Synthesis of Calamitic Fluorinated Mesogens with Complex Crystallization Behavior
Abstract
:1. Introduction
2. Results and Discussion
2.1. Ethylenation of the Iodofluorocompounds
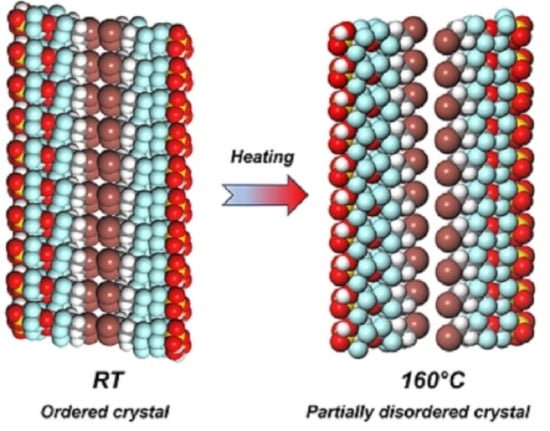
2.2. Structural Characteristics of I(CH2)2(CF2)2O(CF2)2SO2OH
3. Materials and Methods
3.1. Chemicals
3.2. Synthesis
3.3. Methods of Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gielen, D.; Boshell, F.; Saygin, D.; Bazilian, M.D.; Wagner, N.; Gorini, R. The Role of Renewable Energy in the Global Energy Transformation. Energy Strategy Rev. 2019, 24, 38–50. [Google Scholar] [CrossRef]
- Jacobson, M.Z. The Health and Climate Impacts of Carbon Capture and Direct Air Capture. Energy Environ. Sci. 2019, 12, 3567–3574. [Google Scholar] [CrossRef]
- Lew, D.; Miller, N.; Clark, K.; Jordan, G.; Gao, Z. Impact of High Solar Penetration in the Western Interconnection; National Renewable Energy Agency: Masdar City, Abu Dhabi, 2010; pp. 1–9.
- Inganäs, O. Organic Photovoltaics over Three Decades. Adv. Mater. 2018, 30, 1800388. [Google Scholar] [CrossRef]
- Zhang, G.; Lin, F.R.; Qi, F.; Heumüller, T.; Distler, A.; Egelhaaf, H.J.; Li, N.; Chow, P.C.Y.; Brabec, C.J.; Jen, A.K.; et al. Renewed Prospects for Organic Photovoltaics. Chem. Rev. 2022, 122, 14180–14274. [Google Scholar] [CrossRef]
- Armand, M.; Tarascon, J.-M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef]
- Sharma, P.K.; Kumar, D.A.; William, P.; Obulesu, D.; Pandian, P.M.; Khan, T.K.H.; Manikandan, G. Energy Storage System Based on Hybrid Wind and Photovoltaic Technologies. Meas. Sens. 2023, 30, 100915. [Google Scholar] [CrossRef]
- Okonkwo, P.C.; Belgacem, I.B.; Emori, W.; Uzoma, P.C. Nafion degradation mechanisms in proton exchange membrane fuel cell (PEMFC) system: A review. Int. J. Hydrog. Energy 2021, 46, 27956–27973. [Google Scholar] [CrossRef]
- Kusoglu, A.; Weber, A.Z. New Insights into Perfluorinated Sulfonic-Acid Ionomers. Chem. Rev. 2017, 117, 987–1104. [Google Scholar] [CrossRef]
- Fan, L.; Tu, Z.; Chan, S.H. Recent development of hydrogen and fuel cell technologies: A review. Energy Rep. 2021, 7, 8421–8446. [Google Scholar] [CrossRef]
- Tanaka, Y. Development of the MIRAI Fuel Cell vehicle. In Hydrogen Energy Engineering, A Japanese Perspective; Sasaki, K., Li, H.W., Hayashi, A., Yamabe, J., Ogura, T., Lyth, S.M., Eds.; Springer: Japan, Tokyo, 2016; Volume 34, pp. 461–476. [Google Scholar] [CrossRef]
- Jinnouchi, R.; Kudo, K.; Kodama, K.; Kitano, N.; Suzuki, T.; Minami, S.; Shinozaki, K.; Hasegawa, N.; Shinohara, A. The role of oxygen-permeable ionomer for polymer electrolyte fuel cells. Nat. Commun. 2021, 12, 4956. [Google Scholar] [CrossRef]
- Suzuki, T.; Tsushima, S.; Hirai, S. Effects of Nafion® Ionomer and Carbon Particles on Structure Formation in a Proton-Exchange Membrane Fuel Cell Catalyst Layer Fabricated by the Decal-Transfer Method. Int. J. Hydrog. Energy 2011, 36, 12361–12369. [Google Scholar] [CrossRef]
- Liu, S.; Lin, R.; Lu, J.; Wang, Y.; Cai, X. The Impact of Different Side Chain Ionomer on Membrane Electrode Assembly Performance and Durability. Chem. Eng. J. 2023, 472, 145050. [Google Scholar] [CrossRef]
