Bio-Prospecting of Crude Leaf Extracts from Thirteen Plants of Brazilian Cerrado Biome on Human Glioma Cell Lines
Abstract
:1. Introduction
2. Results
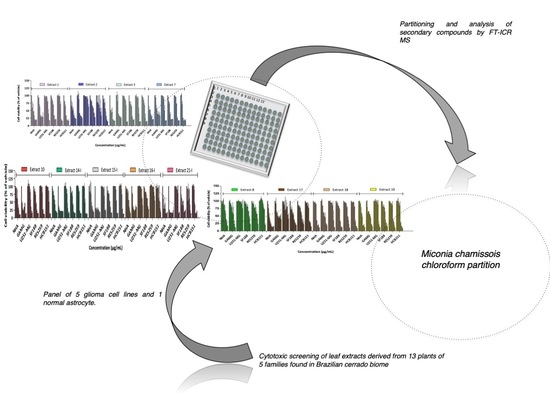
2.1. Cytotoxicity Profile Analysis of Crude Extracts
2.2. Cytotoxicity Profile Analysis of Partitions from Miconia Chamissois Crude Extracts
2.3. Characterization of the Crude Extracts and Partitions from Miconia Chamissois
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Cell Culture
5.2. Plant Extracts and Partitions
5.3. Cytotoxic Screening
5.3.1. Cell Viability
5.3.2. Kinetics Assay
5.3.3. Inhibitory Concentration of 50% of Cells (IC50)
5.4. Selectivity Index
5.5. Analysis of Secondary Compounds Present in the Crude Extract and Partitions in Miconia Chamissois by FT-ICR MS
5.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Horbinski, C.; Berger, T.; Packer, R.J.; Wen, P.Y. Clinical implications of the 2021 edition of the WHO classification of central nervous system tumours. Nat. Rev. Neurol. 2022, 18, 515–529. [Google Scholar] [CrossRef] [PubMed]
- Lapointe, S.; Perry, A.; Butowski, N.A. Primary brain tumours in adults. Lancet 2018, 392, 432–446. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.R. Brain Tumors in Children. N. Engl. J. Med. 2022, 386, 1922–1931. [Google Scholar] [CrossRef]
- Little, S.E.; Popov, S.; Jury, A.; Bax, D.A.; Doey, L.; Al-Sarraj, S.; Jurgensmeier, J.M.; Jones, C. Receptor tyrosine kinase genes amplified in glioblastoma exhibit a mutual exclusivity in variable proportions reflective of individual tumor heterogeneity. Cancer Res. 2012, 72, 1614–1620. [Google Scholar] [CrossRef]
- Germano, I.; Swiss, V.; Casaccia, P. Primary brain tumors, neural stem cell, and brain tumor cancer cells: Where is the link? Neuropharmacology 2010, 58, 903–910. [Google Scholar] [CrossRef]
- Liu, P.; Griffiths, S.; Veljanoski, D.; Vaughn-Beaucaire, P.; Speirs, V.; Bruning-Richardson, A. Preclinical models of glioblastoma: Limitations of current models and the promise of new developments. Expert Rev. Mol. Med. 2021, 23, e20. [Google Scholar] [CrossRef]
- Mittal, S.; Pradhan, S.; Srivastava, T. Recent advances in targeted therapy for glioblastoma. Expert Rev. Neurother. 2015, 15, 935–946. [Google Scholar] [CrossRef]
- Alrwas, A.; Papadopoulos, N.E.; Cain, S.; Patel, S.P.; Kim, K.B.; Deburr, T.L.; Bassett, R., Jr.; Hwu, W.J.; Bedikian, A.Y.; Davies, M.A.; et al. Phase I trial of biochemotherapy with cisplatin, temozolomide, and dose escalation of nab-paclitaxel combined with interleukin-2 and interferon-alpha in patients with metastatic melanoma. Melanoma Res. 2014, 24, 342–348. [Google Scholar] [CrossRef]
- Hermisson, M.; Klumpp, A.; Wick, W.; Wischhusen, J.; Nagel, G.; Roos, W.; Kaina, B.; Weller, M. O6-methylguanine DNA methyltransferase and p53 status predict temozolomide sensitivity in human malignant glioma cells. J. Neurochem. 2006, 96, 766–776. [Google Scholar] [CrossRef]
- Weller, M.; van den Bent, M.; Tonn, J.C.; Stupp, R.; Preusser, M.; Cohen-Jonathan-Moyal, E.; Henriksson, R.; le Rhun, E.; Balana, C.; Chinot, O.; et al. European Association for Neuro-Oncology (EANO) guideline on the diagnosis and 719 treatment of adult astrocytic and oligodendroglial gliomas. Lancet Oncol. 2017, 18, e315–e720. [Google Scholar] [CrossRef]
- Alves, A.L.V.; Gomes, I.N.F.; Carloni, A.C.; Rosa, M.N.; da Silva, L.S.; Evangelista, A.F.; Reis, R.M.; Silva, V.A.O. Role of glioblastoma stem cells in cancer therapeutic resistance: A perspective on antineoplastic agents from natural sources and chemical derivatives. Stem Cell Res. Ther. 2021, 12, 206. [Google Scholar] [CrossRef]
- Cragg, G.M.; Pezzuto, J.M. Natural Products as a Vital Source for the Discovery of Cancer Chemotherapeutic and Chemopreventive Agents. Med. Princ. Pract. 2016, 25 (Suppl. 2), 41–59. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Pezzuto, J.M. Plant-derived anticancer agents. Biochem. Pharm. 1997, 53, 121–133. [Google Scholar] [CrossRef]
