Physicochemical Characterizations, Digestibility, and Lipolysis Inhibitory Effects of Highland Barley Resistant Starches Prepared by Physical and Enzymatic Methods
Abstract
:1. Introduction
2. Results
2.1. Chemical Compositions of HBRSs Prepared by Different Methods
2.2. Solubilities, Swelling Power, and Water-Binding Capacities of HBRSs Prepared by Different Methods
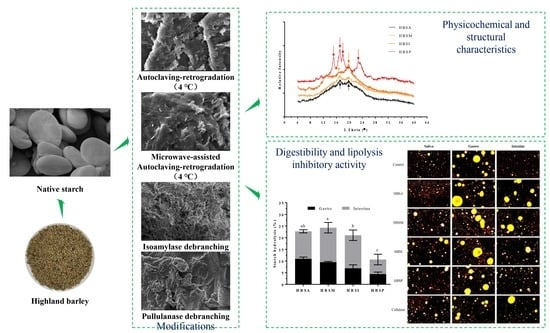
2.3. Morphological Properties of HBRSs Prepared by Different Methods
2.4. FT-IR Spectra of HBRSs Prepared by Different Methods
2.5. XRD Patterns of HBRSs Prepared by Different Methods
2.6. Digestibility of HBRSs Prepared by Different Methods In Vitro
2.7. Inhibitory Effects of HBRSs on the Microstructure of Lipid Emulsion
2.8. Inhibitory Effects of HBRSs on the Release of FFAs
3. Discussion
4. Materials and Methods
4.1. Materials and Chemicals
4.2. Preparation of Native Starch from Highland Barley
4.3. Physical and Enzymatic Modifications of Native Starch from Highland Barley
4.3.1. Preparation of Autoclaving Modified Resistant Starch (HBSA)
4.3.2. Preparation of Microwave-Assisted Autoclaving Modified Resistant Starch (HBSM)
4.3.3. Preparation of Enzyme-Debranched Starch
4.4. Characterization of the Modified Starches
4.4.1. Chemical Composition Analysis
4.4.2. Morphological Property
4.4.3. Fourier Transform Infrared (FT-IR) Spectroscopy
4.4.4. X-ray Diffraction (XRD) Analysis
4.4.5. Solubility, Swelling Power, and Water-Binding Capacity
4.5. Simulated Gastrointestinal Digestion of the Modified Starches In Vitro
4.6. Lipolysis Inhibitory Activity of the Modified Starches
4.6.1. Digestion of Lipid Emulsion In Vitro
4.6.2. Microstructure Analysis
4.6.3. Release of Free Fatty Acids (FFAs)
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Wang, C.; Zhang, Z.; Zhang, X.; Tian, X.; Chen, K.; Zeng, X. Characterization of volatile compounds by HS-GC-IMS and chemical composition analysis of colored highland barley roasted at different temperatures. Foods 2022, 11, 2921. [Google Scholar] [CrossRef]
- Obadi, M.; Sun, J.; Xu, B. Highland barley: Chemical composition, bioactive compounds, health effects, and applications. Food Res. Int. 2021, 140, 110065. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Horvath, C.; Chen, L.; Chen, J.; Zheng, B. Understanding the nutrient composition and nutritional functions of highland barley (Qingke): A review. Trends Food Sci. Technol. 2020, 103, 109–117. [Google Scholar] [CrossRef]
- Yang, Y.; Jiao, A.; Zhao, S.; Liu, Q.; Fu, X.; Jin, Z. Effect of removal of endogenous non-starch components on the structural, physicochemical properties, and in vitro digestibility of highland barley starch. Food Hydrocoll. 2021, 117, 106698. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, B.; Chen, L.; Li, X.; Zheng, B. Hierarchical structure and physicochemical properties of highland barley starch following heat moisture treatment. Food Chem. 2019, 271, 102–108. [Google Scholar] [CrossRef]
- Shah, A.; Masoodi, F.A.; Gani, A.; Ashwar, B.A. In-vitro digestibility, rheology, structure, and functionality of RS3 from oat starch. Food Chem. 2016, 212, 749–758. [Google Scholar] [CrossRef]
- Bojarczuk, A.; Skąpska, S.; Mousavi Khaneghah, A.; Marszałek, K. Health benefits of resistant starch: A review of the literature. J. Funct. Foods 2022, 93, 105094. [Google Scholar] [CrossRef]
