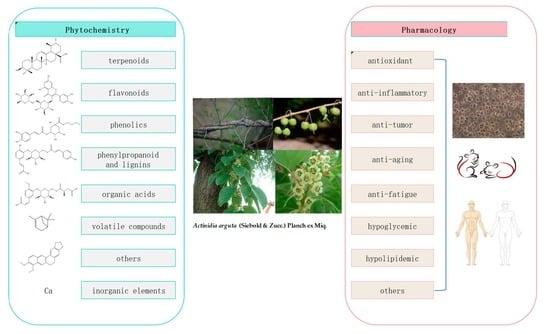
Actinidia arguta (Sieb. et Zucc.) Planch. ex Miq.: A Review of Phytochemistry and Pharmacology
Abstract
:1. Introduction
2. Materials and Methods
3. Phytochemistry
3.1. Terpenoids
3.2. Flavonoids
3.3. Phenolic Compounds
3.4. Phenylpropanoid and Lignin Compounds
3.5. Organic Acids (Esters)
3.6. Volatile Compounds
3.7. Other Compounds
3.8. Inorganic Elements
4. Pharmacological Activities
4.1. Antioxidant Activity
4.2. Anti-Inflammatory Activity
4.3. Anti-Tumor Activity
4.4. Anti-Aging Activity
4.5. Anti-Fatigue Effect
4.6. Hypoglycemic Activity
4.7. Hypolipidemic Activity
4.8. Other Pharmacological Effects
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, J.P.; Wang, Y.L.; Lu, X.L.; Shen, Z.H.; Wang, M.T.; Li, Q.; Wang, R.L. Climatic suitable area analysis and response to climate change of Actinidia arguta in China. Chin. J. Eco-Agric. 2020, 28, 1523–1532. [Google Scholar] [CrossRef]
- He, X.; Fang, J.; Chen, X.; Zhao, Z.; Li, Y.; Meng, Y.; Huang, L. Actinidia chinensis Planch.: A review of chemistry and pharmacology. Front. Pharmacol. 2019, 10, 1236. [Google Scholar] [CrossRef] [PubMed]
- Medic, A.; Hudina, M.; Veberic, R. The effect of cane vigour on the kiwifruit (Actinidia chinensis) and kiwiberry (Actinidia arguta) quality. Sci. Rep. 2021, 11, 12749. [Google Scholar] [CrossRef] [PubMed]
- Di, X.; Wang, H.B.; Zhang, J.K.; Zhai, Y.J.; Wang, Y. Radix Actinidiae Chinensis’ herbal textual. J. Liaoning Univ. Tradit. Chin. Med. 2014, 16, 132–134. [Google Scholar] [CrossRef]
- Zhao, J.H.; Wang, Y.P.; Ai, J.; Gao, B.; Bi, H.H. Processing technology of Actinidia arguta leaf tea. Spec. Econ. Anim. Plants 2014, 17, 53–54. [Google Scholar]
- Tang, Y.N.; Tian, M.X.; Li, Y.Y.; Li, L.Y.; De, X.T.; Zhang, Y. Research progress on development and utilization of Actinidia arguta. Storage Process 2023, 23, 76–80. [Google Scholar]
- Bai, L. Study on the Brewing Technology of Actinidia arguta Brandy. Master’s Thesis, Shenyang Agricultural University, Shenyang, China, 2020. [Google Scholar] [CrossRef]
- Shao, X.R.; Sun, H.T.; Li, H.K. Development of wild Actinidia arguta jam in Changbai Mountains. Mod. Food Sci. Technol. 2012, 28, 1548–1550+1565. [Google Scholar] [CrossRef]
- Lv, M.; Wang, J.W. Study on the technology of compound beverage made of hawthorn and kiwifruit. Beverage Ind. 2015, 18, 49–52. [Google Scholar] [CrossRef]
- Wang, H.L.; Ding, H.; An, D.P.; Wang, Z.; Ma, S.; Wang, J.Q. Research on fermentation process of Actinidia arguta complex ferment beverage and its antioxidant activities. Food Res. Dev. 2021, 42, 130–135. [Google Scholar] [CrossRef]
- Wang, X.T. Study on Production Process of Actinidia Pectin Oral. Master’s Thesis, Shenyang Agricultural University, Shenyang, China, 2017. [Google Scholar]
- Ding, Y.P.; Wang, M.Z.; Liu, Y.X.; Skrypchenko, N.V.; Liu, D.J. Research progress on product development and utilization of Actinidia arguta. Food Ferment. Ind. 2023, 49, 308–314+323. [Google Scholar] [CrossRef]
- Niu, Q.; Shen, J.; Liu, Y.; Nie, C.Y.; Skrypchenko, N.V.; Liu, D.J. Research progress on main active constituents and pharmacological activities of Actinidia arguta. Sci. Technol. Food Ind. 2019, 40, 333–338+344. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, H.L.; Ma, B.R. Studies on the chemical constituents of Actinidia arguta (Sieb. Et Zucc.) Planch. Ex Miquel. China J. Chin. Mater. Med. 1992, 17, 36–38+64. [Google Scholar]
- Di, X.; Wang, H.B.; Zhai, Y.J.; Pan, L. Determination of oleanolic acid and ursolic acid in Radix Actinidia by RP-HPLC. Chin. J. Exp. Tradit. Med. Formulae 2012, 18, 76–78. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, H.L.; Ma, B.R. Isolation and identification of triterpenoids from Actinidia aryguta. Chin. Tradit. Herb. Drugs 1993, 24, 386–387. [Google Scholar]
- Shi, Y.; Wang, Z.B. Studies on the chemical constituents of Actinidia aryguta (Sieb. et Zucc.) Planch ex Miquel. J. Zhangjiakou Med. Coll. 1994, 11, 34–35. [Google Scholar]
- Teng, K.; Zhang, H.F.; Zang, H.; Shen, P.; Sun, J.M. Antioxidant activities in vitro and components analysis of methanol extract of Actinidia arguta by HPLC-DAD-ESI-MS/MS. Chin. Tradit. Herb. Drugs 2019, 50, 4384–4388. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, B.Z.; Ma, B.R.; Sun, J.Y. Studies on the chemical constituents of the stem of Actinidia arguta (Sieb. Et Zucc.) Planch. Ex Miquel. Chin. Pharm. J. 1994, 29, 523–524. [Google Scholar]
- Ahn, J.H.; Park, Y.K.; Yeon, S.W.; Jo, Y.H.; Han, Y.K.; Turk, A.; Ryu, S.H.; Hwang, B.Y.; Lee, K.Y.; Lee, M.K. Phenylpropanoid-conjugated triterpenoids from the leaves of Actinidia arguta and their inhibitory activity on α-glucosidase. J. Nat. Prod. 2020, 83, 1416–1423. [Google Scholar] [CrossRef]
- Li, S.F.; Liu, H.; Zhang, X.L.; Li, D.Y.; Li, Z.L. A new norsesquiterpene glycoside from fruits of Actinidia arguta. Chin. Tradit. Herb. Drugs 2020, 51, 299–306. [Google Scholar] [CrossRef]
- Kim, J.G.; Beppu, K.J.; Kataoka, I. Varietal differences in phenolic content and astringency in skin and flesh of hardy kiwifruit resources in Japan. Sci. Hortic. 2009, 120, 551–554. [Google Scholar] [CrossRef]
- Tan, C.H.; Wang, Z.G.; Muhammad, I.; Liu, C.J. Analysis of flavonoids biosynthesis-related genes expression reveals the mechanism of difference of flavonoid content in different tissues of Actinidia arguta. Braz. J. Bot. 2021, 44, 513–523. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Tian, J.L.; Gao, N.X.; Gong, E.S.; Xin, G.; Liu, C.J.; Si, X.; Sun, X.Y.; Li, B. Assessment of the phytochemical profile and antioxidant activities of eight kiwiberry (Actinidia arguta (Siebold & Zuccarini) Miquel) varieties in China. Food Sci. Nutr. 2021, 9, 5616–5625. [Google Scholar] [CrossRef]
- Jang, D.S.; Lee, G.Y.; Lee, Y.M.; Kim, Y.S.; Sun, H.; Kim, D.H.; Kim, J.S. Flavan-3-ols having a γ-lactam from the roots of Actinidia arguta inhibit the formation of advanced glycation end products in vitro. Chem. Pharm. Bull. 2009, 57, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Webby, R.F.; Markham, K.R. A flavonol 3-O-triglycoside from Actinidia species. Phytochemistry 1990, 29, 289–292. [Google Scholar] [CrossRef]
- Webby, R.F. A flavonol triglycoside from Actinidia arguta var. Giraldii. Phytochemistry 1991, 30, 2443–2444. [Google Scholar] [CrossRef] [PubMed]
- Santos-Buelga, C.; González-Paramás, A.M.; Oludemi, T.; Ayuda-Durán, B.; González-Manzano, S. Plant phenolics as functional food ingredients. Adv. Food Nutr. Res. 2019, 90, 183–257. [Google Scholar] [CrossRef]
- Ahn, J.H.; Ryu, S.H.; Lee, S.; Yeon, S.W.; Turk, A.; Han, Y.K.; Lee, K.Y.; Hwang, B.Y.; Lee, M.K. Aromatic constituents from the leaves of Actinidia arguta with antioxidant and α-glucosidase inhibitory activity. Antioxidants 2021, 10, 1896. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.D.; Lee, J.Y.; Auh, J.H. Metabolomic screening of anti-inflammatory compounds from the leaves of Actinidia arguta (hardy kiwi). Foods 2019, 8, 47. [Google Scholar] [CrossRef]
- Jong, H.A.; Se, H.R.; Sang, W.Y.; Solip, L.; Seon, B.K.; Bang, Y.H.; Mi, K.L. Phenyldilactones from the leaves of hardy kiwifruit (Actinidia arguta). Biochem. Syst. Ecol. 2023, 108, 104636. [Google Scholar] [CrossRef]
- Ahn, J.H.; Yeon, S.W.; Ryu, S.H.; Lee, S.; Turk, A.; Hwang, Y.B.; Lee, M.K. Three new succinate-phenolic conjugates from the fruits of Actinidia arguta. Phytochem. Lett. 2022, 48, 128–131. [Google Scholar] [CrossRef]
- Cui, Q.; Du, R.; Liu, M.; Rong, L. Lignans and their derivatives from plants as antivirals. Molecules 2020, 25, 183. [Google Scholar] [CrossRef]
- Guo, L.L.; Li, L.L.; Fu, X.; Shen, J.; Tian, L.J.; Yang, C.J.; Liu, D.J. Extraction process and antioxidant activity of chlorogenic acid from leaves of Actinidia arguta (Sieb. & Zucc) Planch. ex Miq. Mol. Plant Breed. 2023. Available online: https://kns.cnki.net/kcms/detail//46.1068.S.20230202.1019.002.html (accessed on 29 October 2023).