- Yan, X.; Xu, Z.; Yuan, S.; Han, A.; Shen, Y.; Cheng, X.; Liang, Y.; Shen, S.; Zhang, J. Structural and Transport Properties of Ultrathin Perfluorosulfonic Acid Ionomer Film in Proton Exchange Membrane Fuel Cell Catalyst Layer: A Review. J. Power Sources 2022, 536, 231523. [Google Scholar] [CrossRef]
- Antipin, I.; Alémov, M.; Arslanov, V.; Burilov, V.; Vatsadze, S.; Voloshin, Y.; Volcho, K.; Gorbatchuk, V.; Gorbunova, Y.; Gromov, S. Functional supramolecular systems: Design and applications. Russ. Chem. Rev. 2021, 90, 895–1107. [Google Scholar] [CrossRef]
- Alfutimie, A.; Curtis, R.; Tiddy, G.J.T. Lyotropic Surfactant Liquid Crystals: Micellar Systems. In Handbook of Liquid Crystals, 2nd ed.; Goodby, J., Collings, P., Kato, T., Tschierske, C., Gleeson, H., Raynes, P., Vill, V., Eds.; Wiley-VCH: Weinheim, Germany, 2014; Volume 6. [Google Scholar] [CrossRef]
- Illy, N.; Urayeneza, D.; Maryasevskaya, A.V.; Michely, L.; Boileau, S.; Brissault, B.; Bersenev, E.A.; Anokhin, D.V.; Ivanov, D.A.; Penelle, J. Synthesis and Solid-State Properties of PolyC3 (Co)polymers Containing (CH2-CH2-C(COOR)2) Repeat Units with Densely Packed Fluorocarbon Lateral Chains. Macromolecules 2019, 52, 9199–9207. [Google Scholar] [CrossRef]
- Berrod, Q.; Lyonnard, S.; Guillermo, A.; Ollivier, J.; Frick, B.; Manseri, A.; Ameduri, B.; Gebel, G. Nanostructure and transport properties of proton conducting self-assembled perfluorinated surfactants: A bottom-up approach towards PFSA fuel cell membranes. Macromolecules 2015, 48, 6166–6176. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Yang, S. Multi-functional liquid crystal elastomer composites. Appl. Phys. Rev. 2022, 9, 011301. [Google Scholar] [CrossRef]
- Doostmohammadi, A.; Ladoux, B. Physics of liquid crystals in cell biology. Trends Cell Biol. 2022, 32, 140–150. [Google Scholar] [CrossRef]
- Kapernaum, N.; Lange, A.; Ebert, M.; Grunwald, M.A.; Haege, C.; Marino, S.; Zens, A.; Taubert, A.; Giesselmann, F.; Laschat, S. Current topics in ionic liquid crystals. ChemPlusChem 2022, 87, e202100397. [Google Scholar] [CrossRef]
- Komolkin, A.V.; Laaksonen, A.; Maliniak, A. Molecular dynamics simulation of a nematic liquid crystal. J. Chem. Phys. 1994, 101, 4103–4116. [Google Scholar] [CrossRef]
- Affouard, F.; Kröger, M.; Hess, S. Molecular dynamics of model liquid crystals composed of semiflexible molecules. Phys. Rev. E 1996, 54, 5178. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.R. Molecular simulation of liquid crystals: Progress towards a better understanding of bulk structure and the prediction of material properties. Chem. Soc. Rev. 2007, 36, 1881–1888. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Hooper, J.B.; Bedrov, D. Influence of electrostatic interactions on the properties of cyanobiphenyl liquid crystals predicted from atomistic molecular dynamics simulations. Liq. Cryst. 2017, 44, 332–347. [Google Scholar] [CrossRef]
- Ahmadpour-Samani, P.; Zahedi, P. An investigation on nematic-isotropic phase transition, viscosity, and diffusion coefficient of liquid crystalline elastomers at different temperatures using molecular dynamics simulation. J. Mol. Liq. 2022, 367, 120403. [Google Scholar] [CrossRef]