- Cortelo, P.C.; Demarque, D.P.; Dusi, R.G.; Albernaz, L.C.; Braz-Filho, R.; Goncharova, E.I.; Bokesch, H.R.; Gustafson, K.R.; Beutler, J.A.; Espindola, L.S. A Molecular Networking Strategy: High-Throughput Screening and Chemical Analysis of Brazilian Cerrado Plant Extracts against Cancer Cells. Cells 2021, 10, 691. [Google Scholar] [CrossRef]
- Valli, M.; Russo, H.M.; Bolzani, V.S. The potential contribution of the natural products from Brazilian biodiversity to bioeconomy. Acad. Bras. Cienc. 2018, 90 (Suppl. 1), 763–778. [Google Scholar] [CrossRef]
- Sano, E.E.; Rodrigues, A.A.; Martins, E.S.; Bettiol, G.M.; Bustamante, M.M.C.; Bezerra, A.S.; Couto, A.F., Jr.; Vasconcelos, V.; Schuler, J.; Bolfe, E.L. Cerrado ecoregions: A spatial framework to assess and prioritize Brazilian savanna environmental diversity for conservation. J. Environ. Manag. 2019, 232, 818–828. [Google Scholar] [CrossRef]
- da Silva Lima, L.; Olivares, F.L.; Rodrigues de Oliveira, R.; Vega, M.R.G.; Aguiar, N.O.; Canellas, L.P. Root exudate profiling of maize seedlings inoculated with Herbaspirillum seropedicaeand humic acids. Chem. Biol. Technol. Agric. 2014, 1, 1–18. [Google Scholar] [CrossRef]
- Ratter, J.A.; Ribeiro, J.F.; Bridgewater, S. The Brazilian cerrado vegetation and threats to its biodiversity. Ann. Bot. 1997, 80, 223–230. [Google Scholar] [CrossRef] [Green Version]
- Santos, L.D.; Viel, A.M.; Tarosso, L.F.; Momesso, L.d.S.; Palmieri, D.A.; Spera, K.D. Medicinal plants of the Brazilian cerrado: Knowing to preserve. Biosci. J. 2020, 556–567. [Google Scholar] [CrossRef]
- Malara, F.A.; Matos, D.C.; Ribeiro, L.C.; Falcoski, T.O.; Andrade, T.J.; Santos, V.N.; Lima, N.M.; Carlos, I.Z. Medicinal Plants from Brazilian Cerrado Biome: Potential sources of new anti-inflammatory compounds and antitumor agents on Ehrlich carcinoma. Acad. Bras. Ciênc. 2021, 93, e20191101. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.H.S.; Castro-Gamboa, I.; Bolzani, V.D.S. Plant diversity from Brazilian Cerrado and Atlantic Forest as a tool for prospecting potential therapeutic drugs. Compr. Nat. Prod. II Chem. Biol. 2010, 3, 95–133. [Google Scholar]
- de Mesquita, M.L.; de Paula, J.E.; Pessoa, C.; de Moraes, M.O.; Costa-Lotufo, L.V.; Grougnet, R.; Michel, S.; Tillequin, F.; Espindola, L.S. Cytotoxic activity of Brazilian Cerrado plants used in traditional medicine against cancer cell lines. J. Ethnopharmacol. 2009, 123, 439–445. [Google Scholar] [CrossRef]
- Ribeiro Neto, J.A.; Pimenta Taroco, B.R.; Batista Dos Santos, H.; Thome, R.G.; Wolfram, E.; de A Ribeiro, R.I. Using the plants of Brazilian Cerrado for wound healing: From traditional use to scientific approach. J. Ethnopharmacol. 2020, 260, 112547. [Google Scholar] [CrossRef]
- de Giffoni de Carvalho, J.T.; da Silva Baldivia, D.; Leite, D.F.; de Araujo, L.C.A.; de Toledo Espindola, P.P.; Antunes, K.A.; Rocha, P.S.; de Picoli Souza, K.; Dos Santos, E.L. Medicinal Plants from Brazilian Cerrado: Antioxidant and Anticancer Potential and Protection against Chemotherapy Toxicity. Oxid. Med. Cell. Longev. 2019, 2019, 3685264. [Google Scholar] [CrossRef]
- Bailao, E.F.; Devilla, I.A.; da Conceicao, E.C.; Borges, L.L. Bioactive Compounds Found in Brazilian Cerrado Fruits. Int. J. Mol. Sci. 2015, 16, 23760–23783. [Google Scholar] [CrossRef]
- Fank-de-Carvalho, S.; Somavilla, N.; Marchioretto, M.; Báo, S. Biodiversity in Ecosystems—Linking Structure and Function; BoD -Books on Demand; IntechOpen: London, UK, 2015; 642p. [Google Scholar]
- Albuquerque, U.P.; Ramos, M.A.; Melo, J.G. New strategies for drug discovery in tropical forests based on ethnobotanical and chemical ecological studies. J. Ethnopharmacol. 2012, 140, 197–201. [Google Scholar] [CrossRef]
- de Melo, J.G.; Santos, A.G.; de Amorim, E.L.; do Nascimento, S.C.; de Albuquerque, U.P. Medicinal plants used as antitumor agents in Brazil: An ethnobotanical approach. Evid. Based Complement. Altern. Med. 2011, 2011, 365359. [Google Scholar] [CrossRef]
