Rapid Identification and Analysis of Ochratoxin-A in Food and Agricultural Soil Samples Using a Novel Semi-Automated In-Syringe Based Fast Mycotoxin Extraction (FaMEx) Technique Coupled with UHPLC-MS/MS
Abstract
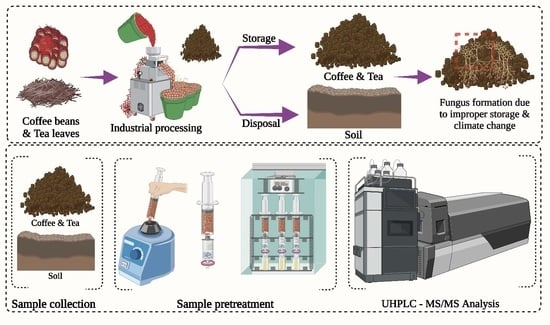
:1. Introduction
2. Results and Discussion
2.1. Optimization of the Extraction Process
2.1.1. Effect of Extraction Solvent
2.1.2. pH of the Extraction Medium
2.1.3. Effect of Extraction Solvent Volume
2.1.4. Effect of Extraction (Vortex) Time
2.2. Optimization of the Clean-Up Process
2.2.1. Effect of Sorbent and Sorbent Amount
2.2.2. Effect of Water Content
2.2.3. Optimization of Plunger Speed
2.3. Method Performances
2.3.1. Analytical Performances of the Developed Method
2.3.2. Matrix Effect
2.3.3. Real Sample Analysis
2.4. Comparison with Previously Reported Methods
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Instrument Conditions
3.2.1. UHPLC Conditions
3.2.2. Mass Spectrometer Conditions
3.2.3. Automated Plunger Device Set-Up for the Clean-Up Process
3.3. Semi-Automated FaMEx Procedure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El-Aty, A.M.A.; Choi, J.H.; Rahman, M.M.; Kim, S.W.; Tosun, A.; Shim, J.H. Residues and contaminants in tea and tea infusions: A review. Food Addit. Contam. Part A 2014, 31, 1794–1804. [Google Scholar] [CrossRef] [PubMed]
- Mogensen, J.M.; Varga, J.; Thrane, U.; Frisvad, J.C. Aspergillus acidus from Puerh tea and black tea does not produce ochratoxin A and fumonisin B2. Int. J. Food Microbiol. 2009, 132, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Monbaliu, S.; Wu, A.; Zhang, D.; van Peteghem, C.; de Saeger, S. Multimycotoxin UPLC- MS/MS for tea, herbal infusions and the derived drinkable products. J. Agric. Food Chem. 2010, 58, 12664–12671. [Google Scholar] [CrossRef] [PubMed]
- Bokhari, F.M.; Aly, M.M. Unexpected hazard due to Fumonisins contaminating herbal teas used traditionally by Saudi people. Afr. J. Microbiol. Res. 2013, 7, 35–40. [Google Scholar]
- Haas, D.; Pfeifer, B.; Reiterich, C.; Partenheimer, R.; Reck, B.; Buzina, W. Identification and quantification of fungi and mycotoxins from Pu-erh tea. Int. J. Food Microbiol. 2013, 166, 316–322. [Google Scholar] [CrossRef]
- Taniwaki, M.H.; Pitt, J.I.; Teixeira, A.A.; Iamanaka, B.T. The source of ochratoxin A in Brazilian coffee and its formation in relation to processing methods. Int. J. Food Microbiol. 2003, 82, 173–179. [Google Scholar] [CrossRef] [PubMed]
- The Commission of the European Communities. Commission regulation (EC) No. 1881/2006 of 19 December 2006. Setting maximum levels for certain contaminants in foodstuffs (Text with EEA relevance). Off. J. Eur. Comm. 2006, 364, 1881. [Google Scholar]
- Batista, L.R.; Chalfoun, S.M.; Silva, C.F.; Cirillo, M.; Varga, E.A.; Schwan, R.F. Ochratoxin A in coffee beans (Coffea arabica L.) processed by dry and wet methods. Food Control. 2009, 20, 784–790. [Google Scholar] [CrossRef]
- Vargas, E.A.; Santos, E.A.; Pittet, A.; da Rocha, A.P.P.; Diaz, G.J.; Gorni, R.; Koch, P.; Lombaert, G.A.; MacDonald, S.; Mallmann, C.A.; et al. Determination of ochratoxin A in green coffee by immunoaffinity column cleanup and liquid chromatography: Collaborative study. J. AOAC Int. 2005, 88, 773–779. [Google Scholar] [CrossRef]