- Lv, Y.; Ma, S.; Yan, J.; Sun, B.; Wang, X. Effect of heat–moisture treatment on the physicochemical properties, structure, morphology, and starch digestibility of highland barley (Hordeum vulgare L. var. nudum Hook. f) flour. Foods 2022, 11, 3511. [Google Scholar] [CrossRef]
- Dundar, A.N.; Gocmen, D. Effects of autoclaving temperature and storing time on resistant starch formation and its functional and physicochemical properties. Carbohydr. Polym. 2013, 97, 764–771. [Google Scholar] [CrossRef] [Green Version]
- Guo, Z.; Zeng, S.; Lu, X.; Zhou, M.; Zheng, M.; Zheng, B. Structural and physicochemical properties of lotus seed starch treated with ultra-high pressure. Food Chem. 2015, 186, 223–230. [Google Scholar] [CrossRef]
- Li, J.; Han, W.; Zhang, B.; Zhao, S.; Du, H. Structure and physicochemical properties of resistant starch prepared by autoclaving-microwave. Starch/Staerke 2018, 70, 1800060. [Google Scholar] [CrossRef]
- Liu, G.; Hong, Y.; Gu, Z.; Li, Z.; Cheng, L.; Li, C. Preparation and characterization of pullulanase debranched starches and their properties for drug controlled-release. RSC Adv. 2015, 5, 97066–97075. [Google Scholar] [CrossRef]
- Chang, Y.; Lv, Y. Structure, functionality, and digestibility of acetylated hulless barley starch. Int. J. Food Prop. 2017, 20, 1818–1828. [Google Scholar] [CrossRef]
- Magallanes-Cruz, P.A.; Flores-Silva, P.C.; Bello-Perez, L.A. Starch structure influences its digestibility: A review. J. Food Sci. 2017, 82, 2016–2023. [Google Scholar] [CrossRef] [Green Version]
- Ma, Z.; Yin, X.; Chang, D.; Hu, X.; Boye, J.I. Long- and short-range structural characteristics of pea starch modified by autoclaving, α-amylolysis, and pullulanase debranching. Int. J. Biol. Macromol. 2018, 120, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ma, M.; Liu, X.; Xu, Z.; Zhang, C.; Sui, Z.; Corke, H. Microwave treatment alters the fine molecular structure of waxy hull-less barley starch. Int. J. Biol. Macromol. 2021, 193, 1086–1092. [Google Scholar] [CrossRef] [PubMed]
- Shen, R.L.; Zhang, W.J.; Dong, J.L. Preparation, structural characteristics and digestibility of resistant starches from highland barley, oats and buckwheat starches. J. Food Nutr. Res. 2016, 55, 303–312. [Google Scholar]
- Wang, H.; Li, Y.; Wang, L.; Wang, L.; Li, Z.; Qiu, J. Multi-scale structure, rheological and digestive properties of starch isolated from highland barley kernels subjected to different thermal treatments. Food Hydrocoll. 2022, 129, 107630. [Google Scholar] [CrossRef]
- Bao, C.; Zeng, H.; Zhang, Y.; Zhang, L.; Lu, X.; Guo, Z.; Miao, S.; Zheng, B. Structural characteristics and prebiotic effects of Semen coicis resistant starches (type 3) prepared by different methods. Int. J. Biol. Macromol. 2017, 105, 671–679. [Google Scholar] [CrossRef]
- Zeng, S.; Wu, X.; Lin, S.; Zeng, H.; Lu, X.; Zhang, Y.; Zheng, B. Structural characteristics and physicochemical properties of lotus seed resistant starch prepared by different methods. Food Chem. 2015, 186, 213–222. [Google Scholar] [CrossRef]
- Wang, J.; Wang, C.; Li, S.; Li, W.; Yuan, G.; Pan, Y.; Chen, H. Anti-diabetic effects of Inonotus obliquus polysaccharides in streptozotocin-induced type 2 diabetic mice and potential mechanism via PI3K-Akt signal pathway. Biomed. Pharmacother. 2017, 95, 1669–1677. [Google Scholar] [CrossRef] [PubMed]