- Napolitano, D.R.; Mineo, J.R.; de Souza, M.A.; de Paula, J.E.; Espindola, L.S.; Espindola, F.S. Down-modulation of nitric oxide production in murine macrophages treated with crude plant extracts from the Brazilian Cerrado. J. Ethnopharmacol. 2005, 99, 37–41. [Google Scholar] [CrossRef]
- Ferreira, J.F.; Lopez, M.H.M.; Gomes, J.V.D.; Martins, D.H.N.; Fagg, C.W.; Magalhaes, P.O.; Davies, N.W.; Silveira, D.; Fonseca-Bazzo, Y.M. Seasonal Chemical Evaluation of Miconia chamissois Naudin from Brazilian Savanna. Molecules 2022, 27, 1120. [Google Scholar] [CrossRef]
- Suffness, M. Assays related to cancer drug discovery. Methods Plant Biochem. Assays Bioactivity 1990, 6, 71–133. [Google Scholar]
- Wang, J.; Jia, Z.; Zhang, Z.; Wang, Y.; Liu, X.; Wang, L.; Lin, R. Analysis of Chemical Constituents of Melastoma dodecandrum Lour. by UPLC-ESI-Q-Exactive Focus-MS/MS. Molecules 2017, 22, 476. [Google Scholar] [CrossRef]
- Xu, J.; Tong, C.; Fu, Q.; Guo, K.; Shi, S.; Xiao, Y. Comprehensive Polyphenolic Profile of Plantago depressa using High-Speed Countercurrent Chromatography Off-line with High-Performance Liquid Chromatography–Diode Array Detector–Quadrupole Time-of-flight Tandem Mass Spectrometry. eFood 2019, 1, 94–105. [Google Scholar] [CrossRef]
- Ameachi, N.C.; Chijioke, C.L. Evaluation of bioactive compounds in Pseudarenthemum tunicatum leaves using gas chromatography-mass spectrometry. Evaluation 2018, 18. [Google Scholar]
- Abdelkader, A.F.A.; Aldughaish, A.M. Physiological and Chemical Characteristics of Age-Differed Ficus Benjamina L. Trees Cultivated in El-Ahassa, Saudi Arabia. J. Plant Sci. 2016, 4, 63. [Google Scholar]
- Mala, M.; Saravanakumar, K. GC-MS Analysis of bioactive compounds in the Methanolicleaf extract of Memecylon edule Roxb. from Authukurichisacred grove, Tamilnadu, India. Life Sci. Arch. 2015, 1, 386–393. [Google Scholar]
- Cao, S.-G.; Wu, X.-H.; Sim, K.-Y.; Tan, B.; Pereira, J.; Goh, S.-H. Styryl-lactone derivatives and alkaloids from Goniothalamus borneensis (Annonaceae). Tetrahedron 1998, 54, 2143–2148. [Google Scholar] [CrossRef]
- Farag, M.A.; Al-Mahdy, D.A. Comparative study of the chemical composition and biological activities of Magnolia grandiflora and Magnolia virginiana flower essential oils. Nat. Prod. Res. 2013, 27, 1091–1097. [Google Scholar] [CrossRef]
- Iheagwam, F.N.; Israel, E.N.; Kayode, K.O.; De Campos, O.C.; Ogunlana, O.O.; Chinedu, S.N. GC-MS Analysis and Inhibitory Evaluation of Terminalia catappa Leaf Extracts on Major Enzymes Linked to Diabetes. Evid.-Based Complement. Altern. Med. Ecam 2019, 2019, 6316231. [Google Scholar]
- Seito, L.N.; Ruiz, A.L.; Vendramini-Costa, D.; Tinti, S.V.; de Carvalho, J.E.; Bastos, J.K.; Di Stasi, L.C. Antiproliferative activity of three methoxylated flavonoids isolated from Zeyheria montana Mart. (Bignoniaceae) leaves. Phytother. Res. 2011, 25, 1447–1450. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, M.; Suzuki, T.; Yoshikoshi, A. Stereoselective total synthesis of (-)-picrotoxinin and (-)-picrotin. J. Am. Chem. Soc. 1989, 111, 3728–3734. [Google Scholar] [CrossRef]
- Kim, H.W.; Choi, S.Y.; Jang, H.S.; Ryu, B.; Sung, S.H.; Yang, H. Exploring novel secondary metabolites from natural products using pre-processed mass spectral data. Sci. Rep. 2019, 9, 17430. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Wang, D.; Fan, Z.; Chen, X.; Yu, F.; Hu, X.; Wang, K.; Yuan, L. Blumea balsamifera--a phytochemical and pharmacological review. Molecules 2014, 19, 9453–9477. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Basavarajappa, H.D.; Sulaiman, R.S.; Fei, X.; Seo, S.Y.; Corson, T.W. The first synthesis of the antiangiogenic homoisoflavanone, cremastranone. Org. Biomol. Chem. 2014, 12, 7673–7677. [Google Scholar] [CrossRef]
