethers and Electron-Deficient Arenes. Chem. Commun. 2016, 52, 1490–1493. [Google Scholar] [CrossRef] [PubMed]
- Tauber, J.; Imbri, D.; Opatz, T. Radical Addition to Iminium Ions and Cationic Heterocycles. Molecules 2014, 19, 16190–16222. [Google Scholar] [CrossRef]
- McCallum, T.; Jouanno, L.-A.; Cannillo, A.; Barriault, L. Persulfate-Enabled Direct C–H Alkylation of Heteroarenes with Unactivated Ethers. Synlett 2016, 27, 1282–1286. [Google Scholar] [CrossRef] [Green Version]
- Chupakhin, O.N.; Charushin, V.N. Recent Advances in the Field of Nucleophilic Aromatic Substitution of Hydrogen. Tetrahedron Lett. 2016, 57, 2665–2672. [Google Scholar] [CrossRef]
- Charushin, V.N.; Chupakhin, O.N. Nucleophilic C—H Functionalization of Arenes: A Contribution to Green Chemistry. Russ. Chem. Bull. 2019, 68, 453–471. [Google Scholar] [CrossRef]
- Akulov, A.A.; Varaksin, M.V.; Mampuys, P.; Charushin, V.N.; Chupakhin, O.N.; Maes, B.U.W. C(Sp2)–H Functionalization in Non-Aromatic Azomethine-Based Heterocycles. Org. Biomol. Chem. 2021, 19, 297–312. [Google Scholar] [CrossRef]
- Liu, X.-L.; Jiang, L.-B.; Luo, M.-P.; Ren, Z.; Wang, S.-G. Recent Advances in Catalytic Enantioselective Direct C–H Bond Functionalization of Electron-Deficient N-Containing Heteroarenes. Org. Chem. Front. 2022, 9, 265–280. [Google Scholar] [CrossRef]
- Jeong, J.; Patel, P.; Hwang, H.; Chang, S. Rhodium(III)-Catalyzed C–C Bond Formation of Quinoline N-Oxides at the C-8 Position under Mild Conditions. Org. Lett. 2014, 16, 4598–4601. [Google Scholar] [CrossRef]
- Sharma, U.; Park, Y.; Chang, S. Rh(III)-Catalyzed Traceless Coupling of Quinoline N-Oxides with Internal Diarylalkynes. J. Org. Chem. 2014, 79, 9899–9906. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Qi, Z.; Li, X. Rhodium(III)-Catalyzed C-C and C-O Coupling of Quinoline N-Oxides with Alkynes: Combination of C-H Activation with O-Atom Transfer. Angew. Chem. 2014, 126, 10970–10974. [Google Scholar] [CrossRef]
- Wu, Z.; Pi, C.; Cui, X.; Bai, J.; Wu, Y. Direct C-2 Alkylation of Quinoline N-Oxides with Ethers via Palladium-Catalyzed Dehydrogenative Cross-Coupling Reaction. Adv. Synth. Catal. 2013, 355, 1971–1976. [Google Scholar] [CrossRef]
- Huang, C.; Wang, J.-H.; Qiao, J.; Fan, X.-W.; Chen, B.; Tung, C.-H.; Wu, L.-Z. Direct Arylation of Unactivated Alkanes with Heteroarenes by Visible-Light Catalysis. J. Org. Chem. 2019, 84, 12904–12912. [Google Scholar] [CrossRef] [PubMed]
- Quattrini, M.C.; Fujii, S.; Yamada, K.; Fukuyama, T.; Ravelli, D.; Fagnoni, M.; Ryu, I. Versatile Cross-Dehydrogenative Coupling of Heteroaromatics and Hydrogen Donors via Decatungstate Photocatalysis. Chem. Commun. 2017, 53, 2335–2338. [Google Scholar] [CrossRef] [PubMed]
- Bhakat, M.; Khatua, B.; Guin, J. Photocatalytic Aerobic Coupling of Azaarenes and Alkanes via Nontraditional Cl • Generation. Org. Lett. 2022, 24, 5276–5280. [Google Scholar] [CrossRef]
- Jung, S.; Lee, H.; Moon, Y.; Jung, H.-Y.; Hong, S. Site-Selective C–H Acylation of Pyridinium Derivatives by Photoredox Catalysis. ACS Catal. 2019, 9, 9891–9896. [Google Scholar] [CrossRef]