- Zhang, J.Y.; Gao, N.X.; Shu, C.; Cheng, S.C.; Sun, X.Y.; Liu, C.J.; Xin, G.; Li, B.; Tian, J.L. Phenolics profile and antioxidant activity analysis of kiwi berry (Actinidia arguta) flesh and peel extracts from four regions in China. Front. Plant Sci. 2021, 12, 689038. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.H.; Park, Y.K.; Jo, Y.H.; Kim, S.B.; Yeon, S.W.; Kim, J.G.; Turk, A.; Song, J.Y.; Kim, Y.S.; Hwang, B.Y.; et al. Organic acid conjugated phenolic compounds of hardy kiwifruit (Actinidia arguta) and their NF-κB inhibitory activity. Food Chem. 2020, 308, 125666. [Google Scholar] [CrossRef]
- Park, H.M.; Son, M.W.; Kim, D.; Kim, S.H.; Kim, S.H.; Kwon, H.C.; Kim, S.Y. Fatty acid components of hardy kiwifruit (Actinidia arguta) as IL-4 production inhibitor. Biomol. Ther. 2011, 19, 126–133. [Google Scholar] [CrossRef]
- Wang, B.Z.; Zhao, H.; Ma, B.R.; Zhang, Y.P. Studies on the chemical constituents of the stem of Actinidia arguta (Sieb. Et Zucc.) Planch. Ex Miquel. (Ⅱ). J. Bethune Med. Univ. 1996, 22, 134. [Google Scholar]
- Jin, D.E.; Park, S.K.; Park, C.H.; Seung, T.W.; Choi, S.G.; Heo, H.J. Nutritional components of Korean traditional Actinidia (Actinidia arguta) sprout and in vitro antioxidant effect. Korean J. Food Sci. Technol. 2015, 47, 37–43. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, X. The effect of plant-derived low-ratio linoleic acid/α-linolenic acid on markers of llucosecontrols: A systematic review and meta-analysis. Int. J. Mol. Sci. 2023, 24, 14383. [Google Scholar] [CrossRef]
- Matich, A.J.; Young, H.; Allen, J.M.; Wang, M.; Fielder, S.; McNeilage, M.A.; MacRae, E.A. Actinidia arguta: Volatile compounds in fruit and flowers. Phytochemistry 2003, 63, 285–301. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, B.; Xin, G.; Li, S.Q.; Sun, X.R.; Liu, C.J. Analysis of essential oil from Actinidia arguta by GC/MS with pretreatment of pectinase. Food Res. Dev. 2012, 33, 6–9. [Google Scholar]
- Yang, M.F.; Zhao, Q.Y.; Liu, G.P. Analysis of constituents of essential oil by GC-MS from Actinidia arguta. Bull. Bot. Res. 2006, 26, 127–129. [Google Scholar]
- Xin, G.; Zhang, B.; Feng, F.; Li, T.C.; Liu, C.J.; Xu, J.G. Analysis of aromatic aonstituents of Actinidia arguta Sieb. et Zucc fruit. Food Sci. 2009, 30, 230–232. [Google Scholar]
- Sun, Y.; Zhang, B.; Li, S.Q.; Xin, G.; Sun, X.R.; Liu, C.J. Changes in aroma components of Actinidia arguta during postharvest storage at 20 °C. Food Sci. 2012, 33, 155–158. [Google Scholar]
- Wang, H.L.; Quan, H.X.; Sun, T.L.; Wang, Z.; Yang, Y.H. Chemical composition, antimicrobial, and antioxidant cytotoxic activities of essential oil from Actinidia arguta. Arch. Microbiol. 2022, 204, 239. [Google Scholar] [CrossRef]
- Liang, P.; Li, S.Q.; Zhang, B.; Liu, C.J.; Xin, G. Fatty acid composition in fruit of wild Actinidia arguta Sieb.et Zucc. Food Sci. 2011, 32, 237–239. [Google Scholar]
- Song, M.J.; Hui, R.H.; Hou, D.Y.; Jin, M. Analysis of fatty acids in Actinidia arguta by GC/MS. J. Anshan Norm. Univ. 2013, 15, 44–46. [Google Scholar]
- Jiang, A.L.; Shen, X.; Hu, W.Z.; Jiang, W.W.; Jiang, B. Supercritical CO2 fluid extraction and fatty acid composition analysis of kiwi seed oil. China Oils Fats 2008, 33, 77–79. [Google Scholar]
- Yang, Z.H.; Li, L.Y.; Yin, Z.Y.; Zhang, H. Constituent analysis of volatile oil in radix of Actinidia arguta. Heilongjiang Med. J. 2000, 13, 137–138. [Google Scholar] [CrossRef]
- Liu, Y.Y. Extraction, Purification and Biological Activities of Alkaloid from Actinidia auguta. Ph.D. Thesis, Shenyang Agricultural University, Shenyang, China, 2016. [Google Scholar]
- Yang, Y.H.; Li, X.; Yang, J.; Liu, C.J.; Tang, Z.L. Isolation, purification and detection of anthraquinone compounds from the root of Actinidia arguta Sieb. et Zucc. Food Sci. 2014, 35, 124–127. [Google Scholar]
- Qin, H.Y.; Zhang, B.X.; Ai, J.; Zhao, Y.; Li, X.Y.; Yang, Y.M.; Zhao, H.; Fan, S.T.; Liu, Y.X. Analysis of amino acids in the fruit, fruit wine and jam of Actinidia arguta. Sci. Technol. Food Ind. 2015, 36, 355–358. [Google Scholar] [CrossRef]
- Wang, D.; Liu, Z.S.; Lin, S.S. Isolation and identification of inositol from Actinidia arguta. Chin. Tradit. Herb. Drugs 1991, 22, 59. [Google Scholar]
- Zhang, J.S.; Li, P.Y.; Lu, S.X.; Wang, L.L.; Shi, Y.; Liu, J.Y.; Song, X.H.; Li, L.N.; Tian, L.Y. Determination of contents of inorganic elements in different parts of Actinidia arguta (Sieb. Et zucc) Planch. J. Bethune Med. Univ. 1993, 19, 354–356. [Google Scholar] [CrossRef]
- Liochev, S.I. Reactive oxygen species and the free radical theory of aging. Free Radic. Biol. Med. 2013, 60, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liu, A.; Hu, S.; Ares, I.; Martínez-Larrañaga, M.R.; Wang, X.; Martínez, M.; Anadón, A.; Martínez, M.A. Synthetic phenolic antioxidants: Metabolism, hazards and mechanism of action. Food Chem. 2021, 353, 129488. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Xu, Q.; Li, L.; Lin, L.; Yu, J.L.; Zhu, J.Y.; Zhang, H.; Xia, G.Q.; Zang, H. Antioxidant and enzyme-inhibitory activity of extracts from Erigeron annuus flower. Ind. Crops Prod. 2020, 148, 112283. [Google Scholar] [CrossRef]
- Chen, M.H.; He, X.; Sun, H.; Sun, Y.; Li, L.; Zhu, J.Y.; Xia, G.Q.; Guo, X.; Zang, H. Phytochemical analysis, UPLC-ESI-Orbitrap-MS analysis, biological activity, and toxicity of extracts from Tripleurospermum limosum (Maxim.) pobed. Arabian J. Chem. 2022, 15, 103797. [Google Scholar] [CrossRef]
- Feng, J.X.; Sun, Y.; Wei, Z.B.; Sun, H.; Li, L.; Zhu, J.Y.; Xia, G.Q.; Zang, H. Screening the extract of Laportea bulbifera (Sieb. et Zucc.) wedd. based on active component content, its antioxidant capacity and exploration of hepatoprotective activity in rats. Molecules 2023, 28, 6256. [Google Scholar] [CrossRef]
- Sun, Y.; Feng, J.X.; Wei, Z.B.; Sun, H.; Li, L.; Zhu, J.Y.; Xia, G.Q.; Zang, H. Phytochemical analysis, antioxidant activities in vitro and in vivo, and theoretical calculation of different extracts of Euphorbia fischeriana. Molecules 2023, 28, 5172. [Google Scholar] [CrossRef]
- An, X.X.; Lee, S.G.; Kang, H.; Heo, H.J.; Cho, Y.S.; Kim, D.O. Antioxidant and anti-inflammatory effects of various cultivars of kiwi berry (Actinidia arguta) on lipopolysacharide-stimulated RAW 264.7 cells. J. Microbiol. Biotechnol. 2016, 26, 1367–1374. [Google Scholar] [CrossRef]