- De Deckere, E.A.M.; Kloots, W.J.; Van Amelsvoort, J.M.M. Resistant starch decreases serum total cholesterol and triacylglycerol concentrations in rats. J. Nutr. 1993, 123, 2142–2151. [Google Scholar]
- Park, O.J.; Kang, N.E.; Chang, M.J.; Kim, W.K. Resistant starch supplementation influences blood lipid concentrations and glucose control in overweight subjects. J. Nutr. Sci. Vitaminol. 2004, 50, 93–99. [Google Scholar] [CrossRef] [Green Version]
- Haghikia, A.; Zimmermann, F.; Schumann, P.; Jasina, A.; Roessler, J.; Schmidt, D.; Heinze, P.; Kaisler, J.; Nageswaran, V.; Aigner, A.; et al. Propionate attenuates atherosclerosis by immune-dependent regulation of intestinal cholesterol metabolism. Eur. Heart J. 2022, 43, 518–533. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Ma, R.; Zhan, J.; Wang, F.; Tian, Y. The role of protein and its hydrolysates in regulating the digestive properties of starch: A review. Trends Food Sci. Technol. 2022, 125, 54–65. [Google Scholar] [CrossRef]
- Zeng, H.; Chen, P.; Chen, C.; Huang, C.; Lin, S.; Zheng, B.; Zhang, Y. Structural properties and prebiotic activities of fractionated lotus seed resistant starches. Food Chem. 2018, 251, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Wen, C.; Zhang, J.; Dzah, C.S.; Zhang, H.; He, Y.; Duan, Y. Structural characterization and physicochemical properties of arrowhead resistant starch prepared by different methods. Int. J. Biol. Macromol. 2020, 157, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Otemuyiwa, I.O.; Aina, A.F. Physicochemical properties and in-vitro digestibility studies of microwave assisted chemically modified breadfruit (Artocarpus altilis) starch. Int. J. Food Prop. 2021, 24, 140–151. [Google Scholar] [CrossRef]
- Van, S.J.J.G.; Tournois, H.; Wit, D.D.; Vliegenthart, J.F.G. Short range structure in partially crystalline potato starch determined with attenuated total reflectance FTIR. Carbohydr. Res. 1995, 279, 201–214. [Google Scholar]
- Şencan, A.; Kiliç, M. Investigation of the changes in surface area and FT-IR spectra of activated carbons obtained from hazelnut shells by physicochemical treatment methods. J. Chem. 2015, 2015, 651651. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Wang, Z.W.; Shi, X.M. Effect of microwave heat/moisture treatment on physicochemical properties of Canna edulis Ker starch. J. Sci. Food Agric. 2009, 89, 653–664. [Google Scholar] [CrossRef]
- Agama-Acevedo, E.; Pacheco-Vargas, G.; Bello-Pérez, L.A.; Alvarez-Ramirez, J. Effect of drying method and hydrothermal treatment of pregelatinized Hylon VII starch on resistant starch content. Food Hydrocoll. 2018, 77, 817–824. [Google Scholar] [CrossRef]
- Cai, J.; Cai, C.; Man, J.; Zhou, W.; Wei, C. Structural and functional properties of C-type starches. Carbohydr. Polym. 2014, 101, 289–300. [Google Scholar] [CrossRef]
- Demirkesen-Bicak, H.; Tacer-Caba, Z.; Nilufer-Erdil, D. Pullulanase treatments to increase resistant starch content of black chickpea (Cicer arietinum L.) starch and the effects on starch properties. Int. J. Biol. Macromol. 2018, 111, 505–513. [Google Scholar] [CrossRef]