- Vaclavik, L.; Vaclavikova, M.; Begley, T.H.; Krynitsky, A.J.; Rader, J.I. Determination of multiple mycotoxins in dietary supplements containing green coffee bean extracts using ultrahigh-performance liquid chromatography-tandem mass spectrometry (UHPLC-MS/MS). J. Agric. Food Chem. 2013, 61, 4822–4830. [Google Scholar] [CrossRef]
- Garcia-Moraleja, A.; Font, G.; Mañes, J.; Ferrer, E. Simultaneous determination of mycotoxin in commercial coffee. Food Control 2015, 57, 282–292. [Google Scholar] [CrossRef]
- Chisvert, S.; Cárdenas, S.; Lucena, R. Dispersive micro-solid phase extraction. TrAC Trends Anal. Chem. 2019, 112, 226–233. [Google Scholar] [CrossRef]
- Cina, M.; del Valle Ponce, M.; Martinez, L.D.; Cerutti, S. Development of a novel UHPLC-MS/MS method for the determination of ochratoxin A in tea. Heliyon 2021, 7, e06663. [Google Scholar] [CrossRef] [PubMed]
- Anastassiades, M.; Lehotay, S.J.; Štajnbaher, D.; Schenck, F.J. Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J. AOAC Int. 2003, 86, 412–431. [Google Scholar] [CrossRef] [PubMed]
- Lawal, A.; Wong, R.C.S.; Tan, G.H.; Abdulra’uf, L.B.; Alsharif, A.M.A. Recent modifications and validation of QuEChERS-dSPE coupled to LC-MS and GC-MS instruments for determination of pesticide agrochemical residues in fruits and vegetables. J. Chromatogr. Sci. 2018, 56, 656–669. [Google Scholar] [CrossRef]
- Tighrine, A.; Amir, Y.; Alfaro, P.; Mamou, M.; Nerin, C. Simultaneous extraction and analysis of preservatives and artificial sweeteners in juices by salting out liquid-liquid extraction method prior to ultra-high performance liquid chromatography. Food Chem. 2019, 277, 586–594. [Google Scholar] [CrossRef]
- Wei, Q.; Huang, C.; Lu, P.; Zhang, X.; Chen, Y. Combining magnetic MOFs as a highly adsorbent with homogeneous chemiluminescent immunosensor for rapid and ultrasensitive determination of Ochratoxin A. J. Hazard. Mater. 2023, 441, 129960. [Google Scholar] [CrossRef]
- Anli, E.; Alkis, I.M. Ochratoxin A and Brewing Technology: A Review. J. Inst. Brew. 2010, 116, 23–32. [Google Scholar] [CrossRef]
- Ponnusamy, V.K.; Mani, V.; Chen, S.-M.; Huang, W.-T.; Jen, J.-F. Rapid microwave assisted synthesis of graphene nanosheets polyethyleneimine gold nanoparticle composite and its application to the selective electrochemical determination of dopamine. Talanta 2014, 120, 148–157. [Google Scholar] [CrossRef]
- Hayward, D.G.; Wong, J.W.; Shi, F.; Zhang, K.; Lee, N.S.; Dibenedetto, A.L.; Hengel, M.J. Multiresidue Pesticide Analysis of Botanical Dietary Supplements Using Salt-out Acetonitrile Extraction, Solid-Phase Extraction Cleanup Column, and Gas Chromatography-Triple Quadrupole Mass Spectrometry. Anal. Chem. 2013, 85, 4686–4693. [Google Scholar] [CrossRef]