- Barbara, P.; Grazyna, H.C.; Elwira, W. Effects of solvents and extraction methods on the content and antiradical activity of polyphenols from fruits Actinidia arguta, Crataegus monogyna, Gaultheria procumbens and Schisandra chinensis. Acta Sci. Pol. Technol. Aliment. 2016, 15, 57–63. [Google Scholar] [CrossRef]
- Wen, G.; Liu, H.Y.; Yang, M. Extraction of flavonoids from Actinidia arguta and its antioxidant activity in vitro. North. Hortic. 2015, 37, 140–143. [Google Scholar]
- Zhang, Q.N. Study on the Antioxidative Effect of Quercetin from Actinidia arguta and the Antitumor Effect and Toxicity of Doxorubicin Liposome Containing Quercetin and Vitamin C. Ph.D. Thesis, Shenyang Agricultural University, Shenyang, China, 2019. [Google Scholar] [CrossRef]
- Esposito, E.; Drechsler, M.; Puglia, C.; Cortesi, R. New strategies for the delivery of some natural anti-oxidants with therapeutic properties. Mini-Rev. Med. Chem. 2019, 19, 1030–1039. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Sowndhararajan, K.; Kim, M.; Kim, J.; Kim, D.; Kim, S.; Kim, G.Y.; Kim, S.; Jhoo, J.W. Antioxidant, inhibition of α-glucosidase and suppression of nitric oxide production in LPS-induced murine macrophages by different fractions of Actinidia arguta stem. Saudi J. Biol. Sci. 2014, 21, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Hao, Y.J.; An, X.L.; Sun, H.D.; Lian, M.L.; Jin, M.Y. Comparative analysis of antioxidant activities of different solvent extracts from adventitious roots of Actinidia arguta. Agric. Sci. J. Yanbian Univ. 2019, 41, 8–12. [Google Scholar] [CrossRef]
- Khromykh, N.O.; Lykholat, Y.V.; Didur, O.O.; Sklyar, T.V.; Davydov, V.R.; Lavrentieva, K.V.; Lykholat, T.Y. Phytochemical profiles, antioxidant and antimicrobial activity of Actinidia polygama and A. arguta fruits and leaves. Biosyst. Divers. 2022, 30, 39–45. [Google Scholar] [CrossRef]
- Macedo, C.; Silva, A.M.; Ferreira, A.S.; Cadiz-Gurrea, M.D.; Ferandez-Ochoa, A.; Segura-Carretero, A.; Delerue-Matos, C.; Costa, P.; Rodrigues, F. Insights into the polyphenols extraction from Actinidia arguta fruit (kiwiberry): A source of pro-healthy compounds. Sci. Hortic. 2023, 313, 111910. [Google Scholar] [CrossRef]
- Huang, G.; Mei, X.; Hu, J. The antioxidant activities of natural polysaccharides. Curr. Drug Targets 2017, 18, 1296–1300. [Google Scholar] [CrossRef]
- Liu, C.J.; Pan, S.; Liang, S. In vitro antioxidant activity of polysaccharides from Actinidia arguta sieb. et zucc. fruits. Food Sci. 2012, 33, 79–82. [Google Scholar]
- Ji, X.G.; Xie, P.; Yang, D.H.; Liu, D.C.; Yang, X.W.; Zhou, Q.B.; Liu, D.J.; Yang, H.S. Extraction technology optimization and antioxidant activity of polysaccharide from Actinidia arguta fruit. Chin. Wild Plant Resour. 2023, 42, 59–66. [Google Scholar] [CrossRef]
- Chang, Q.Q.; Wang, H.L.; Bai, X.X.; Liu, D.Y.; Yuan, Y.; Lu, J. Extraction of polysaccharide and antioxidant activit from Actinidia arguta stems and leaves in Changbai Mountain. North. Hortic. 2018, 40, 136–144. [Google Scholar]
- Gu, S.T.; Jiang, A.L.; Li, X.M.; Jin, H.; Hu, W.Z. Effects of different storage temperatures on postharvest physiological quality and antioxidative capacity of Actinidia arguta. Food Ferment. Ind. 2019, 45, 178–184. [Google Scholar] [CrossRef]
- Kim, H.Y.; Hwang, K.W.; Park, S.Y. Extracts of Actinidia arguta stem inhibited LPS-induced inflammatory responses through nuclear factor-κB pathway in RAW 264.7 cells. Nutr. Res. 2014, 34, 1008–1016. [Google Scholar] [CrossRef] [PubMed]
- Heoa, K.H.; Xiao, S.; Shim, D.W.; Kim, M.K.; Koppula, S.; Yu, S.H.; Kim, H.B.; Kim, T.J.; Kang, T.B.; Lee, K.H. Actinidia arguta extract attenuates inflammasome activation: Potential involvement in NLRP3 ubiquitination. J. Ethnopharmacol. 2018, 213, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.L.; Shi, D.Y.; Li, L.L.; Xia, J.; Zaimenko, N.V.; Skrypchenko, N.V.; Liu, D.J. Optimization of extraction technology of total triterpenes from the branches of Actinidia arguta by response surface methodology and its in vitro anti-inflammatory activity. Sci. Technol. Food Ind. 2021, 42, 189–197. [Google Scholar] [CrossRef]
- Takano, F.; Tanaka, T.; Tsukamoto, E.; Yahagi, N.; Fushiya, S. Isolation of (+)-catechin and (−)-epicatechin from Actinidia arguta as bone marrow cell proliferation promoting compounds. Planta Med. 2003, 69, 321–326. [Google Scholar] [CrossRef]
- Takano, F.; Tanaka, T.; Aoi, J.; Yahagi, N.; Fushiy, S. Protective effect of (+)-catechin against 5-fluorouracil-induced myelosuppression in mice. Toxicology 2004, 201, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Han, S.H.; Kim, J.; Lee, H.J.; Lee, J.G.; Lee, E.J. Inhibition of hardy kiwifruit (Actinidia arguta) ripening by 1-methylcyclopropene during cold storage and anticancer properties of the fruit extract. Food Chem. 2016, 190, 150–157. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Wang, X.; Ding, T.T.; Cui, H.P.; Jiang, S.; Du, P.G. Extraction and in vitro anti-tumor experiment of anthraquinone compounds from the roots of Actinidia arguta. Chin. J. Gerontol. 2011, 31, 4630–4631. [Google Scholar]
- Hou, F.Y.; Chen, F.; Lu, Y.; Sun, J.Y. Study on the anti-infective and antitumor effects of Actinidia arguta stem polysaccharides produced in Changbai Mountain. J. Bethune Med. Univ. 1995, 21, 472–475. [Google Scholar]
- Fang, Y.Z.; Sun, X.C.; Hui, J.L.; Song, P.J.; Hu, J.F. Experimental study on the anticancer effect of Actinidia arguta juice. Harbin Med. J. 1990, 16, 49–50. [Google Scholar]
- Zuo, L.L.; Wang, Z.Y.; Fan, Z.L.; Tian, S.Q.; Liu, J.R. Evaluation of antioxidant and antiproliferative properties of three Actinidia (Actinidia kolomikta, Actinidia arguta, Actinidia chinensis) extracts in vitro. Int. J. Mol. Sci. 2012, 13, 5506–5518. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.P.; Han, J.X.; Jiang, C.P.; Zhang, Y. Biomarkers, oxidative stress and autophagy in skin aging. Ageing Res. Rev. 2020, 59, 101036. [Google Scholar] [CrossRef] [PubMed]
- Zouboulis, C.C.; Ganceviciene, R.; Liakou, A.I.; Theodoridis, A.; Elewa, R.; Makrantonaki, E. Aesthetic aspects of skin aging, prevention, and local treatment. Clin. Dermatol. 2019, 37, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.L.; Wang, J.C.; Zhao, H. Research progress of anti-aging in traditional chinese medicine. Shenzhen J. Integr. Tradit. Chin. West. Med. 2023, 33, 129–133. [Google Scholar] [CrossRef]