- Tian, H.; Yang, H.; Tian, C.; An, G.; Li, G. Cross-Dehydrogenative Coupling of Strong C(Sp3)–H with N -Heteroarenes through Visible-Light-Induced Energy Transfer. Org. Lett. 2020, 22, 7709–7715. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-Y.; Li, J.; Liu, W.; Li, C.-J. Diacetyl as a “Traceless” Visible Light Photosensitizer in Metal-Free Cross-Dehydrogenative Coupling Reactions. Chem. Sci. 2019, 10, 5018–5024. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Song, X.; Qi, M.-F.; Sun, B. Weak Brønsted Base-Promoted Photoredox Catalysis for C-H Alkylation of Heteroarenes Mediated by Triplet Excited Diaryl Ketone. Tetrahedron Lett. 2022, 99, 153846. [Google Scholar] [CrossRef]
- Bhakat, M.; Biswas, P.; Dey, J.; Guin, J. Heteroarylation of Ethers, Amides, and Alcohols with Light and O2. Org. Lett. 2021, 23, 6886–6890. [Google Scholar] [CrossRef]
- Zhao, H.; Li, Z.; Jin, J. Green Oxidant H2O2 as a Hydrogen Atom Transfer Reagent for Visible Light-Mediated Minisci Reaction. New J. Chem. 2019, 43, 12533–12537. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, G.; Li, Y.; Wang, S.; Lei, A. The Synergistic Effect of Self-Assembly and Visible-Light Induced the Oxidative C–H Acylation of N-Heterocyclic Aromatic Compounds with Aldehydes. Chem. Commun. 2018, 54, 5744–5747. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, C.; Guo, S.; Wang, W.; Zhang, Y. PIFA-Mediated Cross-Dehydrogenative Coupling of N -Heteroarenes with Cyclic Ethers: Ethanol as an Efficient Promoter. Eur. J. Org. Chem. 2021, 2021, 411–421. [Google Scholar] [CrossRef]
- Utepova, I.A.; Trestsova, M.A.; Chupakhin, O.N.; Charushin, V.N.; Rempel, A.A. Aerobic Oxidative C–H/C–H Coupling of Azaaromatics with Indoles and Pyrroles in the Presence of TiO2 as a Photocatalyst. Green Chem. 2015, 17, 4401–4410. [Google Scholar] [CrossRef]
- Li, Z.; Wu, L.; Guo, J.; Shao, Y.; Song, Y.; Ding, Y.; Zhu, L.; Yao, X. Light-Promoted Minisci Coupling Reaction of Ethers and Aza Aromatics Catalyzed by Au/TiO2 Heterogeneous Photocatalyst. ChemCatChem 2021, 13, 3671–3678. [Google Scholar] [CrossRef]
- Qiao, J.; Song, Z.; Huang, C.; Ci, R.; Liu, Z.; Chen, B.; Tung, C.; Wu, L. Direct, Site-Selective and Redox-Neutral A-C−H Bond Functionalization of Tetrahydrofurans via Quantum Dots Photocatalysis. Angew. Chem. Int. Ed. 2021, 60, 27201–27205. [Google Scholar] [CrossRef] [PubMed]
- Vijeta, A.; Reisner, E. Carbon Nitride as a Heterogeneous Visible-Light Photocatalyst for the Minisci Reaction and Coupling to H2 Production. Chem. Commun. 2019, 55, 14007–14010. [Google Scholar] [CrossRef] [PubMed]
- Boyd, A.A.; Flaud, P.-M.; Daugey, N.; Lesclaux, R. Rate Constants for RO2 + HO2 Reactions Measured under a Large Excess of HO2. J. Phys. Chem. A 2003, 107, 818–821. [Google Scholar] [CrossRef]
- Opeida, I.A.; Sheparovych, R.B. Inhibition by Hydrogen Peroxide in the Radical Chain Oxidation of Hydrocarbons by Molecular Oxygen. Theor. Exp. Chem. 2019, 55, 36–42. [Google Scholar] [CrossRef]