- Ye, Z.; Wang, X.; Fu, R.; Yan, H.; Han, S.; Gerelt, K.; Cui, P.; Chen, J.; Qi, K.; Zhou, Y. Determination of six groups of mycotoxins in Chinese dark tea and the associated risk assessment. Environ. Pollut. 2020, 261, 114180. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Jin, F.; Zhang, P.; Zhang, Y.; Wang, J.; Shao, H.; Jin, M.; Wang, S.; Zheng, L.; Wang, J. Simultaneous determination of perfluorinated compounds in edible oil by gel-permeation chromatography combined with dispersive solid-phase extraction and liquid chromatography-tandem mass spectrometry. J. Agric. Food Chem. 2015, 63, 8364–8371. [Google Scholar] [CrossRef] [PubMed]
- Moraleja, A.; Font, G.; JordiManes; Ferrer, E. Development of a new method for the simultaneous determination of 21 mycotoxins in coffee beverages by liquid chromatography-tandem mass spectrometry. Food Res. Int. 2015, 72, 247–255. [Google Scholar] [CrossRef]
- Chen, M.-J.; Liu, Y.-T.; Lin, C.-W.; Ponnusamy, V.K.; Jen, J.-F. Rapid determination of triclosan in personal care products using new in-tube based ultrasound-assisted salt-induced liquid–liquid microextraction coupled with high-performance liquid chromatography-ultraviolet detection. Anal. Chim. Acta 2013, 767, 81–87. [Google Scholar] [CrossRef]
- Tozlovanu, M.; Pfohl-Leszkowicz, A. Ochratoxin A in roasted coffee purchased in french super market. Transfer in coffee beverage: Comparison of several methods. Toxins 2010, 2, 1928–1949. [Google Scholar] [CrossRef]
- Graziani, G.; Santini, A.; Ferracane, R.; Ritieni, A. Microwave-assisted Extraction of Ochratoxin A from Roasted Coffee Beans: An Alternative Analytical Approach. J. Food Res. 2012, 1, 121. [Google Scholar] [CrossRef]
- TBessaire; Mujahid, C.; Mottier, P.; Desmarchelier, A. Multiple Mycotoxins Determination in Food by LC-MS/MS: An International Collaborative Study. Toxins 2019, 11, 658. [Google Scholar] [CrossRef]
- Nielsen, K.F.; Ngemela, A.F.; Jensen, L.B.; de Medeiros, L.S.; Rasmussen, P.H. UHPLC-MS/MS Determination of Ochratoxin A and Fumonisins in Coffee Using QuEChERS Extraction Combined with Mixed-Mode SPE Purification. J. Agric. Food Chem. 2015, 63, 1029–1034. [Google Scholar] [CrossRef]
- Pallares, N.; Font, G.; Manes, J.; Ferrer, E. Multimycotoxin LC-MS/MS analysis in tea beverages after dispersive liquid-liquid microextraction (DLLME). J. Agric. Food Chem. 2017, 65, 10282–10289. [Google Scholar] [CrossRef]
- Benites, A.J.; Fernandes, M.; Boleto, A.R.; Azevedo, S.; Silva, S.; Leitao, A.L. Occurrence of ochratoxin A in roasted coffee samples commercialized in Portugal. Food Control 2017, 73, 1223–1228. [Google Scholar] [CrossRef]
- Vanesa, D.; Ana, P. Occurrence of Ochratoxin A in coffee beans, ground roasted coffee, and soluble coffee and method validation. Food Control 2013, 30, 675–678. [Google Scholar] [CrossRef]






| Sample Type | Spiked Conc (ng/g) | Intraday Recovery (%) | RSD % | Interday Recovery (%) | RSD % |
|---|---|---|---|---|---|
| Coffee sample-1 | 0 | BQL | - | BQL | - |
| 1 | 82.48 | 3.06 | 88.05 | 6.31 | |
| 10 | 85.55 | 1.15 | 85.84 | 2.75 | |
| 25 | 90.37 | 7.34 | 94.95 | 6.81 | |
| Coffee sample-2 | 0 | BQL | - | BQL | - |
| 1 | 92.14 | 5.06 | 90.29 | 4.46 | |
| 10 | 87.48 | 2.74 | 84.14 | 1.86 | |
| 25 | 82.82 | 3.34 | 91.56 | 5.26 | |
| Coffee sample-3 | 0 | BQL | - | BQL | - |
| 1 | 83.84 | 1.67 | 85.87 | 6.82 | |