- Kiatponglarp, W.; Tongta, S.; Rolland-Sabaté, A.; Buléon, A. Crystallization and chain reorganization of debranched rice starches in relation to resistant starch formation. Carbohydr. Polym. 2015, 122, 108–114. [Google Scholar] [CrossRef]
- Polesi, L.F.; Sarmento, S.B.S. Structural and physicochemical characterization of RS prepared using hydrolysis and heat treatments of chickpea starch. Starch/Staerke 2011, 63, 226–235. [Google Scholar] [CrossRef]
- Ma, Z.; Hu, X.; Boye, J.I. Research advances on the formation mechanism of resistant starch type III: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 276–297. [Google Scholar] [CrossRef]
- Yang, Q.; Qi, L.; Luo, Z.; Kong, X.; Xiao, Z.; Wang, P.; Peng, X. Effect of microwave irradiation on internal molecular structure and physical properties of waxy maize starch. Food Hydrocoll. 2017, 69, 473–482. [Google Scholar] [CrossRef]
- Xia, W.; Zhang, K.; Su, L.; Wu, J. Microbial starch debranching enzymes: Developments and applications. Biotechnol. Adv. 2021, 50, 107786. [Google Scholar] [CrossRef] [PubMed]
- Eerlingen, R.C.; Crombez, M.; Delcour, J.A. Enzyme-resistant starch. I. Quantitative and qualtitative influence of incubation time and temperature of autoclaved starch on resistant starch formation. Cereal Chem. 1993, 70, 339–344. [Google Scholar]
- Cornejo-Ramírez, Y.I.; Martínez-Cruz, O.; Del Toro-Sánchez, C.L.; Wong-Corral, F.J.; Borboa-Flores, J.; Cinco-Moroyoqui, F.J. The structural characteristics of starches and their functional properties. CYTA—J. Food 2018, 16, 1003–1017. [Google Scholar] [CrossRef]
- Wang, S.; Li, C.; Copeland, L.; Niu, Q.; Wang, S. Starch retrogradation: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 568–585. [Google Scholar] [CrossRef]
- Miao, M.; Jiang, B.; Zhang, T. Effect of pullulanase debranching and recrystallization on structure and digestibility of waxy maize starch. Carbohydr. Polym. 2009, 76, 214–221. [Google Scholar] [CrossRef]
- Li, S.; Ward, R.; Gao, Q. Effect of heat-moisture treatment on the formation and physicochemical properties of resistant starch from mung bean (Phaseolus radiatus) starch. Food Hydrocoll. 2011, 25, 1702–1709. [Google Scholar] [CrossRef]
- Englyst, H.N.; Kingman, S.M.; Cummings, J.H. Classification and measurement of nutritionally important starch fractions. Eur. J. Clin. Nutr. 1992, 46, S33–S50. [Google Scholar]
- You, S.Y.; Lim, S.T.; Lee, J.H.; Chung, H.J. Impact of molecular and crystalline structures on in vitro digestibility of waxy rice starches. Carbohydr. Polym. 2014, 112, 729–735. [Google Scholar] [CrossRef]
- Chung, H.J.; Lim, H.S.; Lim, S.T. Effect of partial gelatinization and retrogradation on the enzymatic digestion of waxy rice starch. J. Cereal Sci. 2006, 43, 353–359. [Google Scholar] [CrossRef]
- Nie, Y.; Luo, F. Dietary fiber: An opportunity for a global control of hyperlipidemia. Oxid. Med. Cell. Longev. 2021, 2021, 5542342. [Google Scholar] [CrossRef]
- Dongowski, G.; Jacobasch, G.; Schmiedl, D. Structural stability and prebiotic properties of resistant starch type 3 increase bile acid turnover and lower secondary bile acid formation. J. Agric. Food Chem. 2005, 53, 9257–9267. [Google Scholar] [CrossRef]
- Hu, Z.; Zhao, L.; Hu, Z.; Wang, K. Hierarchical structure, gelatinization, and digestion characteristics of starch from longan (Dimocarpus longan Lour.) seeds. Molecules 2018, 23, 3262. [Google Scholar] [CrossRef] [Green Version]