- Hurtado Fernández, E. Avocado (Persea Americana): Complementarity of Different Omics Technologies for Its Metabolic Characterization; Universidad de Granada: Granada, Spain, 2014. [Google Scholar]
- Chen, Y.C.; Chiu, H.L.; Chao, C.Y.; Lin, W.H.; Chao, L.K.; Huang, G.J.; Kuo, Y.H. New Anti-Inflammatory Aromatic Components from Antrodia camphorata. Int. J. Mol. Sci. 2013, 14, 4629–4639. [Google Scholar] [CrossRef] [Green Version]
- Miceli, A.; Moncada, A.; Sabatino, L.; Vetrano, F. Effect of Gibberellic Acid on Growth, Yield, and Quality of Leaf Lettuce and Rocket Grown in a Floating System. Agronomy 2019, 9, 382. [Google Scholar] [CrossRef]
- Grimbs, A.; Shrestha, A.; Rezk, A.S.; Grimbs, S.; Hakeem Said, I.; Schepker, H.; Hütt, M.; Albach, D.C.; Brix, K.; Kuhnert, N.; et al. Bioactivity in Rhododendron: A Systemic Analysis of Antimicrobial and Cytotoxic Activities and Their Phylogenetic and Phytochemical Origins. Front. Plant Sci. 2017, 8, 551. [Google Scholar] [CrossRef]
- Kanchanapoom, T.; Kasai, R.; Yamasaki, K. Iridoid and phenolic diglycosides from Canthium berberidifolium. Phytochemistry 2002, 61, 461–464. [Google Scholar] [CrossRef]
- Kenfack, J.N.; Ponou, B.K.; Kühlborn, J.; Teponno, R.B.; Nono, R.N.; Fouedjou, R.T.; Opatz, T.; Park, H.J.; Tapondjou, L.A. A New Flavonol Glycoside from Tristemma hirtum (Melastomataceae). Nat. Prod. Sci. 2018, 24, 213–218. [Google Scholar] [CrossRef]
- Al-Zikri, P.N.H.; Taher, M.; Susanti, D.; Rezali, M.F.; Read, R.W.; Sohrab, M.; Hasan, C.M.; Ahmad, F. Cytotoxic tirucallane triterpenes from the stem of Luvunga scandens. Rev. Bras. Farm. 2014, 24, 561–564. [Google Scholar] [CrossRef]
- Hooi Poay, T.; Sui Kiong, L.; Cheng Hock, C. Characterisation of galloylated cyanogenic glucosides and hydrolysable tannins from leaves of Phyllagathis rotundifolia by LC-ESI-MS/MS. Phytochem. Anal. 2011, 22, 516–525. [Google Scholar] [CrossRef]
- Costa, M.F.; Jesus, T.I.; Lopes, B.R.; Angolini, C.F.; Montagnolli, A.; Gomes, L.P.; Pereira, G.S.; Ruiz, A.L.; Carvalho, J.E.; Eberlin, M.N.; et al. Eugenia aurata and Eugenia punicifolia HBK inhibit inflammatory response by reducing neutrophil adhesion, degranulation and NET release. Bmc Complement. Altern. Med. 2016, 16, 403. [Google Scholar] [CrossRef]
- Fank-de-Carvalho, S.M.; Somavilla, N.S.; Marchioretto, M.S.; Báo, S.N. Plant structure in the Brazilian neotropical savannah species. In Biodiversity in Ecosystems—Linking Structure and Function; BoD -Books on Demand; IntechOpen: London, UK, 2015; pp. 425–459. [Google Scholar]
- Silva, V.A.O.; Alves, A.L.V.; Rosa, M.N.; Silva, L.R.V.; Melendez, M.E.; Cury, F.P.; Gomes, I.N.F.; Tansini, A.; Longato, G.B.; Martinho, O.; et al. Hexane partition from Annona crassiflora Mart. promotes cytotoxity and apoptosis on human cervical cancer cell lines. Investig. New Drugs 2019, 37, 602–615. [Google Scholar] [CrossRef]
- dos Santos, K.M.; de Fatima Nunes, D.A.; Gomes, I.N.F.; da Silva, S.L.; de Azambuja Ribeiro, R.I.M. Inhibition of gelatinase activity of MMP-2 and MMP-9 by extracts of Bauhinia ungulata L. Biosci. J. 2015, 31, 584–590. [Google Scholar] [CrossRef]
- Faria, I.; Silva, A.; Lima, G.; Longatti, T.; Carmo, L.; Villar, J.; Araujo, A.; Thomé, R.; Santos, H.; Ribeiro, R. Alkaloid and phenolic compounds of Xylopia aromatica inhibits tumor growth by down-regulating matrix metalloproteinase-2 expression. Pak. J. Pharm. Sci. 2019, 34, 599–606. [Google Scholar]
- Silva-Oliveira, R.J.; Lopes, G.F.; Camargos, L.F.; Ribeiro, A.M.; Santos, F.V.; Severino, R.P.; Severino, V.G.; Terezan, A.P.; Thome, R.G.; Santos, H.B.; et al. Tapirira guianensis Aubl. Extracts Inhibit Proliferation and Migration of Oral Cancer Cells Lines. Int. J. Mol. Sci. 2016, 17, 1839. [Google Scholar] [CrossRef] [Green Version]
- Santos, K.; Gomes, I.; Silva-Oliveira, R.; Pinto, F.; Oliveira, B.; Chagas, R.; Romão, W.; Reis, R.M.; Ribeiro, R. Bauhinia variegata candida Fraction Induces Tumor Cell Death by Activation of Caspase-3, RIP, and TNF-R1 and Inhibits Cell Migration and Invasion In Vitro. Biomed Res. Int. 2018, 2018, 4702481. [Google Scholar] [CrossRef]