- Zhu, D.Q.; Liu, Z.S. An investigation in the antiaging effect of Actinidia arguta. J. Hainan Med. Univ. 1996, 2, 54–57. [Google Scholar] [CrossRef]
- Liu, Z.S.; Zhu, D.Q. Preliminary study on the antiaging effect of Actinidia arguta. Chin. J. Gerontol. 1987, 7, 45–46. [Google Scholar]
- Gan, Z.W.; Zhang, D.X.; Zhang, Y.J.; Chen, Q.L.; Liu, H.F.; Ma, X.Y. Nutritional components and aging-delaying action of some wild berries in Changbai Mountainous Area. J. Xi’an Jiaotong Univ. Med. Sci. 2004, 25, 343–345. [Google Scholar]
- Jin, Y.; Mao, D.R.; Qiao, S.P.; Hong, W.Q. Preparation of effervescent tablet of Actinidia arguta fruits and its anti-fatigue effect. J. Beihua Univ. Nat. Sci. 2022, 23, 167–173. [Google Scholar]
- Liu, Y.Y.; Liu, C.J. Antifatigue and increasing exercise performance of Actinidia arguta crude alkaloids in mice. J. Food Drug Anal. 2016, 24, 738–745. [Google Scholar] [CrossRef]
- Xue, H.; Hao, Z.; Gao, Y.; Cai, X.; Tang, J.; Liao, X.; Tan, J. Research progress on the hypoglycemic activity and mechanisms of natural polysaccharides. Int. J. Biol. Macromol. 2023, 252, 126199. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Shen, M.; Song, Q.; Xie, J. Biological activities and pharmaceutical applications of polysaccharide from natural resources: A review. Carbohydr. Polym. 2018, 183, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Liu, J.F. Actinidia arguta polysaccharide component analysis and blood sugar and lipid decline. In Proceedings of the 4th Conference of Chinese Society for Horticultural Science on Kiwifruit, Chengdu, China, 26 October 2010; p. 53. [Google Scholar]
- Yao, J.Q. Optimization of Flavonoids Extraction Technology from Actinidia arguta and Its Hypoglycemic Activity. Master’s Thesis, Liaoning University, Shenyang, China, 2023. [Google Scholar] [CrossRef]
- Kurakane, S.; Yamada, N.; Sato, H.; Igarashi, K. Anti-diabetic effects of Actinidia arguta polyphenols on rats and KK-Ay mice. Food Sci. Technol. Res. 2011, 17, 93–102. [Google Scholar] [CrossRef]
- Niu, Q. Study on the Separation, Purification and Hypoglycemic Activity of Polysaccharides from the Branches of Actinidia arguta. Master’s Thesis, Jiamusi University, Jiamusi, China, 2021. [Google Scholar] [CrossRef]
- Liu, Y.J.; Liu, J.F.; Tian, X.Y.; Wang, X.D.; Wang, L.X.; Ren, D.M. Polysaccharide of Actinidia arguta and activity of blood glucose and lipid of decline. J. Food Sci. Biotechnol. 2012, 31, 86–89. [Google Scholar]
- Lee, A.Y.; Kang, M.J.; Choe, E.; Kim, J.I. Hypoglycemic and antioxidant effects of daraesoon (Actinidia arguta shoot) in animal models of diabetes mellitus. Nutr. Res. Pract. 2015, 9, 262–267. [Google Scholar] [CrossRef]
- Wojdyło, A.; Nowicka, P.; Oszmiański, J.; Golis, T. Phytochemical compounds and biological effects of Actinidia fruits. J. Funct. Foods 2017, 30, 194–202. [Google Scholar] [CrossRef]
- Stewart, J.; McCallin, T.; Martinez, J.; Chacko, S.; Yusuf, S. Hyperlipidemia. Pediatr. Rev. 2020, 41, 393–402. [Google Scholar] [CrossRef]
- Lan, J.; Zhao, Y.; Dong, F.; Yan, Z.; Zheng, W.; Fan, J.; Sun, G. Meta-analysis of the effect and safety of berberine in the treatment of type 2 diabetes mellitus, hyperlipemia and hypertension. J. Ethnopharmacol. 2015, 161, 69–81. [Google Scholar] [CrossRef]
- Osadnik, T.; Goławski, M.; Lewandowski, P.; Morze, J.; Osadnik, K.; Pawlas, N.; Lejawa, M.; Jakubiak, G.K.; Mazur, A.; Schwingschackl, L.; et al. A network meta-analysis on the comparative effect of nutraceuticals on lipid profile in adults. Pharmacol. Res. 2022, 183, 106402. [Google Scholar] [CrossRef]
- Leontowicz, M.; Leontowicz, H.; Jesion, I.; Bielecki, W.; Najman, K.; Latocha, P.; Park, Y.S.; Gorinstein, S. Kiwifruit Actinidia arguta supplementation protects aorta and liver in rats with induced hypercholesterolemia. Nutr. Res. 2016, 36, 1231–1242. [Google Scholar] [CrossRef]
- Chen, G.; Wang, Y.; Zhu, R.G.; Liu, Y.F.; He, L.; Gao, Y.X. Study on the effect of Actinidia arguta polysaccharides on lipid metabolism in rats with high diet. J. Shenyang Agric. Univ. 2020, 51, 321–327. [Google Scholar] [CrossRef]
- Zheng, Y.Y.; Yang, Y.; Liu, X.H.; Zhang, C.H. Antimicrobial activities of Actinidia arguta polysaccharides. Food Sci. Technol. 2012, 37, 171–173. [Google Scholar] [CrossRef]
- Zhang, C.H.; Yang, Y.; Xu, N.; Liu, C.J. A study on antimicrobial activities of Actinidia arguta total flavones. Food Ind. 2012, 33, 113–115. [Google Scholar]
- Zhu, R.G.; Zhang, X.Y.; Wang, Y.; Zhang, L.J.; Zhao, J.; Chen, G.; Fan, J.G.; Jia, Y.F.; Yan, F.W.; Ning, C. Characterization of polysaccharide fractions from fruit of Actinidia arguta and assessment of their antioxidant and antiglycated activities. Carbohydr. Polym. 2019, 210, 73–84. [Google Scholar] [CrossRef]
- Li, X.X.; Liang, J.W.; Nie, Y.; Chen, X.Y.; Han, X.F.; Han, C.R. Extraction of polysaccharides from tara vine (Actinidia arguta) and its anti-ultraviolet effect on yeast. Farm Prod. Process. 2020, 501, 10–13. [Google Scholar] [CrossRef]
- Hou, F.Y.; Sun, Y.B.; Chen, F.; Li, X.A. Studies on immunopharmacological effects of polysaccharide from the stem of Actinidia arguta (Sieb.et Zucc.) ex Miq. China J. Chin. Mater. Med. 1995, 20, 42–44+63. [Google Scholar]
- Liu, Y.F. The effect of Actinidia arguta polysaccharides on the immune system of mice. Liaoning For. Sci. Technol. 2021, 48, 32–33+40. [Google Scholar]
- Xuan, L. Preliminary Structure Identification, Antioxidant Activity and Immunity Activity of the Polysaccharides from Actinidia arguta. Ph.D. Thesis, Shenyang Agricultural University, Shenyang, China, 2013. [Google Scholar]
- Almeida, D.; Pinto, D.; Santos, J.; Vinha, A.F.; Palmeira, J.; Ferreira, H.N.; Rodrigues, F.; Oliveira, M.B.P.P. Hardy kiwifruit leaves (Actinidia arguta): An extraordinary source of value-added compounds for food industry. Food Chem. 2018, 259, 113–121. [Google Scholar] [CrossRef]












| No. | Name | Formula | Exact Theoretical Molecular Weight | Source | Characterization Method | Refs. |
|---|---|---|---|---|---|---|
| 1 | 3β-Hydroxyurs-12-en-28-oic acid | C30H48O3 | 456.3603 | Leaves | EI-MS, 1H-NMR, 13C-NMR | [16] |
| 2 | 3β,24-Dihydroxyurs-12-en-28-oic acid | C30H48O4 | 472.3553 | Leaves | IR, EI-MS, 1H-NMR, 13C-NMR | [16] |
