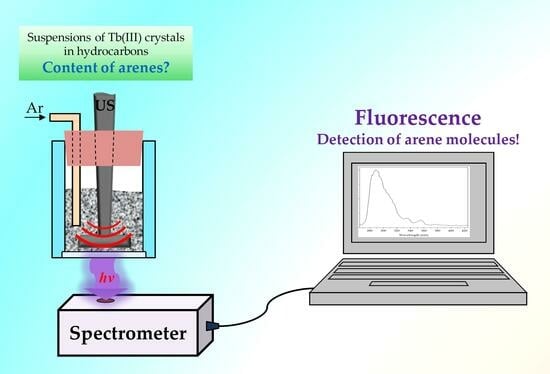
Application of Sonotriboluminescence to Determine Arene Molecules in Hydrocarbons
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tukhbatullin, A.A.; Sharipov, G.L.; Burangulova, N.F.; Mustafin, A.G. Luminescence of aromatic hydrocarbon molecules in the sonication of terbium sulfate suspensions. Ultrason. Sonochem. 2019, 50, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Tukhbatullin, A.A.; Sharipov, G.L.; Burangulova, N.F. Scintillation activation of luminescence of terbium sulfate suspensions in aromatic hydrocarbons under sonication. J. Mol. Liq. 2019, 289, 110973. [Google Scholar] [CrossRef]
- Tukhbatullin, A.A.; Sharipov, G.L. Luminescence of aromatic compounds during ultrasonic treatment of Tb2(SO4)3 suspension in commercial gasoline. Appl. Spectrosc. 2022, 76, 1216–1221. [Google Scholar] [CrossRef] [PubMed]
- Ashokkumar, M. The characterization of acoustic cavitation bubbles—An overview. Ultrason. Sonochem. 2011, 18, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Rooze, J.; Rebrov, E.V.; Schouten, J.C.; Keurentjes, J.T.F. Dissolved gas and ultrasonic cavitation—A review. Ultrason. Sonochem. 2013, 20, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Borisenok, V.A. Sonoluminescence: Experiments and models (Review). Acoust. Phys. 2015, 61, 308–332. [Google Scholar] [CrossRef]
- Suslick, K.S.; Eddingsaas, N.C.; Flannigan, D.J.; Hopkins, S.D.; Xu, H. The chemical history of a bubble. Acc. Chem. Res. 2018, 51, 2169–2178. [Google Scholar] [CrossRef]
- Eddingsaas, N.C.; Suslick, K.S. Mechanoluminescence: Light from sonication of crystal slurries. Nature 2006, 444, 163. [Google Scholar] [CrossRef]
- Eddingsaas, N.C.; Suslick, K.S. Intense mechanoluminescence and gas phase reactions from the sonication of an organic slurry. J. Am. Chem. Soc. 2007, 129, 6718–6719. [Google Scholar] [CrossRef]
- Nakayama, K. Microplasma generation in gap of sliding contact through discharging of ambient gas due to triboelectrification. Int. J. Plasma Environ. Sci. Technol. 2010, 4, 148–153. [Google Scholar] [CrossRef]
- Collins, A.L.; Camara, C.G.; Cleve, E.V.; Putterman, S.J. Simultaneous measurement of triboelectrification and triboluminescence of crystalline materials. Rev. Sci. Instrum. 2018, 89, 013901. [Google Scholar] [CrossRef]
- Wong, H.-Y.; Chan, W.T.; Law, G.-L. Triboluminescence of centrosymmetric lanthanide β-diketonate complexes with aggregation-induced emission. Molecules 2019, 24, 662. [Google Scholar] [CrossRef]
- Virot, M.; Pflieger, R.; Skorb, E.V.; Ravaux, J.; Zemb, T.; Möhwald, H. Crystalline silicon under acoustic cavitation: From mechanoluminescence to amorphization. J. Phys. Chem. C 2012, 116, 15493–15499. [Google Scholar] [CrossRef]
- Eddingsaas, N.C.; Suslick, K.S. Plasma characteristics of the discharge produced during mechanoluminescence. Phys. Rev. Lett. 2007, 99, 234301. [Google Scholar] [CrossRef]
- Sharipov, G.L.; Tukhbatullin, A.A. Triboluminescence of inorganic lanthanide salts. In Triboluminescence: Theory, Synthesis, and Application; Olawale, D.O., Okoli, O.O.I., Fontenot, R.S., Hollerman, W.A., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 273–303. [Google Scholar]