- Rosa, M.N.; LRV, E.S.; Longato, G.B.; Evangelista, A.F.; Gomes, I.N.F.; Alves, A.L.V.; de Oliveira, B.G.; Pinto, F.E.; Romao, W.; de Rezende, A.R.; et al. Bioprospecting of Natural Compounds from Brazilian Cerrado Biome Plants in Human Cervical Cancer Cell Lines. Int. J. Mol. Sci. 2021, 22, 3383. [Google Scholar] [CrossRef]
- Lemee, J.M.; Clavreul, A.; Menei, P. Intratumoral heterogeneity in glioblastoma: Don’t forget the peritumoral brain zone. Neuro-Oncol. 2015, 17, 1322–1332. [Google Scholar] [CrossRef]
- Bermejo, A.; Figadere, B.; Zafra-Polo, M.C.; Barrachina, I.; Estornell, E.; Cortes, D. Acetogenins from Annonaceae: Recent progress in isolation, synthesis and mechanisms of action. Nat. Prod. Rep. 2005, 22, 269–303. [Google Scholar] [CrossRef] [PubMed]
- Ishimaru, C.; Takeuchi, T.; Yonezawa, Y.; Kuriyama, I.; Takemura, M.; Kato, I.; Sugawara, F.; Yoshida, H.; Mizushina, Y. Inhibitory effect of acetogenins on mammalian DNA polymerase and human cancer cell growth. Lett. Drug Des. Discov. 2007, 4, 239–245. [Google Scholar] [CrossRef]
- Takahashi, S.; Yonezawa, Y.; Kubota, A.; Ogawa, N.; Maeda, K.; Koshino, H.; Nakata, T.; Yoshida, H.; Mizushina, Y. Pyranicin, a non-classical annonaceous acetogenin, is a potent inhibitor of DNA polymerase, topoisomerase and human cancer cell growth. Int. J. Oncol. 2008, 32, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, M.A.; Royo, V.A.; Ferreira, D.S.; Crotti, A.E.; Andrade e Silva, M.L.; Carvalho, J.C.; Bastos, J.K.; Cunha, W.R. In vivo analgesic and anti-inflammatory activities of ursolic acid and oleanoic acid from Miconia albicans (Melastomataceae). Z. Fur Nat. C J. Biosci. 2006, 61, 477–482. [Google Scholar] [CrossRef]
- Serna, D.M.; Martinez, J.H. Phenolics and Polyphenolics from Melastomataceae Species. Molecules 2015, 20, 17818–17847. [Google Scholar] [CrossRef]
- Sharma, S.B.; Gupta, R. Drug development from natural resource: A systematic approach. Mini Rev. Med. Chem. 2015, 15, 52–57. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, R.F.; Xiao, Q.G.; Li, R.; Shen, X.L.; Zhu, X.G. Hedyotis diffusa Willd extract inhibits the growth of human glioblastoma cells by inducing mitochondrial apoptosis via AKT/ERK pathways. J. Ethnopharmacol. 2014, 158 Pt A, 404–411. [Google Scholar] [CrossRef]
- Chang, H.F.; Huang, W.T.; Chen, H.J.; Yang, L.L. Apoptotic effects of gamma-mangostin from the fruit hull of Garcinia mangostana on human malignant glioma cells. Molecules 2010, 15, 8953–8966. [Google Scholar] [CrossRef]
- Liu, B.; Gao, Y.Q.; Wang, X.M.; Wang, Y.C.; Fu, L.Q. Germacrone inhibits the proliferation of glioma cells by promoting apoptosis and inducing cell cycle arrest. Mol. Med. Rep. 2014, 10, 1046–1050. [Google Scholar] [CrossRef]
- Gentile, M.T.; Ciniglia, C.; Reccia, M.G.; Volpicelli, F.; Gatti, M.; Thellung, S.; Florio, T.; Melone, M.A.; Colucci-D’Amato, L. Ruta graveolens L. induces death of glioblastoma cells and neural progenitors, but not of neurons, via ERK 1/2 and AKT activation. PLoS ONE 2015, 10, e0118864. [Google Scholar] [CrossRef]
- Flores, B.C.; Klinger, D.R.; Welch, B.G.; White, J.A.; Batjer, H.H.; Samson, D.S. Management of intracranial aneurysms associated with arteriovenous malformations. Neurosurg. Focus 2014, 37, E11. [Google Scholar] [CrossRef]
- Oberoi, R.K.; Parrish, K.E.; Sio, T.T.; Mittapalli, R.K.; Elmquist, W.F.; Sarkaria, J.N. Strategies to improve delivery of anticancer drugs across the blood-brain barrier to treat glioblastoma. Neuro Oncol. 2016, 18, 27–36. [Google Scholar] [CrossRef]
- Patel, A.P.; Tirosh, I.; Trombetta, J.J.; Shalek, A.K.; Gillespie, S.M.; Wakimoto, H.; Cahill, D.P.; Nahed, B.V.; Curry, W.T.; Martuza, R.L.; et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science 2014, 344, 1396–1401. [Google Scholar] [CrossRef]