| 3 | 2α,3α,24-Trihydroxyurs-12-en-28-oic acid | C30H48O5 | 488.3502 | Leaves | IR, EI-MS, 1H-NMR | [17] |
| 4 | 2α,3β-Dihydroxyurs-12-en-28,30-olide | C30H46O4 | 470.3396 | Roots | HPLC-DAD-ESI-MS | [18] |
| 5 | 12α-Chloro-2α,3β,23-tetrahydroxyolean-28-oic acid-13-lactone | C30H47O5Cl | 522.3112 | Roots | HPLC-DAD-ESI-MS | [18] |
| 6 | Oleanolic acid | C30H48O3 | 456.3603 | Roots | HPLC | [14,15] |
| 7 | Ursolic acid | C30H48O3 | 456.3603 | Roots, stems | IR, MS, 13C-NMR | [14,15,19] |
| 8 | Acetyl oleanolic acid | C32H50O4 | 4983709 | Stems | IR, MS, 1H-NMR, 13C-NMR | [19] |
| 9 | Actinidic acid | C30H46O5 | 486.3345 | Leaves | UV, IR, HR-ESI-TOF-MS, 1H-NMR, 13C-NMR, HSQC, HMBC, NOESY | [20] |
| 10 | Actiniargupene A | C30H46O4 | 470.3396 | Leaves | UV, IR, HR-ESI-TOF-MS, 1H-NMR, 13C-NMR, HSQC, HMBC, NOESY | [20] |
| 11 | Actiniargupene B | C39H52O7 | 632.3713 | Leaves | UV, IR, HR-ESI-TOF-MS, 1H-NMR, 13C-NMR, HSQC, HMBC, NOESY | [20] |
| 12 | Actiniargupene C | C39H52O7 | 632.3713 | Leaves | UV, IR, HR-ESI-TOF-MS, 1H-NMR, 13C-NMR, HSQC, HMBC, NOESY | [20] |
| 13 | 3-O-trans-p-Coumaroyl actinidic acid | C39H52O7 | 632.3713 | Leaves | IR, HR-ESI-TOF-MS, 1H-NMR, 13C-NMR, HSQC, HMBC, NOESY | [20] |
| 14 | 3-O-cis-p-Coumaroyl actinidic acid | C39H52O7 | 632.3713 | Leaves | IR, HR-ESI-TOF-MS, 1H-NMR, 13C-NMR, HSQC, HMBC, NOESY | [20] |
| 15 | 2α,3α,23-Trihydroxyursa-12,20(30)-dien-28-oic acid | C39H52O7 | 632.3713 | Leaves | IR, HR-ESI-TOF-MS, 1H-NMR, 13C-NMR, HSQC, HMBC, NOESY | [20] |
| 16 | Dehydroisoactinidic acid | C30H46O5 | 486.3345 | Leaves | UV, IR, HR-ESI-TOF-MS, 1H-NMR, 13C-NMR, HSQC, HMBC, NOESY | [20] |
| 17 | Actiniargupene D | C39H54O7 | 634.3870 | Leaves | UV, IR, HR-ESI-TOF-MS, 1H-NMR, 13C-NMR, HSQC, HMBC, NOESY | [20] |
| 18 | Actiniargupene E | C39H54O8 | 650.3819 | Leaves | UV, IR, HR-ESI-TOF-MS, 1H-NMR, 13C-NMR, HSQC, HMBC, NOESY | [20] |
| 19 | Actiniargupene F | C39H54O7 | 634.3870 | Leaves | UV, IR, HR-ESI-TOF-MS, 1H-NMR, 13C-NMR, HSQC, HMBC, NOESY | [20] |
| 20 | 3-O-trans-p-Coumaroylasiatic acid | C39H54O7 | 634.3870 | Leaves | UV, IR, HR-ESI-TOF-MS, 1H-NMR, 13C-NMR, HSQC, HMBC, NOESY | [20] |
| 21 | 23-O-trans-p-Coumaroylasiatic acid | C39H54O7 | 634.3870 | Leaves | UV, IR, HR-ESI-TOF-MS, 1H-NMR, 13C-NMR, HSQC, HMBC, NOESY | [20] |
| 22 | 11α-Methoxyurs-12-ene-3β,12-diol | C31H52O3 | 472.3916 | Leaves | UV, IR, HR-ESI-TOF-MS, 1H-NMR, 13C-NMR, HSQC, HMBC, NOESY | [20] |
| 23 | Ilelatifol A | C30H48O3 | 456.3603 | Leaves | UV, IR, HR-ESI-TOF-MS, 1H-NMR, 13C-NMR, HSQC, HMBC, NOESY | [20] |
| 24 | (2R,6R,9R)-Trihydroxy-megastigmane-4,7E-dien-3-one-9-O-β-D-glucopyranoside | C19H30O9 | 402.1890 | Fruit | UV, 1H-NMR, 13C-NMR, HRESI-TOF-MS, HMBC, NOESY, HPLC, ECD | [21] |
| 25 | (6S,9R)-Roseoside | C19H30O8 | 386.0941 | Fruit | ESI-MS, 1H-NMR, 13C-NMR, ECD | [21] |
| No. | Name | Formula | Exact Theoretical Molecular Weight | Source | Characterization Method | Refs. |
|---|---|---|---|---|---|---|
| 26 | Rutin | C27H30O16 | 610.1534 | Skin and flesh of the ripe fruit | HPLC | [22] |
| 27 | Quercetin | C15H10O7 | 302.0427 | Skin and flesh of the ripe fruit | HPLC | [22] |
| 28 | (−)-epi-Catechin | C15H14O6 | 290.0790 | Roots | ESI-MS, 1H-NMR, 13C-NMR | [25] |
| 29 | (+)-Catechin | C15H14O6 | 290.0790 | Roots | ESI-MS, 1H-NMR, 13C-NMR | [25] |
| 30 | Proanthocyanidin B2 | C30H26O12 | 578.1424 | Fruit | UV, HPLC-MS | [24] |
| 31 | Proanthocyanidin C1 | C45H38O18 | 866.2058 | Fruit | UV, HPLC-MS | [24] |
| 32 | (+)-Gallocatechin | C15H14O7 | 306.0740 | Fruit | UV, HPLC-MS | [24] |
| 33 | Quercetin-3-O-galactoside | C21H20O12 | 464.0955 | Fruit | UV, HPLC-MS | [24] |
| 34 | Quercetin-3-O-rutinoside | C27H30O16 | 610.1534 | Fruit | UV, HPLC-MS | [24] |
| 35 | Quercetin-3-O-glucoside | C21H20O12 | 464.0955 | Fruit | UV, HPLC-MS | [24] |
| 36 | Astragalin | C21H20O11 | 448.1006 | Fruit | ESI-MS, 1H-NMR, 13C-NMR | [21] |
| 37 | Procyanidin B4 | C30H26O12 | 578.1424 | Roots | ESI-MS, 1H-NMR, 13C-NMR | [25] |
| 38 | 6-(2-Pyrrolidinone-5-yl)-(−)-epicatechin | C19H19NO7 | 373.1162 | Roots | IR, ESI-MS, HR-ESI-MS, 1H-NMR, 13C-NMR, HMBC | [25] |
| 39 | 8-(2-Pyrrolidinone-5-yl)-(−)-epicatechin | C19H19NO7 | 373.1162 | Roots | IR, FAB-MS, HR-FAB-MS, 1H-NMR, 13C-NMR, HMBC | [25] |
| 40 | Kaempferol-3-O-rutinoside (+) | C27H30O15 | 594.1585 | Fruit | LC-MS/MS | [23] |
| 41 | Kaempferol-3-O-rutinoside (−) | C27H30O15 | 594.1585 | Fruit | LC-MS/MS | [23] |
| 42 | Kaempferol-3-O-neohesperidoside | C27H30O15 | 594.1585 | Fruit | LC-MS/MS | [23] |
| 43 | Isorhamnetin-3-O-neohesperidoside (+) | C28H32O16 | 624.1690 | Fruit | LC-MS/MS | [23] |
| 44 | Isorhamnetin-3-O-neohesperidoside (−) | C28H32O16 | 624.1690 | Fruit | LC-MS/MS | [23] |
| 45 | Isorhamnetin-3-O-rutinoside | C28H32O16 | 624.1690 | Fruit | LC-MS/MS | [23] |
| 46 | Isorhamnetin-3-O-neohespeidoside | C28H32O16 | 624.1690 | Fruit | LC-MS/MS | [23] |
| 47 | Quercetin-3-O-rhamnoglucoside | C25H28O15 | 568.1428 | Fruit | LC-MS/MS | [23] |
| 48 | Quercetin-3-O-β-D-galactopyranoside | C21H20O12 | 464.0955 | Fruit | ESI-MS, 1H-NMR, 13C-NMR | [21] |
| 49 | Quercetin-3-O-[α-rhamnopyranosyl-(1-4)-rhamnopyranosyl-(1-6)-β-galactopyranoside | C37H48O20 | 812.2739 | Plant material | UV, MS, 1H-NMR, 13C-NMR | [26] |
| 50 | Kaempferol-3-O-[α-rhamnopyranosyl-(1-4)-rhamnopyranosyl-(1-6)-β-galactopyranoside | C37H48O19 | 796.2790 | Plant material | 1H-NMR, 13C-NMR | [26] |
| 51 | Quercetin 3-sambubioside | C26H28O16 | 596.1377 | Leaves | PC, GC, UV, 1H-NMR, 13C-NMR | [27] |
| 52 | Quercetin 3-O-β-D-[2-O-β-D-xylopyranosy-6-O-α-L-rhamnopyranosyl] glucopyranoside | C32H38O20 | 742.1956 | Leaves | PC, GC, UV, 1H-NMR, 13C-NMR | [27] |
| 53 | Isorhamnetin-3-O-α-L-rhamnopyranosyl-(1-3)-α-L-rhamnopyranosyl-(1-6)-β-D-galactopyranoside | C34H42O20 | 770.2269 | Fruit | LC-MS/MS | [23] |
| No. | Name | Formula | Exact Theoretical Molecular Weight | Source | Characterization Method | Refs. |
|---|---|---|---|---|---|---|
| 54 | Planchol A | C14H14O6 | 278.0790 | Roots | HPLC-DAD-ESI-MS | [18] |
| 55 | Planchol B | C15H16O6 | 292.0947 | Roots | HPLC-DAD-ESI-MS | [18] |
| 56 | Isotachioside | C13H18O8 | 302.1002 | Roots | HPLC-DAD-ESI-MS | [18] |
| 57 | p-Hydroxybenzoic acid | C7H6O3 | 138.0317 | Roots, leaves | ESI-MS, 1H-NMR, 13C-NMR | [25,29] |