- Ma, M.; Zhang, Y.; Chen, X.; Li, H.; Sui, Z.; Corke, H. Microwave irradiation differentially affect the physicochemical properties of waxy and non-waxy hull-less barley starch. J. Cereal Sci. 2020, 95, 103072. [Google Scholar] [CrossRef]
- Arijaje, E.O.; Wang, Y.J.; Shinn, S.; Shah, U.; Proctor, A. Effects of chemical and enzymatic modifications on starch-stearic acid complex formation. J. Agric. Food Chem. 2014, 62, 2963–2972. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Huang, H.; Li, X.; Wang, T.; Chen, X.; Gao, W. Physicochemical characterisation, digestibility and anticonstipation activity of some high-resistant untraditional starches from zingiberaceae plants. Int. J. Food Sci. Technol. 2017, 52, 617–625. [Google Scholar] [CrossRef]
- Wang, C.; Li, W.; Chen, Z.; Gao, X.; Yuan, G.; Pan, Y.; Chen, H. Effects of simulated gastrointestinal digestion in vitro on the chemical properties, antioxidant activity, α-amylase and α-glucosidase inhibitory activity of polysaccharides from Inonotus obliquus. Food Res. Int. 2018, 103, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhang, Z.; Zhang, H.; Decker, E.A.; McClements, D.J. Influence of lipid type on gastrointestinal fate of oil-in-water emulsions: In vitro digestion study. Food Res. Int. 2015, 75, 71–78. [Google Scholar] [CrossRef]
- Sarkar, A.; Ye, A.; Singh, H. On the role of bile salts in the digestion of emulsified lipids. Food Hydrocoll. 2016, 60, 77–84. [Google Scholar] [CrossRef] [Green Version]
- Sánchez, A.; Maceiras, R.; Cancela, A.; Rodríguez, M. Influence of n-hexane on in Situ transesterification of marine macroalgae. Energies 2012, 5, 243–257. [Google Scholar] [CrossRef]





| Samples | HBSA | HBSM | HBSI | HBSP |
|---|---|---|---|---|
| Amylose content (% 1) | 24.53 ± 4.43 c | 29.25 ± 2.63 c | 51.29 ± 1.32 a | 36.02 ± 4.32 b |
| Protein content (%) | nd 2 | nd | nd | nd |
| RS content (%) | 21.81 ± 0.22 a | 21.37 ± 0.48 a | 22.57 ± 1.96 a | 21.39 ± 0.89 a |
| Solubility (%) | 3.86 ± 0.07 b | 3.46 ± 0.25 b | 63.24 ± 0.01 a | 61.34 ± 2.87 a |
| Swelling power (g/g) | 16.96 ± 0.97 a | 14.58 ± 1.33 b | 9.58 ± 0.10 c | 11.51 ± 0.43 c |
| Water-binding capacity (g/g) | 2.51 ± 0.13 a | 2.52 ± 0.31 a | 1.37 ± 0.31 b | 1.48 ± 0.15 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Tian, X.; Zhang, X.; Zhang, Z.; Zhang, X.; Zeng, X. Physicochemical Characterizations, Digestibility, and Lipolysis Inhibitory Effects of Highland Barley Resistant Starches Prepared by Physical and Enzymatic Methods. Molecules 2023, 28, 1065. https://doi.org/10.3390/molecules28031065
Wang C, Tian X, Zhang X, Zhang Z, Zhang X, Zeng X. Physicochemical Characterizations, Digestibility, and Lipolysis Inhibitory Effects of Highland Barley Resistant Starches Prepared by Physical and Enzymatic Methods. Molecules. 2023; 28(3):1065. https://doi.org/10.3390/molecules28031065
Chicago/Turabian StyleWang, Cong, Xinyi Tian, Xiayin Zhang, Zhiming Zhang, Xiaoyu Zhang, and Xiaoxiong Zeng. 2023. "Physicochemical Characterizations, Digestibility, and Lipolysis Inhibitory Effects of Highland Barley Resistant Starches Prepared by Physical and Enzymatic Methods" Molecules 28, no. 3: 1065. https://doi.org/10.3390/molecules28031065