- Carocho, M.; Ferreira, I.C. The role of phenolic compounds in the fight against cancer--a review. Anti-Cancer Agents Med. Chem. 2013, 13, 1236–1258. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, W.; He, Q.; Wu, Y.; Lu, Z.; Sun, J.; Liu, Z.; Shao, Y.; Wang, A. Oleic acid induces apoptosis and autophagy in the treatment of Tongue Squamous cell carcinomas. Sci. Rep. 2017, 7, 11277. [Google Scholar] [CrossRef]
- Zhu, S.; Jiao, W.; Xu, Y.; Hou, L.; Li, H.; Shao, J.; Zhang, X.; Wang, R.; Kong, D. Palmitic acid inhibits prostate cancer cell proliferation and metastasis by suppressing the PI3K/Akt pathway. Life Sci. 2021, 286, 120046. [Google Scholar] [CrossRef]
- Shin, H.E.; Lee, S.; Choi, Y.; Park, S.; Kwon, S.; Choi, J.K.; Seo, S.Y.; Lee, Y. Synthetic Homoisoflavane Derivatives of Cremastranone Suppress Growth of Colorectal Cancer Cells through Cell Cycle Arrest and Induction of Apoptosis. Biomol. Ther. 2022, 30, 576–584. [Google Scholar] [CrossRef]
- Diantini, A.; Subarnas, A.; Lestari, K.; Halimah, E.; Susilawati, Y.; Supriyatna, S.; Julaeha, E.; Achmad, T.H.; Suradji, E.W.; Yamazaki, C.; et al. Kaempferol-3-O-rhamnoside isolated from the leaves of Schima wallichii Korth. inhibits MCF-7 breast cancer cell proliferation through activation of the caspase cascade pathway. Oncol. Lett. 2012, 3, 1069–1072. [Google Scholar] [CrossRef]
- Silva, A.G.; Silva, V.A.O.; Oliveira, R.J.S.; de Rezende, A.R.; Chagas, R.C.R.; Pimenta, L.P.S.; Romao, W.; Santos, H.B.; Thome, R.G.; Reis, R.M.; et al. Matteucinol, isolated from Miconia chamissois, induces apoptosis in human glioblastoma lines via the intrinsic pathway and inhibits angiogenesis and tumor growth in vivo. Investig. New Drugs 2020, 38, 1044–1055. [Google Scholar] [CrossRef]
- Teixeira, S.A.; Luzzi, M.C.; Martin, A.C.B.M.; Duarte, T.T.; Leal, M.O.; Teixeira, G.R.; Reis, M.T.; Junior, C.R.A.; Santos, K.; Melendez, M.E.; et al. The Barretos Cancer Hospital Animal Facility: Implementation and Results of a Dedicated Platform for Preclinical Oncology Models. Vet. Sci. 2022, 9, 636. [Google Scholar] [CrossRef]
- Cruvinel-Carloni, A.; SilvaOliveira, R.; Torrieri, R.; Bidinotto, L.T.; Berardinelli, G.N.; Oliveira-Silva, V.A.; Clara, C.A.; de Almeida, G.C.; Martinho, O.; Squire, J.A.; et al. Molecular characterization of short-term primary cultures and comparison with corresponding tumor tissue of Brazilian glioblastoma patients. Transl. Cancer Res. 2017, 6, 332–345. [Google Scholar] [CrossRef] [Green Version]

| IC50 (µg/mL) Mean ± Standard Deviation (SD) | ||||||
|---|---|---|---|---|---|---|
| Crude Extract/Chemotherapy | NHA | GAMG | U251-MG | SF188 | RES259 | HCB151 |
| 1 | 36.38 ± 11.3 | <1.5 | 146.1 ± 35.8 | 72.72 ± 8.3 | 89.92 ± 71.5 | 25.93 ± 12.9 |
| 2 | 13.4 ± 2.3 | <1.5 | >300 | 118.5 ± 1.7 | 124.7 ± 188.1 | 72.85 ± 26.9 |
| 3 | >200 | <1.5 | 120.6 ± 10.9 | 57.45 ± 13.7 | 70.93 ± 40 | 54.78 ± 22 |
| 7 | 41.30 ± 18.21 | <1.5 | 117.2 ± 53.5 | 43.75 ± 1.4 | 50.40 ± 39 | 28.69 ± 13.4 |
| 8 | 36.19 ± 20.1 | 6.66 ± 4.3 | 176.5 ± 74.88 | 66.20 ± 5.3 | 86.80 ± 43.5 | 125.7 ± 10.9 |
| 10 | 21.46 ± 1.9 | 18.83 ± 4.67 | 70.52 ± 61 | 18.45 ± 16.9 | 44.15 ± 43.9 | 145.7 ± 19.7 |
| 14-I | 22.84 ± 14.05 | <1.5 | 77.68 ± 20.1 | 26.55 ± 12.4 | 74.76 ± 1.5 | 55.30 ± 0 |
| 15-I | 35.68 ± 37.1 | <1.5 | 136.4 ± 33.6 | 34.31 ± 8 | 106.3 ± 5.2 | 81.95 ± 0 |
| 16-I | 62.67 ± 50.2 | <1.5 | 296.9 ± 23.2 | 100.5 ± 16.6 | >200 | 208.1 ± 30.1 |
| 17 | 2.9 ± 1.4 | 25.7 ± 12.9 | 28.9 ± 5.5 | 13.6 ± 5.7 | 29.3 ± 17.4 | 30.2 ± 10.6 |
| 18 | 12.05 ± 1.1 | 19.94 ± 0.4 | 70.21 ± 7.4 | 23.08 ± 3.6 | 38.07 ± 5.7 | 30.05 ± 3.9 |
| 19 | 12.11 ± 5.3 | 8.37 ± 11.8 | 89.85 ± 5 | 19.28 ± 8.3 | 33.04 ± 12.0 | 27.43 ± 6.2 |
| 21-I | 3.73 ± 3.7 | <1.5 | 132.5 ± 32.5 | 6.56 ± 0.6 | 37.04 ± 8.4 | 47.52 ± 0 |
| TMZ | 1.28 ± 26.26 | 7.06 ± 31.84 | 39.78 ± 23.67 | 30.74 ± 27.91 | 16.93 ± 27.55 | 38.22 ± 26.02 |
| Crude Extract/Chemotherapy | Glioma Cell Lines | ||||
|---|---|---|---|---|---|