| 58 | Vanillic acid | C8H8O4 | 168.0423 | Roots, leaves | EI-MS, 1H-NMR, 13C-NMR | [29] |
| 59 | Protocatechuic acid | C7H6O4 | 154.0266 | Leaves, fruit | IR, HRESI-TOF-MS, 1H-NMR, 13C-NMR, HMBC | [21,29] |
| 60 | Isovanillic acid | C8H8O4 | 168.0423 | Leaves | IR, HRESI-TOF-MS, 1H-NMR, 13C-NMR, HMBC | [29] |
| 61 | Hydroxytyrosol | C12H16O7 | 154.0630 | Leaves | IR, HRESI-TOF-MS, 1H-NMR, 13C-NMR, HMBC | [29] |
| 62 | Caffeoylthreonic acid | C12H16O7 | 298.0689 | Leaves | HPLC-MS/MS | [30] |
| 63 | salvianic acid A | C10H12O5 | 212.0685 | Leaves | HPLC-MS/MS | [30] |
| 64 | Maysedilactone A | C15H16O8 | 324.0845 | Leaves | IR, ESI-MS, HR-ESI-MS, 1H-NMR, 13C-NMR | [31] |
| 65 | Maysedilactone D | C15H16O9 | 340.0794 | Leaves | IR, ESI-MS, HR-ESI-MS 1H-NMR, 13C-NMR, HMBC, NOESY | [31] |
| 66 | Maysedilactone B | C16H18O9 | 354.0951 | Leaves | IR, ESI-MS, HR-ESI-MS, 1H-NMR, 13C-NMR | [31] |
| 67 | Argutinoside J | C18H22O11 | 414.1162 | Fruit | HRESI-TOF-MS, IR, UV, 1H-NMR, 13C-NMR, HMBC | [32] |
| 68 | Argutinoside K | C19H24O12 | 444.1268 | Fruit | HRESI-TOF-MS, IR, UV, 1H-NMR, 13C-NMR, HMBC | [32] |
| 69 | Argutinoside L | C20H28O10 | 428.1682 | Fruit | HRESI-TOF-MS, IR, UV, 1H-NMR, 13C-NMR, HMBC | [32] |
| 70 | Vanillic acid-4-O-β-D-glucopyranoside | C14H18O9 | 330.0951 | Fruit | ESI-MS, 1H-NMR, 13C-NMR | [21] |
| 71 | 1-O-Feruloyl-β-D-glucopyranoside | C16H20O9 | 356.1107 | Fruit | ESI-MS, 1H-NMR, 13C-NMR | [21] |
| 72 | Ferulic acid-4-O-β-D-glucopyranoside | C16H20O9 | 356.1107 | Fruit | ESI-MS, 1H-NMR, 13C-NMR | [21] |
| 73 | Rhodioloside | C14H20O7 | 300.1209 | Leaves | ESI-MS, 1H-NMR, 13C-NMR | [21] |
| 74 | 5-O-Caffeoyl quinic acid methyl ester | C17H20O9 | 368.1107 | Fruit | ESI-MS, 1H-NMR, 13C-NMR | [21] |
| 75 | 5-O-Caffeoyl quinic acid butyl ester | C20H26O9 | 410.1577 | Fruit | ESI-MS, 1H-NMR, 13C-NMR | [21] |
| 76 | 5-O-Feruloyl quinic acid methyl ester | C18H22O9 | 382.1264 | Fruit | ESI-MS, 1H-NMR, 13C-NMR | [21] |
| 77 | 5-O-Coumaroyl quinic acid methyl ester | C16H20O8 | 340.1158 | Fruit | ESI-MS, 1H-NMR, 13C-NMR | [21] |
| No. | Name | Formula | Exact Theoretical Molecular Weight | Source | Characterization Method | Refs. |
|---|---|---|---|---|---|---|
| 78 | Chlorogenic acid | C16H18O9 | 354.0951 | Leaves | HPLC | [34] |
| 79 | Quinic acid | C7H12O6 | 192.0634 | Fruit | HPLC-DAD-MS/MS | [35] |
| 80 | Caffeic acid | C9H8O4 | 180.0423 | Roots, leaves | EI-MS, 1H-NMR, 13C-NMR | [29] |
| 81 | trans-4-Hydroxycinnamic acid | C9H8O3 | 164.0473 | Roots, leaves | EI-MS, 1H-NMR | [29] |
| 82 | Epipinoresinol | C20H22O6 | 358.1416 | Roots | HPLC-DAD-ESI-MS | [18] |
| 83 | Pinoresinol | C20H22O6 | 358.1416 | Roots, leaves | TLC, ESI-MS, 1H-NMR, 13C-NMR | [25,29] |
| 84 | Argutoside A | C24H24O11 | 488.1319 | Leaves | IR, HRESI-TOF-MS, 1H-NMR, 13C-NMR, HMBC | [29] |
| 85 | Argutoside B | C23H26O11 | 465.1526 | Leaves | IR, HRESI-TOF-MS, 1H-NMR, 13C-NMR, HMBC | [29] |
| 86 | Argutoside C | C23H26O11 | 465.1526 | Leaves | IR, ESI-MS, HRESI-TOF-MS, 1H-NMR, 13C-NMR, HMBC | [29] |
| 87 | Argutoside D | C23H26O11 | 478.1475 | Leaves | IR, HRESI-TOF-MS, 1H-NMR, 13C-NMR, HMBC, HSQC | [29] |
| 88 | (−)-Rhodolatouchol | C10H14O3 | 182.0943 | Leaves | IR, HRESI-TOF-MS, 1H-NMR, 13C-NMR, HMBC | [29] |
| 89 | p-E-Coumaric acid-9-O-glucopyranoside | C15H18O8 | 326.1002 | Leaves | IR, HRESI-TOF-MS, 1H-NMR, 13C-NMR, HMBC | [29] |
| 90 | E-Ferulic acid | C10H10O4 | 194.0579 | Leaves | IR, HRESI-TOF-MS, 1H-NMR, 13C-NMR, HMBC | [29] |
| 91 | 3,5-Dimethoxy-4-hydroxycinnamic alcohol | C12H16O4 | 224.1049 | Leaves | IR, HRESI-TOF-MS, 1H-NMR, 13C-NMR, HMBC | [29] |
| 92 | 7S,8R-Cedrusin | C20H24O5 | 344.1624 | Leaves | IR, HRESI-TOF-MS, 1H-NMR, 13C-NMR, HMBC | [29] |
| 93 | Dehydroconiferyl alcohol | C21H26O5 | 358.1780 | Leaves | IR, HRESI-TOF-MS, 1H-NMR, 13C-NMR, HMBC | [29] |
| 94 | (7S,8S)-3-Methoxy-3′,7-epoxy-8,4′-oxyneoligna-4,9,9′-triol | C19H22O6 | 346.1416 | Leaves | IR, HRESI-TOF-MS, 1H-NMR, 13C-NMR, HMBC | [29] |
| 95 | Pinoresinol 4-O-β-glucopyranoside | C26H32O11 | 520.1945 | Leaves | IR, HRESI-TOF-MS, 1H-NMR, 13C-NMR, HMBC | [29] |
| 96 | Alutaceuol | C30H36O11 | 572.2258 | Leaves | IR, HRESI-TOF-MS, 1H-NMR, 13C-NMR, HMBC | [29] |
| 97 | Alutaceuol isomer | C30H36O11 | 572.2258 | Leaves | IR, HRESI-TOF-MS, 1H-NMR, 13C-NMR, HMBC | [29] |
| 98 | (−)-(2R,3R)-Secoisolariciresinol | C20H26O6 | 362.1729 | Leaves | IR, HRESI-TOF-MS, 1H-NMR, 13C-NMR, HMBC | [29] |
| 99 | Glehlinoside F | C35H42O14 | 686.2575 | Leaves | IR, HRESI-TOF-MS, 1H-NMR, 13C-NMR, HMBC | [29] |
| No. | Name | Formula | Exact Theoretical Molecular Weight | Source | Characterization Method | Refs. |
|---|---|---|---|---|---|---|
| 100 | Argutinoside A | C20H24O12 | 456.1238 | Fruit | HPLC | [36] |
| 101 | Argutinoside B | C21H26O12 | 470.1424 | Fruit | HPLC-DAD-MS/MS | [36] |
| 102 | Argutinoside C | C20H24O11 | 440.1319 | Fruit | EI-MS, 1H-NMR, 13C-NMR | [36] |
| 103 | Argutinoside D | C21H26O11 | 454.1475 | Fruit | EI-MS, 1H-NMR | [36] |
| 104 | Argutinoside E | C21H26O11 | 454.1475 | Fruit | HPLC-DAD-ESI-MS | [36] |
| 105 | Argutinoside F | C21H26O12 | 470.1424 | Fruit | TLC, ESI-MS, 1H-NMR, 13C-NMR | [36] |
| 106 | Argutinoside G | C21H26O12 | 470.1424 | Fruit | IR, HRESI-TOF-MS, 1H-NMR, 13C-NMR, HMBC | [36] |
| 107 | Argutinoside H | C22H28O12 | 484.1581 | Fruit | IR, HRESI-TOF-MS, 1H-NMR, 13C-NMR, HMBC | [36] |
| 108 | Argutinoside I | C21H26O12 | 470.1424 | Fruit | IR, ESI-MS, HRESI-TOF-MS, 1H-NMR, 13C-NMR, HMBC | [36] |
| 109 | Butyl 2-hydroxysuccinate | C8H14O5 | 190.0841 | Fruit | IR, HRESI-TOF-MS, 1H-NMR, 13C-NMR, HMBC, HSQC | [36] |
| 110 | 3-O-trans-p-Coumaroyl quinic acid methyl ester | C18H22O8 | 366.1315 | Fruit | IR, HRESI-TOF-MS, 1H-NMR, 13C-NMR, HMBC | [36] |
| 111 | 3-O-cis-p-Coumaroyl quinic acid methyl ester | C18H22O8 | 366.1315 | Fruit | IR, HRESI-TOF-MS, 1H-NMR, 13C-NMR, HMBC | [36] |
| 112 | 3-O-Trans-p-Caffeoyl quinic acid methylester | C18H22O9 | 382.1264 | Fruit | IR, HRESI-TOF-MS, 1H-NMR, 13C-NMR, HMBC | [36] |
| 113 | 5-O-trans-p-Caffeoyl quinic acid methyl ester | C18H22O8 | 366.1315 | Fruit | IR, HRESI-TOF-MS, 1H-NMR, 13C-NMR, HMBC | [36] |
| 114 | 5-O-cis-p-Caffeoyl quinic acid methyl ester | C18H22O8 | 366.1315 | Fruit | IR, HRESI-TOF-MS, 1H-NMR, 13C-NMR, HMBC | [36] |