| 10 | 82.50 | 1.11 | 98.85 | 2.89 | |
| 25 | 95.32 | 4.77 | 91.45 | 2.35 | |
| Tea sample-1 | 0 | BQL | - | BQL | - |
| 1 | 86.02 | 5.75 | 88.64 | 2.06 | |
| 10 | 100.59 | 4.18 | 85.24 | 4.91 | |
| 25 | 90.24 | 2.48 | 86 | 2.52 | |
| Tea sample-2 | 0 | BQL | - | BQL | - |
| 1 | 85.86 | 3.60 | 89.22 | 3.39 | |
| 10 | 87.58 | 1.07 | 92.88 | 5.46 | |
| 25 | 86.29 | 1.59 | 90.60 | 6.96 | |
| Tea sample-3 | 0 | BQL | - | BQL | - |
| 1 | 84.73 | 3.58 | 98.57 | 2.81 | |
| 10 | 91.54 | 1.32 | 86.59 | 6.63 | |
| 25 | 88.27 | 1.22 | 86.54 | 2.71 | |
| Soil sample-1 | 0 | BQL | - | BQL | - |
| 1 | 86.92 | 3.41 | 90.42 | 5.36 | |
| 10 | 90.76 | 5.26 | 85.06 | 3.67 | |
| 25 | 92.42 | 4.78 | 97.81 | 6.42 | |
| Soil sample-2 | 0 | BQL | - | BQL | - |
| 1 | 89.53 | 3.80 | 95.89 | 3.45 | |
| 10 | 93.68 | 4.96 | 87.66 | 6.41 | |
| 25 | 87.27 | 5.88 | 88.44 | 2.90 | |
| Soil sample-3 | 0 | BQL | - | BQL | - |
| 1 | 89.46 | 2.74 | 92.48 | 3.69 | |
| 10 | 87.67 | 6.45 | 97.52 | 5.63 | |
| 25 | 97.51 | 5.29 | 104.61 | 6.05 |
| Analyte Studied | Extraction Method | Analytical Technique | Solvent Volume (mL) | Extraction Time (min) | Sample Amount | LOD (ng/g) | Ref. |
|---|---|---|---|---|---|---|---|
| OTA | SPE | LC-FD | 100 | >60 | 20 # | 0.4 | [25] |
| OTA | MWE | LC-FD | 50 | 20 | 2.5 # | 5 | [26] |
| OTA, AFs, DON, ZEL, FB1, FB2, T-2 & HT-2 | QuEChERS | LC-MSMS | 10 | 20 | 5 # | - | [27] |
| AFs & OTA | QuEChERS-SPE | LC-MSMS | 40 | 35 | 5 # | 0.5 | [28] |
| OTA | DLLME–SFO | LC-MSMS | - | 20 | 5 * | 0.5 | [5] |
| AFs, DONs, NIV, T-2, HT-2, ZEA, OTA & ENNs | DLLME | LC-MSMS | 4.14 | 25 | 5 # | <0.1 | [29] |
| OTA | SPE | LC-FD | 150 | 40 | 15 # | 0.266 | [30] |
| OTA | SPE | LC-FD | 100 | - | 5 # | 0.02 | [31] |
| OTA | SPE | LC-FD | 40 | >60 | 10 # | <0.01 | [9] |
| OTA | SPE | LC-MSMS | 2.5 | 6 | 0.25 # | 0.3 | * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prakasham, K.; Gurrani, S.; Shiea, J.-T.; Wu, M.-T.; Wu, C.-F.; Ku, Y.-J.; Tsai, T.-Y.; Hua, H.-T.; Lin, Y.-J.; Huang, P.-C.; et al. Rapid Identification and Analysis of Ochratoxin-A in Food and Agricultural Soil Samples Using a Novel Semi-Automated In-Syringe Based Fast Mycotoxin Extraction (FaMEx) Technique Coupled with UHPLC-MS/MS. Molecules 2023, 28, 1442. https://doi.org/10.3390/molecules28031442
Prakasham K, Gurrani S, Shiea J-T, Wu M-T, Wu C-F, Ku Y-J, Tsai T-Y, Hua H-T, Lin Y-J, Huang P-C, et al. Rapid Identification and Analysis of Ochratoxin-A in Food and Agricultural Soil Samples Using a Novel Semi-Automated In-Syringe Based Fast Mycotoxin Extraction (FaMEx) Technique Coupled with UHPLC-MS/MS. Molecules. 2023; 28(3):1442. https://doi.org/10.3390/molecules28031442
Chicago/Turabian StylePrakasham, Karthikeyan, Swapnil Gurrani, Jen-Taie Shiea, Ming-Tsang Wu, Chia-Fang Wu, Yi-Jia Ku, Tseng-Yu Tsai, Hung-Ta Hua, Yu-Jia Lin, Po-Chin Huang, and et al. 2023. "Rapid Identification and Analysis of Ochratoxin-A in Food and Agricultural Soil Samples Using a Novel Semi-Automated In-Syringe Based Fast Mycotoxin Extraction (FaMEx) Technique Coupled with UHPLC-MS/MS" Molecules 28, no. 3: 1442. https://doi.org/10.3390/molecules28031442