- Eddingsaas, N.C. Mechanoluminescence induced by acoustic cavitation. In Triboluminescence: Theory, Synthesis, and Application; Olawale, D.O., Okoli, O.O.I., Fontenot, R.S., Hollerman, W.A., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 237–271. [Google Scholar]
- Sage, I.; Bourhill, G. Triboluminescent materials for structural damage monitoring. J. Mater. Chem. 2001, 11, 231–245. [Google Scholar] [CrossRef]
- Bunzli, J.C.G.; Wong, K.L. Lanthanide mechanoluminescence. J. Rare Earths 2018, 36, 1–41. [Google Scholar] [CrossRef]
- Berlman, I.B. Handbook of Fluorescence Spectra of Aromatic Molecules, 2nd ed.; Academic Press: New York, NY, USA, 1971; p. 473. [Google Scholar]
- Koban, W.; Koch, J.D.; Hanson, R.K.; Schulz, C. Absorption and fluorescence of toluene vapor at elevated temperatures. Phys. Chem. Chem. Phys. 2004, 6, 2940–2945. [Google Scholar] [CrossRef]
- Nakayama, K. Triboplasma generation and triboluminescence: Influence of stationary sliding partner. Tribol. Lett. 2010, 37, 215–228. [Google Scholar] [CrossRef]
- Nevshupa, R. Effect of gas pressure on the triboluminescence and contact electrification under mutual sliding of insulating materials. J. Phys. D Appl. Phys. 2013, 46, 185501. [Google Scholar] [CrossRef]
- Camara, C.G.; Escobar, J.V.; Hird, J.R.; Putterman, S.J. Correlation between nanosecond X-ray flashes and stick–slip friction in peeling tape. Nature 2008, 455, 1089–1092. [Google Scholar] [CrossRef]
- Goryacheva, I.Y.; Mel’nikov, G.V.; Shtykov, S.N.; Ponomarev, A.S. Phosphorimetric determination of polynuclear aromatic hydrocarbons in gasoline. J. Anal. Chem. 2000, 55, 795–798. [Google Scholar] [CrossRef]
- Zoccolillo, L.; Babi, D.; Felli, M. Evaluation of polycyclic aromatic hydrocarbons in gasoline by HPLC and GC-MS. Chromatographia 2000, 52, 373–376. [Google Scholar] [CrossRef]
- Flumignan, D.L.; Boralle, N.; De Oliveira, J.E. Screening Brazilian commercial gasoline quality by hydrogen nuclear magnetic resonance spectroscopic fingerprintings and pattern-recognition multivariate chemometric analysis. Talanta 2010, 82, 99–105. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tukhbatullin, A.A.; Panova, N.A.; Galimov, D.I.; Gareev, B.M.; Tukhbatullina, A.A.; Vasilyuk, K.S.; Sharipov, G.L. Application of Sonotriboluminescence to Determine Arene Molecules in Hydrocarbons. Molecules 2023, 28, 7932. https://doi.org/10.3390/molecules28237932
Tukhbatullin AA, Panova NA, Galimov DI, Gareev BM, Tukhbatullina AA, Vasilyuk KS, Sharipov GL. Application of Sonotriboluminescence to Determine Arene Molecules in Hydrocarbons. Molecules. 2023; 28(23):7932. https://doi.org/10.3390/molecules28237932
Chicago/Turabian StyleTukhbatullin, Adis A., Nadezhda A. Panova, Dim I. Galimov, Bulat M. Gareev, Alina A. Tukhbatullina, Kristina S. Vasilyuk, and Glyus L. Sharipov. 2023. "Application of Sonotriboluminescence to Determine Arene Molecules in Hydrocarbons" Molecules 28, no. 23: 7932. https://doi.org/10.3390/molecules28237932