| 115 | 5-O-trans-p-Coumaroyl quinic acid methyl ester | C18H22O9 | 382.1264 | Fruit | IR, HRESI-TOF-MS, 1H-NMR, 13C-NMR, HMBC | [36] |
| 116 | 5-O-cis-p-Coumaroyl quinic acid methyl ester | C18H22O9 | 382.1264 | Fruit | IR, HRESI-TOF-MS, 1H-NMR, 13C-NMR, HMBC | [36] |
| 117 | 3-O-trans-p-Caffeoyl quinic acid butyl ester | C21H28O9 | 424.1733 | Fruit | IR, HRESI-TOF-MS, 1H-NMR, 13C-NMR, HMBC | [36] |
| 118 | 4-O-trans-p-Caffeoyl quinic acid butyl ester | C21H28O9 | 424.1733 | Fruit | IR, HRESI-TOF-MS, 1H-NMR, 13C-NMR, HMBC | [36] |
| 119 | 5-O-trans-p-Caffeoyl quinic acid butyl ester | C21H28O8 | 408.1784 | Fruit | IR, HRESI-TOF-MS, 1H-NMR, 13C-NMR, HMBC | [36] |
| 120 | 5-O-trans-p-Coumaroyl quinic acid butyl ester | C21H28O9 | 424.1733 | Fruit | IR, HRESI-TOF-MS, 1H-NMR, 13C-NMR, HMBC | [36] |
| 121 | 4-O-trans-p-Coumaroyl shikimic acid | C17H20O7 | 336.1209 | Fruit | IR, HRESI-TOF-MS, 1H-NMR, 13C-NMR, HMBC | [36] |
| 122 | 3-O-cis-p-Coumaroyl shikimic acid | C17H20O7 | 336.1209 | Fruit | HPLC | [36] |
| 123 | 1-Methyl-5-ethyl citrate | C9H14O7 | 234.0740 | Fruit | HPLC-DAD-MS/MS | [36] |
| 124 | 1,6-Dimethyl citrate | C8H12O7 | 220.0583 | Fruit | EI-MS, 1H-NMR, 13C-NMR | [36] |
| 125 | 1,5,6-Trimethyl citrate | C9H14O7 | 234.0740 | Fruit | EI-MS, 1H-NMR | [36] |
| 126 | 1,6-Dimethyl-5-ethyl citrate | C10H16O7 | 248.0896 | Fruit | HPLC-DAD-ESI-MS | [36] |
| 127 | 5-Butyl citrate | C10H16O7 | 248.0896 | Fruit | TLC, ESI-MS, 1H-NMR, 13C-NMR | [36] |
| 128 | 1-Methyl-6-butyl citrate | C11H18O7 | 262.1053 | Fruit | IR, HRESI-TOF-MS, 1H-NMR, 13C-NMR, HMBC | [36] |
| 129 | Succinic acid | C20H24O12 | 118.0266 | Leaves | IR, HRESI-TOF-MS, 1H-NMR, 13C-NMR, HMBC | [14] |
| 130 | γ-Quinide | C7H10O5 | 174.0528 | Roots | IR, ESI-MS, HRESI-TOF-MS, 1H-NMR, 13C-NMR, HMBC | [18] |
| 131 | Octeyl-10-undecylenate | C19H36O2 | 296.2715 | Stems | IR, HRESI-TOF-MS, 1H-NMR, 13C-NMR, HMBC, HSQC | [38] |
| 132 | Palmitoleic acid | C16H30O2 | 254.2246 | Sprouts | IR, HRESI-TOF-MS, 1H-NMR, 13C-NMR, HMBC | [39] |
| 133 | Stearic acid | C18H36O2 | 284.2715 | Sprouts | IR, HRESI-TOF-MS, 1H-NMR, 13C-NMR, HMBC | [39] |
| 134 | Oleic acid | C18H34O2 | 282.2559 | Sprouts | IR, HRESI-TOF-MS, 1H-NMR, 13C-NMR, HMBC | [39] |
| 135 | α-Linoleic acid | C18H32O2 | 280.2402 | Fruit, sprouts | IR, HRESI-TOF-MS, 1H-NMR, 13C-NMR, HMBC | [37,39] |
| 136 | α-Linolenic acid | C18H30O2 | 278.2246 | Fruit, sprouts | IR, HRESI-TOF-MS, 1H-NMR, 13C-NMR, HMBC | [37,39] |
| 137 | Eicosadienoic acid | C20H36O2 | 308.2715 | Sprouts | IR, HRESI-TOF-MS, 1H-NMR, 13C-NMR, HMBC | [39] |
| 138 | Ethyl stearate | C19H37O2 | 297.2794 | Fruit | IR, HRESI-TOF-MS, 1H-NMR, 13C-NMR, HMBC | [37] |
| No. | Name | Formula | Exact Theoretical Molecular Weight | Source | Characterization Method | Refs. |
|---|---|---|---|---|---|---|
| 139 | m-Xylene | C8H10 | 106.0783 | Roots | GC-MS | [50] |
| 140 | Naphthalene | C10H8 | 128.0626 | Roots, flowers | GC-MS | [41,50] |
| 141 | n-Undecane | C11H24 | 156.1878 | Roots | GC-MS | [50] |
| 142 | n-Dodecane | C12H26 | 170.2035 | Roots, fruit | GC-MS | [41,50] |
| 143 | n-Tetradecane | C14H30 | 198.2348 | Roots, fruit | GC-MS | [41,50] |
| 144 | n-Heptadecane | C17H36 | 240.2817 | Roots, flowers, fruit | GC-MS | [41,46,50] |
| 145 | n-Eicosane | C20H42 | 282.3287 | Roots, flowers | GC-MS | [41,46,50] |
| 146 | 2,6,10-Trimethyldodecane | C15H32 | 212.2504 | Roots | GC-MS | [50] |
| 147 | 2,6,10,14-Tetramethylpentadecane | C19H40 | 268.3130 | Roots | GC-MS | [50] |
| 148 | 8-Methylheptadecane | C18H38 | 254.2974 | Roots | GC-MS | [50] |
| 149 | Ethylmethylundecanol | C14H30O | 214.2297 | Roots | GC-MS | [50] |
| 150 | Methyl pentadecanoate | C16H32O2 | 256.2402 | Roots | GC-MS | [50] |
| 151 | Dibutyl phthalate | C16H22O4 | 278.1518 | Roots | GC-MS | [50] |
| 152 | 2,4,6-Trimethyl decanoic acid | C13H26O2 | 214.1933 | Roots | GC-MS | [50] |
| 153 | 3,7-Dimethyl-1,8-Nonadiene | C11H20 | 152.1565 | Roots | GC-MS | [50] |
| 154 | (3E)-3-Undecene | C11H22 | 154.1722 | Roots | GC-MS | [50] |
| 155 | 1,6-Nonadien-3-ol,3,7-dimethyl-,acetate | C13H22O2 | 210.1620 | Roots | GC-MS | [50] |
| 156 | Ethyl acetate | C4H8O2 | 88.0524 | Flowers, fruit | GC-MS | [41,43] |
| 157 | Butanoic acid, methyl ester | C5H10O2 | 102.0681 | Fruit | GC-MS | [43] |
| 158 | Pyridine | C5H5N | 79.0422 | Fruit | GC-MS | [43] |
| 159 | (E)-2-Hexenal | C6H10O | 98.0732 | Fruit | GC-MS | [43] |
| 160 | 1-Hexanol | C6H14O | 102.1045 | Flowers, fruit | GC-MS | [41,42,43,46] |
| 161 | Hexanoic acid, ethyl ester | C8H16O2 | 144.1150 | Fruit | GC-MS | [43] |
| 162 | 3-Cyclohexen-1-ol,4-methyl-1-(methylethyl) | C10H18O | 154.1358 | Fruit | GC-MS | [43] |
| 163 | Ethyl butyrate | C6H12O2 | 116.0837 | Fruit | GC-MS | [42,46] |
| 164 | 2-Furaldehyde | C5H4O2 | 96.0211 | Fruit | GC-MS | [42] |
| 165 | 2-Hexenal | C6H10O | 98.0732 | Fruit | GC-MS | [42,46] |
| 166 | (E)-3-Hexen-1-ol | C6H12O | 100.0888 | Fruit | GC-MS | [42] |
| 167 | cis-Hex-2-en-1-ol | C6H12O | 100.0888 | Fruit | GC-MS | [42] |
| 168 | 2-Hexen-1-ol | C6H12O | 100.0888 | Fruit | GC-MS | [42,43] |
| 169 | Dihydrofuran-2(3H)-one | C4H6O2 | 86.0386 | Fruit | GC-MS | [42] |
| 170 | (1S)-(−)-α-Pinene | C10H16 | 136.1252 | Fruit | GC-MS | [42] |
| 171 | Benzaldehyde | C7H6O | 106.0419 | Fruit | GC-MS | [42] |
| 172 | 5-Methylfurfural | C6H6O2 | 110.0368 | Fruit | GC-MS | [42] |
| 173 | 1-Octen-3-ol | C8H16O | 128.1201 | Fruit | GC-MS | [42] |
| 174 | Benzene,1-methyl-2-(1-methylethyl)- | C10H14 | 134.1096 | Fruit | GC-MS | [42] |
| 175 | 1-Methyl-4-methyl ethenyl cyclohexene | C10H16 | 136.1252 | Fruit | GC-MS | [42] |
| 176 | 1,3,3-Trimethyl-2-oxabicyclo[2.2.2]octane | C10H18O | 154.1358 | Fruit | GC-MS | [42] |
| 177 | Benzyl alcohol | C7H8O | 108.0575 | Flowers, fruit | GC-MS | [42] |
| 178 | o-Cresol | C7H8O | 108.0575 | Fruit | GC-MS | [42] |
| 179 | Phenylacetaldehyde | C8H8O | 120.0575 | Fruit | GC-MS | [42] |
| 180 | 4-Isopropyl-1-methyl-1,4-cyclohexadiene | C10H16 | 136.1252 | Fruit | GC-MS | [42] |
| 181 | α-Terpinolene | C10H16 | 136.1252 | Flowers, fruit | GC-MS | [41,42,45] |
| 182 | 1-Methyl-4-(prop-1-en-2-yl)benzene | C10H12 | 132.0939 | Fruit | GC-MS | [42] |
| 183 | Methyl benzoate | C8H8O2 | 136.0524 | Fruit | GC-MS | [41,42] |
| 184 | 1,6-Octadien-3-ol,3,7-dimethyl | C10H18O | 154.1358 | Fruit | GC-MS | [42] |