- Opeida, I.A.; Sheparovych, R.B.; Hrynda, Y.M.; Khavunko, O.Y.; Kompanets, M.O.; Shendryk, A.N. Kinetics of Oxidation of Benzyl Alcohols with Molecular Oxygen Catalyzed by N-hydroxyphthalimide: Role of Hydroperoxyl Radicals. Int. J. Chem. Kinet. 2019, 51, 679–688. [Google Scholar] [CrossRef]
- Patil, S.V.; Tanko, J.M. Radical Additions of Acyclic and Cyclic Ethers to Alkenes via an Allyl Transfer Reaction Involving Phthalimido-N-Oxyl Radical. Tetrahedron 2016, 72, 7849–7858. [Google Scholar] [CrossRef]
- Terent’ev, A.O.; Platonov, M.M.; Krylov, I.B.; Chernyshev, V.V.; Nikishin, G.I. Synthesis of 1-Hydroperoxy-1′-Alkoxyperoxides by the Iodine-Catalyzed Reactions of Geminal Bishydroperoxides with Acetals or Enol Ethers. Org. Biomol. Chem. 2008, 6, 4435. [Google Scholar] [CrossRef] [PubMed]
- Terent’ev, A.O.; Sharipov, M.Y.; Krylov, I.B.; Gaidarenko, D.V.; Nikishin, G.I. Manganese Triacetate as an Efficient Catalyst for Bisperoxidation of Styrenes. Org. Biomol. Chem. 2015, 13, 1439–1445. [Google Scholar] [CrossRef] [PubMed]
- Terent’ev, A.; Borisov, D.; Semenov, V.; Chernyshev, V.; Dembitsky, V.; Nikishin, G. Selective Synthesis of Unsymmetrical Peroxides: Transition-Metal-Catalyzed Oxidation of Malononitrile and Cyanoacetic Ester Derivatives by Tert-Butyl Hydroperoxide at the α-Position. Synthesis 2011, 2011, 2091–2100. [Google Scholar] [CrossRef]
- Pastori, N.; Gambarotti, C.; Punta, C. Recent Developments in Nucleophilic Radical Addition to Imines: The Key Role of Transition Metals and the New Porta Radical-Type Version of the Mannich and Strecker Reactions. MROC 2009, 6, 184–195. [Google Scholar] [CrossRef]
- Vil’, V.A.; Grishin, S.S.; Baberkina, E.P.; Kostyagina, V.A.; Kovalenko, A.E.; Terent’ev, A.O. Radical Addition of Tetrahydrofuran to Imines Assisted by Tert-Butyl Hydroperoxide. Tetrahedron Lett. 2020, 61, 152150. [Google Scholar] [CrossRef]
- Clerici, A.; Cannella, R.; Pastori, N.; Panzeri, W.; Porta, O. A Free Radical Mannich Type Reaction: Selective α-CH Aminomethylation of Ethers by Ti(III)/t-BuOOH System under Aqueous Acidic Conditions. Tetrahedron 2006, 62, 5986–5994. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, S.; Xu, G.; Li, M.; Tang, C.; Fan, W. Cu-Catalyzed Carbamoylation versus Amination of Quinoline N-Oxide with Formamides. Org. Biomol. Chem. 2019, 17, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Godugu, K.; Nallagondu, C.G.R. Solvent and Catalyst-free Synthesis of Imidazo[1,2-a]Pyridines by Grindstone Chemistry. J. Heterocycl. Chem. 2021, 58, 250–259. [Google Scholar] [CrossRef]
- Wen, J.; Li, X.; Li, H.; Ma, S.; He, K.; Xu, Y.; Fang, Y.; Liu, W.; Gao, Q. Enhanced Visible-Light H2 Evolution of g-C3N4 Photocatalysts via the Synergetic Effect of Amorphous NiS and Cheap Metal-Free Carbon Black Nanoparticles as Co-Catalysts. Appl. Surf. Sci. 2015, 358, 204–212. [Google Scholar] [CrossRef]
- Yao, S.; Xue, S.; Peng, S.; Jing, M.; Qian, X.; Shen, X.; Li, T.; Wang, Y. Synthesis of Graphitic Carbon Nitride at Different Thermal-Pyrolysis Temperature of Urea and It Application in Lithium–Sulfur Batteries. J. Mater. Sci. Mater. Electron. 2018, 29, 17921–17930. [Google Scholar] [CrossRef]