| GAMG | U251-MG | SF188 | RES259 | HCB151 | |
| 1 | UD | 0.2 | 0.5 | 0.4 | 1.4 |
| 2 | UD | UD | 0.1 | 0.1 | 0.2 |
| 3 | UD | ID | UD | UD | UD |
| 7 | UD | 0.4 | 0.9 | 0.8 | 1.4 |
| 8 | 5.4 | 0.2 | 0.5 | 0.4 | 0.3 |
| 10 | 1.1 | 0.3 | 1.2 | 0.5 | 0.1 |
| 14-I | UD | 0.3 | 0.9 | 0.3 | 0.4 |
| 15-I | UD | 0.3 | 1.0 | 0.3 | 0.4 |
| 16-I | UD | 0.2 | 0.6 | UD | 0.3 |
| 17 | 0.1 | 0.1 | 0.2 | 0.1 | 0.1 |
| 18 | 0.6 | 0.2 | 0.5 | 0.3 | 0.4 |
| 19 | 1.4 | 0.1 | 0.6 | 0.4 | 0.4 |
| 21-I | UD | 0.03 | 0.6 | 0.1 | 0.1 |
| TMZ | 0.2 | 0.03 | 0.0 | 0.1 | 0.03 |
| IC50 (µg/mL) Mean ± Standard Deviation (SD) | ||||||
|---|---|---|---|---|---|---|
| Crude Extract/Partitions/Chemotherapy | NHA | GAMG | U251-MG | SF188 | RES259 | HCB151 |
| CE | 12.11 ± 5.35 | 8.37 ± 11.81 | 89.85 ± 5.09 | 19.28 ± 8.28 | 33.04 ± 12.08 | 27.43 ± 6.20 |
| HDP | >200 | >200 | >200 | >200 | >200 | >200 |
| HP | 9.17 ± 0.99 | 37.39 ± 5.22 | 34.66 ± 5.45 | 5.35 ± 2.27 | 18.22 ± 4.58 | 21.76 ± 6.74 |
| CP | 14.06 ± 2.41 | 34.31 ± 5.50 | 21.54 ± 1.80 | 7.28 ± 3.40 | 20.83 ± 4.91 | 15.00 ± 1.00 |
| AEP | 31.22 ± 10.40 | 2.57 ± 3.34 | 102.4 ± 2.61 | 41.69 ± 6.06 | 27.40 ± 4.25 | 34.26 ± 4.20 |
| TMZ | 1.28 ± 26.26 | 7.06 ± 31.84 | 39.78 ± 23.67 | 30.74 ± 27.91 | 16.93 ± 27.55 | 38.22 ± 26.02 |
| Crude Extract/Partitions/Chemotherapy | Cell Lines | ||||
|---|---|---|---|---|---|
| GAMG | U251-MG | SF188 | RES259 | HCB151 | |
| CE | 1.4 | 0.1 | 0.6 | 0.4 | 0.4 |
| HDP | UD | UD | UD | UD | UD |
| HP | 0.2 | 0.3 | 1.7 | 0.5 | 0.4 |
| CP | 0.4 | 0.7 | 1.9 | 0.7 | 0.9 |
| AEP | 12.1 | 0.3 | 0.7 | 1.1 | 0.9 |
| TMZ | 0.2 | 0.03 | 0.04 | 0.1 | 0.03 |
| IC50 (µg/mL) Mean ± Standard Deviation (SD) | |||||
|---|---|---|---|---|---|
| Cell Lines | Crude Extract/Partition | 6 h | 12 h | 24 h | 48 h |
| NHA | CE | >300 | >300 | >300 | 64.94 ± 23.29 |
| HP | >200 | 77.66 ± 24.02 | 37.64 ± 32.21 | 30.55 ± 37.02 | |
| CP | >200 | 61.39 ± 21.98 | 35.13 ± 26.58 | 20.93 ± 32.62 | |
| GAMG | CE | >300 | >300 | 89.29 ± 20.33 | 35.98 ± 26.61 |
| HP | >200 | 49.20 ± 21.85 | 55.95 ± 26.24 | 34.78 ± 32.96 | |
| CP | 51.52 ± 18.43 | 32.17 ± 24.64 | 40.11 ± 26.13 | 39.14 ± 23.52 | |
| U251-MG | CE | >300 | >300 | >300 | >300 |
| HP | >200 | >200 | >200 | >200 | |
| CP | >200 | >200 | >200 | 69.03 ± 30.89 | |
| SF188 | CE | >300 | 91.88 ± 17.86 | 56.87 ± 24.38 | 15.42 ± 32.78 |
| HP | >200 | 51.88 ± 24.84 | 26.54 ± 31.35 | 16.46 ± 34.98 | |
| CP | >200 | 41.58 ± 22.61 | 22.35 ± 30.20 | 6.34 ± 32.81 | |
| RES259 | CE | >300 | >300 | >300 | 20.45 ± 24.31 |
| HP | >200 | 93.91 ± 19.89 | 66.21 ± 30.07 | 22.89 ± 32.60 | |
| CP | >200 | >200 | 64.72 ± 25.95 | 9.16 ± 35.78 | |
| m/z Measured | m/z Theoretical | Error (ppm) | DBE | [M-H] | Proposed Compound | Reference |
|---|---|---|---|---|---|---|
| 191.05620 | 191.05602 | −0.45 | 2 | [C7H11O6] | chlorogenic acid | [34] |
| 197.04549 | 197.16511 | 0.27 | 5 | [C9H9O5] | syringic acid | [35] |
| 219.05098 | 219.16907 | 0.23 | 3 | [C8H11O7] | 2-hydroxypropane-1,2,3-tricarboxylic acid, dimethyl ester | [36] |
| 229.07151 | 229.07130 | 1.11 | 4 | [C10H13O6] | D-(-) Erythrose | [37] |
| 255.23294 | 255.23295 | 0.06 | 1 | [C16H31O2] | palmitic acid | [38] |
| 265.10783 | 266.10789 | 1.19 | 6 | [C14H17O5] | goniothalesdiol | [39] |
| 277.21771 | 277.25421 | −1.46 | 4 | [C19H29O2] | linolenic acid | [40] |
| 281.24860 | 281.45410 | 0.01 | 2 | [C18H33O2] | oleic acid | [41] |
| 299.09247 | 299.29864 | 0.11 | 10 | [C17H15O5] | 5-hydroxy-6,7-dimethoxyflavanone | [42] |
| 309.09756 | 311.37850 | 1.35 | 7 | [C15H17O7] | picrotin | [43] |
| 313.10808 | 313.32526 | 0.20 | 10 | [C18H17O5] | agrimonolide | [44] |
| 331.08231 | 331.29745 | 0.06 | 10 | [C17H15O7] | dihydroquercetin-7,4’-dimethylether | [45] |
| 345.09851 | 345.32300 | −1.55 | 10 | [C18H17O7] | cremastranone | [46] |
| 351.12974 | 351.32698 | −0.19 | 3 | [C14H23O10] | methyl 4-(6.7-dideoxy-galacto-hept-6-enopyranosyl)-galactopyranoside | [47] |