- Manseri, A.; Ameduri, B.; Boutevin, B.; Caporiccio, G.; Kotora, M.; Hajek, M. Synthesis of Telechelic Dienes from Fluorinated α,ω-Diiodoperfluoroalcanes. Part I. Divinyl and Diallyl Derivatives from Model I(C2F4)nI Compounds. J. Fluor. Chem. 1995, 73, 151–158. [Google Scholar] [CrossRef]
- Sauguet, L.; Ameduri, B.; Boutevin, B. Fluorinated Copolymers and Terpolymers Based on Vinylidene Fluoride and Bearing Sulfonic Acid Side-Group. J. Polym. Sci. Polym. Chem. 2007, 45, 1814–1834. [Google Scholar] [CrossRef]
- Goodby, J.W. Materials and Phase Structures of Calamitic and Discotic Liquid Crystals. In Handbook of Visual Display Technology; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1243–1287. [Google Scholar] [CrossRef]
- Corpart, J.-M.; Girault, S.; Juhué, D. Structure and Surface Properties of Liquid Crystalline Fluoroalkyl Polyacrylates: Role of the Spacer. Langmuir 2001, 17, 7237–7244. [Google Scholar] [CrossRef]
- Vivien, F.; Wicker, N. Minimal enclosing parallelepiped in 3D. Comput. Geom. 2004, 29, 177–190. [Google Scholar] [CrossRef]
- Ball, J.M.; Majumdar, A. Nematic liquid crystals: From Maier-Saupe to a continuum theory. Mol. Cryst. Liq. Cryst. 2010, 525, 1–11. [Google Scholar] [CrossRef]
- Glagoleva, A.A.; Vasilevskaya, V.V.; Khokhlov, A.R. Microphase separation in the melts of diblock copolymers composed of linear and amphiphilic blocks. Polym. Sci. Ser. A 2010, 52, 182–190. [Google Scholar] [CrossRef]
- Dierking, I.; Al-Zangana, S. Lyotropic liquid crystal phases from anisotropic nanomaterials. Nanomaterials 2017, 7, 305. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.R.; Yu, G.; Potter, T.D.; Walker, M.; Gray, S.J.; Li, J.; Boyd, N.J. Molecular simulation approaches to the study of thermotropic and lyotropic liquid crystals. Crystals 2022, 12, 685. [Google Scholar] [CrossRef]
- Sun, H.; Ren, P.; Fried, J.R. The COMPASS force field: Parameterization and validation for phosphazenes. Comput. Theor. Polym. Sci. 1998, 8, 229–246. [Google Scholar] [CrossRef]
- Beckers, J.V.L.; Lowe, C.P.; De Leeuw, S.W. An iterative PPPM method for simulating Coulombic systems on distributed memory parallel computers. Mol. Simul. 1998, 20, 369–383. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; Postma, J.P.M.; van Gunsteren, W.F.; DiNola, A.; Haak, J.R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 1984, 81, 3684–3690. [Google Scholar] [CrossRef]
- Shuichi, N. Constant temperature molecular dynamics methods. Prog. Theor. Phys. Suppl. 1991, 103, 1–46. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anokhin, D.; Maryasevskaya, A.; Abukaev, A.; Ozkose, U.U.; Buglakov, A.; Ivanov, D.A.; Améduri, B. Synthesis of Calamitic Fluorinated Mesogens with Complex Crystallization Behavior. Molecules 2023, 28, 8002. https://doi.org/10.3390/molecules28248002
Anokhin D, Maryasevskaya A, Abukaev A, Ozkose UU, Buglakov A, Ivanov DA, Améduri B. Synthesis of Calamitic Fluorinated Mesogens with Complex Crystallization Behavior. Molecules. 2023; 28(24):8002. https://doi.org/10.3390/molecules28248002
Chicago/Turabian StyleAnokhin, Denis, Alina Maryasevskaya, Ainur Abukaev, Umut Ugur Ozkose, Alexander Buglakov, Dimitri A. Ivanov, and Bruno Améduri. 2023. "Synthesis of Calamitic Fluorinated Mesogens with Complex Crystallization Behavior" Molecules 28, no. 24: 8002. https://doi.org/10.3390/molecules28248002