- Sankari Devi, E.; Alanthadka, A.; Tamilselvi, A.; Nagarajan, S.; Sridharan, V.; Maheswari, C.U. Metal-Free Oxidative Amidation of Aldehydes with Aminopyridines Employing Aqueous Hydrogen Peroxide. Org. Biomol. Chem. 2016, 14, 8228–8231. [Google Scholar] [CrossRef]





 | |||
| Run | Changes to the General Conditions | Conversion a 1a, % | Yield a 3aa, % |
|---|---|---|---|
| 1 | none | 53 | 45 |
| 2 | no TiO2 | 0 | 0 |
| 3 | no NHPI | 0 | 0 |
| 4 | no TBHP | 6 | 4 |
| 5 | TFA (1.5 mmol) added | 52 | 45 |
| 6 | H2O (0.5 mL) added | 23 | 9 |
| 7 | THF (12.5 mmol) | 38 | 36 |
| 8 | THF (50 mmol) | 32 | 27 |
| 9 | HFIP (1 mL) added | 18 | 16 |
| 10 | MeCN (1 mL) added | 17 | 16 |
| 11 | DCE (1 mL) added | 0 | 0 |
| 12 | H2O2 34% aq. b | 9 | 3 |
| 13 | m-CPBA 75% aq. b | 28 | 0 |
| 14 | PhCH(CH3)2OOH 80% | 15 | 15 |
| 15 | PhCH(CH3)2OOPhCH(CH3)2 98% b | 16 | 0 |
| 16 | BzOOBz 75% aq. (1 mmol) b | 59 | 44 |
| 17 | (NH4)2S2O8 b,c | 39 | 33 |
| 18 | Na2S2O8 b,c | 36 | 22 |
| 19 | K2S2O8 b,c | 44 | 39 |
| 20 | K2S2O8 b,c, 16 h, Argon atmosphere | 90 | 27 |
| 21 | Argon atmosphere | 44 | 39 |
 | ||||||
| Run | TiO2, mg | NHPI, mmol | TBHP, mmol | Time, h | Conversion a 1a, % | Yield a 3aa, % |
|---|---|---|---|---|---|---|
| 1 | 2.5 | 0.1 | 2 | 5 | 5 | 5 |
| 2 | 5 | 0.1 | 2 | 5 | 12 | 11 |
| 3 | 20 | 0.1 | 2 | 5 | 44 | 40 |
| 4 | 40 | 0.1 | 2 | 5 | 55 | 46 |
| 5 | 10 | 0.05 | 2 | 5 | 0 | 0 |
| 6 | 10 | 0.1 | 2 | 5 | 39 | 36 |
| 7 | 10 | 0.2 | 2 | 5 | 44 | 39 |
| 8 | 10 | 0.4 | 2 | 5 | 52 | 41 |
| 9 | 10 | 0.1 | 1 | 5 | 31 | 27 |
| 10 | 10 | 0.1 | 4 | 5 | 49 | 42 |
| 11 | 10 | 0.1 | 6 | 5 | 38 | 38 |
| 12 | 10 | 0.1 | 2 | 1 | 5 | 4 |
| 13 | 10 | 0.1 | 2 | 2 | 20 | 15 |
| 14 | 10 | 0.1 | 2 | 5 | 34 | 28 |
| 15 | 20 | 0.2 | 4 | 8 | 96 | 89 |
| 16 | - b | 0.2 | 4 | 8 | 9 | 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopat’eva, E.R.; Krylov, I.B.; Segida, O.O.; Merkulova, V.M.; Ilovaisky, A.I.; Terent’ev, A.O. Heterogeneous Photocatalysis as a Potent Tool for Organic Synthesis: Cross-Dehydrogenative C–C Coupling of N-Heterocycles with Ethers Employing TiO2/N-Hydroxyphthalimide System under Visible Light. Molecules 2023, 28, 934. https://doi.org/10.3390/molecules28030934
Lopat’eva ER, Krylov IB, Segida OO, Merkulova VM, Ilovaisky AI, Terent’ev AO. Heterogeneous Photocatalysis as a Potent Tool for Organic Synthesis: Cross-Dehydrogenative C–C Coupling of N-Heterocycles with Ethers Employing TiO2/N-Hydroxyphthalimide System under Visible Light. Molecules. 2023; 28(3):934. https://doi.org/10.3390/molecules28030934
Chicago/Turabian StyleLopat’eva, Elena R., Igor B. Krylov, Oleg O. Segida, Valentina M. Merkulova, Alexey I. Ilovaisky, and Alexander O. Terent’ev. 2023. "Heterogeneous Photocatalysis as a Potent Tool for Organic Synthesis: Cross-Dehydrogenative C–C Coupling of N-Heterocycles with Ethers Employing TiO2/N-Hydroxyphthalimide System under Visible Light" Molecules 28, no. 3: 934. https://doi.org/10.3390/molecules28030934