| 363.10909 | 363.10800 | −1.52 | 9 | [C18H19O8] | 4,4’-Dihydroxy-3,3’-dimethoxy-2,2’-dimethyl-5,6,5’,6’-bimethylenedioxybiphenyl | [48] |
| 379.13921 | 375.13410 | 1.67 | 8 | [C19H23O8] | gibberellic Acid | [49] |
| 431.09823 | 431.37037 | 0.33 | 12 | [C21H19O10] | kaempferol-o-rhamnoside | [50] |
| 445.13427 | 445.13400 | 1.97 | 7 | [C19H25O12] | canthoside A | [51] |
| 447.09333 | 447.36978 | −0.09 | 12 | [C21H19O11] | 6-hydroxyapigenin-7-o-β-d-glucopyranoside | [52] |
| 455.35390 | 455.35100 | −1.82 | 7 | [C30H47O3] | 3-Oxotirucalla-7,24-dien-21-oic acid | [53] |
| 483.07802 | 483.35726 | 0.02 | 11 | [C20H19O14] | 3, 6-di-o-galloyl-d-glucose | [54] |
| 593.15123 | 593.51122 | −0.06 | 13 | [C27H29O15] | rutinosylkaempferol | [55] |
| 635.08917 | 635.46179 | −0.28 | 16 | [C27H23O18] | 1,2,3-tri-o-galloyl-β-d-glucose | [54] |
| Cell Lines | Type | Subtype | Grade (OMS) | Description | Sex | Age | Mainly Mutations | Origin | Culture Conditions |
|---|---|---|---|---|---|---|---|---|---|
| GAMG | Established | GBM | IV | Adult | Female | 42 years | TP53 and TERT | DSMZ | DMEM + 10% FBS + 1% P/S |
| U251-MG | Established | GBM | IV | Adult | Male | 75 years | CDKN2A/B, EGFR, TP53, PTEN and TERT | Kindly provided by Dr. Joseph Costello | DMEM + 10% FBS + 1% P/S |
| SF188 | Established | GBM | IV | Paediatric | Male | 8 years | TP53 and NF1 | Kindly provided by Dr. Chris Jones | DMEM + 10% FBS + 1% P/S |
| RES259 | Established | DA | II | Paediatric | Female | 4 years | TERT | Kindly provided by Dr. Chris Jones | DMEM + 10% FBS + 1% P/S |
| HCB151 | Primary | GBM | IV | Adult | Male | 59 years | CDKN2A and PTEN | Barretos Cancer Hospital | DMEM + 10% FBS + 1% P/S |
| NHA | Established | - | - | NA | NA | NA | - | ECACC | DMEM + 10% FBS + 1% P/S |
| Crude Extract Identification | Vernacular Name | Botanical Name | Family | Registration Code |
|---|---|---|---|---|
| 1 | Pau Pombo | Tapirira guianensis | Fabaceae | 143407BHCB |
| 2 | Gonçalo-Alves | Astronium fraxinifolium | Anacardiaceae | 143403BHCB |
| 3 | Pimenta-de-Macaco | Xylopia aromatica | Annonaceae | 43397BHCB |
| 7 | Araticum | Annona crassiflora | Annonaceae | 143400BHCB |
| 8 | Negramina | Siparuna guianensis | Siparunaceae | 143404BHCB |
| 10 | Marcela | Achyrocline alata | Asteraceae | 11486CG/MS |
| 14-I | Pata-de-vaca | Bauhinia variegata | Fabaceae | 161589BHCB |
| 15-I | Pata-de-vaca branca | Bauhinia variegata candida | Fabaceae | 161590BHCB |
| 16-I | Pata-de-viado | Bauhinia ungulata | Fabaceae | 161588BHCB |
| 17 | Pixirica-da-mata | Miconia cuspidata | Melastomataceae | 44998HUFU |
| 18 | Canela de velho | Miconia albicans | Melastomataceae | 56558 HUFU |
| 19 | Pixirica-açu | Miconia chamissois | Melastomataceae | 59592HUFU |
| 21-I | Barbatimão | Stryphnodendron adstringens | Fabaceae | 169871BHCB |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, V.A.O.; Rosa, M.N.; Gomes, I.N.F.; Vital, P.d.S.; Alves, A.L.V.; Evangelista, A.F.; Longato, G.B.; Carloni, A.C.; Oliveira, B.G.; Pinto, F.E.; et al. Bio-Prospecting of Crude Leaf Extracts from Thirteen Plants of Brazilian Cerrado Biome on Human Glioma Cell Lines. Molecules 2023, 28, 1394. https://doi.org/10.3390/molecules28031394
Silva VAO, Rosa MN, Gomes INF, Vital PdS, Alves ALV, Evangelista AF, Longato GB, Carloni AC, Oliveira BG, Pinto FE, et al. Bio-Prospecting of Crude Leaf Extracts from Thirteen Plants of Brazilian Cerrado Biome on Human Glioma Cell Lines. Molecules. 2023; 28(3):1394. https://doi.org/10.3390/molecules28031394
Chicago/Turabian StyleSilva, Viviane A. O., Marcela N. Rosa, Izabela N. F. Gomes, Patrik da Silva Vital, Ana Laura V. Alves, Adriane F. Evangelista, Giovanna B. Longato, Adriana C. Carloni, Bruno G. Oliveira, Fernanda E. Pinto, and et al. 2023. "Bio-Prospecting of Crude Leaf Extracts from Thirteen Plants of Brazilian Cerrado Biome on Human Glioma Cell Lines" Molecules 28, no. 3: 1394. https://doi.org/10.3390/molecules28031394