| 185 | Ethyl benzoate | C9H10O2 | 150.0681 | Fruit | GC-MS | [41,42] |
| 186 | Terpinen-4-ol | C10H18O | 154.1358 | Fruit | GC-MS | [42] |
| 187 | Trimethylbenzene | C9H12 | 120.0939 | Flowers, fruit | GC-MS | [41,42] |
| 188 | α-Terpineol | C10H18O | 154.1358 | Fruit | GC-MS | [42,46] |
| 189 | 4-(2,6,6-Trimethylcyclohex-2-en-1-yl)but-3-en-2-one | C13H20O | 192.1514 | Fruit | GC-MS | [42] |
| 190 | N,N-Dibutylformamide | C9H19NO | 157.1467 | Fruit | GC-MS | [42] |
| 191 | 2-Methoxy-4-vinylphenol | C9H10O2 | 150.0681 | Fruit | GC-MS | [42] |
| 192 | 2-(Benzo[d][1,3]dioxol-5-yl)-6-chloroimidazo[1,2-b]pyridazine | C13H8N3O2Cl | 273.0305 | Fruit | GC-MS | [42] |
| 193 | Triethyl citrate | C12H20O7 | 276.1209 | Fruit | GC-MS | [42] |
| 194 | Methyl tetradecanoate | C15H30O2 | 242.2246 | Fruit | GC-MS | [42] |
| 195 | Myristic acid | C14H28O2 | 228.2089 | Fruit | GC-MS | [42] |
| 196 | 1,2-Benzene-3,4,5,6-d4-dicarboxylicacid, bis(2-methylpropyl) ester | C16H22O4 | 282.1769 | Fruit | GC-MS | [42] |
| 197 | 1-(2,4-Difluorophenyl)piperazine | C10H12F2N2 | 198.0969 | Fruit | GC-MS | [42] |
| 198 | Ethyl palmitate | C18H36O2 | 284.2715 | Fruit | GC-MS | [42] |
| 199 | Methyl linolenate | C19H32O2 | 292.2402 | Fruit | GC-MS | [41,42] |
| 200 | Ethyl linoleate | C20H36O2 | 308.2715 | Fruit | GC-MS | [41,42] |
| 201 | Ethyl linolenate | C20H34O2 | 306.2559 | Fruit | GC-MS | [41,42] |
| 202 | Ethanol | C2H6O | 46.0619 | Fruit | GC-MS | [44] |
| 203 | α-Pinene | C10H16 | 136.1252 | Flowers, fruit | GC-MS | [41,44] |
| 204 | β-Pinene | C10H16 | 136.1252 | Flowers, fruit | GC-MS | [41,44] |
| 205 | β-Myrcene | C10H16 | 136.1252 | Flowers, fruit | GC-MS | [41,44] |
| 206 | Benzene,1-methyl-3-(1-methylethyl)- | C10H14 | 134.1096 | Fruit | GC-MS | [44] |
| 207 | Dipentene | C10H16 | 136.1252 | Fruit | GC-MS | [44] |
| 208 | (1R-trans) 1-Methyl-4-(1-methylethenyl)-2-cyclohexene-1-ol | C10H16O | 152.1201 | Fruit | GC-MS | [44] |
| 209 | 4,6,6-Trimethyl-bicyclo[3.1.1]hept-3-en-2-one | C10H14O | 150.1045 | Fruit | GC-MS | [44] |
| 210 | 9,12-Octadecadienoic acid | C18H32O2 | 280.2402 | Fruit, seeds | GC-MS | [47,48] |
| 211 | Palmitic acid | C16H32O2 | 256.2402 | Fruit, seeds | GC-MS | [48] |
| 212 | Linolenic acid | C18H30O2 | 278.2246 | Fruit, seeds | GC-MS | [48] |
| 213 | Erucic acid | C22H42O2 | 338.3815 | Seeds | GC-MS | [48] |
| 214 | Gondoic acid | C20H38O2 | 310.2872 | Seeds | GC-MS | [48] |
| 215 | (10Z,13Z)-Octadeca-10,13-dienoic acid | C18H32O2 | 280.2402 | Fruit | GC-MS | [47] |
| 216 | Eicosanoic-12,12,13,13-d4-acid | C20H40O2 | 316.3279 | Fruit | GC-MS | [47] |
| 217 | 9-Octadecenoic acid | C18H34O2 | 282.2559 | Seeds | GC-MS | [49] |
| 218 | Eucalyptol | C10H18O | 154.1358 | Flowers, fruit | GC-MS | [41,44] |
| 219 | 1-Methyl-4-(1-methylethenyl)-cyclohexene | C10H16 | 136.1252 | Fruit | GC-MS | [44] |
| 220 | 3,4-Dimethylbicyclo[3.2.1]oct-2-ene | C10H16 | 136.1252 | Fruit | GC-MS | [44] |
| 221 | Methyl acetate | C3H6O2 | 74.0368 | Fruit | GC-MS | [41,45] |
| 222 | Acetic acid | C2H4O2 | 60.0211 | Flowers, fruit | GC-MS | [41,45] |
| 223 | 1,3,5,7-Cyclooctatetraene | C8H8 | 104.0626 | Fruit | GC-MS | [45] |
| 224 | Styrene | C8H8 | 104.0626 | Fruit | GC-MS | [41,45] |
| 225 | 2-Methyl-bicyclo[3.1.0]hexan-2-en | C7H10 | 94.0783 | Fruit | GC-MS | [45] |
| 226 | p-Cymene | C10H14 | 134.1096 | Fruit | GC-MS | [41,45] |
| 227 | 3-Carene | C10H16 | 136.1252 | Fruit | GC-MS | [45] |
| 228 | 4-Isopropyl-1-methyl-1,4-cyclohexadiene | C10H16 | 136.1252 | Fruit | GC-MS | [45] |
| 229 | Methyl heptenone | C8H14O | 126.1045 | Fruit | GC-MS | [45] |
| 230 | 1-Ethyl-3,5-dimethylbenzene | C10H14 | 134.1096 | Fruit | GC-MS | [45] |
| 231 | Camphor | C10H16O | 152.1201 | Flowers, fruit | GC-MS | [41] |
| 232 | β-Caryophyllene | C15H24 | 204.1878 | Flowers | GC-MS | [41] |
| 233 | 2,6-Dimethyl-6-hydroxyocta-2,7-dienal | C10H16O2 | 168.1150 | Flowers | GC-MS | [41] |
| 234 | 2,6-Dimethylocta-3,7-diene-2,6-diol | C10H18O2 | 170.1307 | Flowers | GC-MS | [41] |
| 235 | E,E-α-Farnesene | C15H24 | 204.1878 | Flowers | GC-MS | [41] |
| 236 | Z,E-Farnesol | C15H26O | 222.1984 | Flowers | GC-MS | [41] |
| 237 | E,E-Farnesyl acetate | C17H28O2 | 264.2089 | Flowers | GC-MS | [41] |
| 238 | Geranylacetone | C13H22O | 194.1671 | Flowers | GC-MS | [41] |
| 239 | Germacrene D | C15H28O | 208.2191 | Flowers | GC-MS | [41] |
| 240 | Hexahydrofarnesylacetone | C18H36O | 268.2766 | Flowers | GC-MS | [41] |
| 241 | E-8-Hydroxylinalool | C10H18O2 | 170.1307 | Flowers | GC-MS | [41] |
| 242 | Z-8-Hydroxylinalool | C10H18O2 | 170.1307 | Flowers | GC-MS | [41] |
| 243 | Lilac alcohol a | C10H18O2 | 170.1307 | Flowers | GC-MS | [41] |
| 244 | Lilac alcohol b | C10H18O2 | 170.1307 | Flowers | GC-MS | [41] |
| 245 | Lilac alcohol c | C10H18O2 | 170.1307 | Flowers | GC-MS | [41] |
| 246 | Lilac alcohol d | C10H18O2 | 170.1307 | Flowers | GC-MS | [41] |
| 247 | Lilac aldehyde 1 | C10H16O2 | 168.1150 | Flowers | GC-MS | [41] |
| 248 | Lilac aldehyde 2 | C10H16O2 | 168.1150 | Flowers | GC-MS | [41] |
| 249 | Lilac aldehyde 3 | C10H16O2 | 168.1150 | Flowers | GC-MS | [41] |
| 250 | Lilac aldehyde 4 | C10H16O2 | 168.1150 | Flowers | GC-MS | [41] |
| 251 | Limonene | C10H16 | 136.1252 | Flowers, fruit | GC-MS | [41] |
| 252 | Linalool | C10H18O | 154.1358 | Flowers, fruit | GC-MS | [41] |
| 253 | cis-Linalool oxide | C10H18O2 | 170.1307 | Flowers | GC-MS | [41] |
| 254 | trans-Linalool oxide | C10H18O2 | 170.1307 | Flowers | GC-MS | [41] |
| 255 | 6-Methylhept-5-en-2-one | C8H14O | 126.1045 | Flowers, fruit | GC-MS | [41] |
| 256 | Ocimene | C10H16 | 136.1252 | Flowers | GC-MS | [41] |
| 257 | E-β-Ocimene | C10H16 | 136.1252 | Flowers, fruit | GC-MS | [41] |
| 258 | Phytol | C20H40O | 296.3079 | Flowers | GC-MS | [41] |
| 259 | Squalene | C30H50 | 410.3913 | Flowers, fruit | GC-MS | [41,46] |
| 260 | 3,7,11,15-Tetramethyl hexadeca-6,10,14-trienol | C20H36O | 292.2766 | Flowers | GC-MS | [41] |
| 261 | Camphene | C10H16 | 136.1252 | Fruit | GC-MS | [41] |
| 262 | 2-Carene | C10H16 | 136.1252 | Fruit | GC-MS | [41] |
| 263 | cis-Carveol | C10H16O | 152.1201 | Fruit | GC-MS | [41] |
| 264 | Carvone | C10H14O | 150.1045 | Fruit | GC-MS | [41] |
| 265 | Endo-5,5,6-trimethylnorbornan-2-one | C10H16O | 152.1201 | Fruit | GC-MS | [41] |
| 266 | p-Mentha-1,3,8-triene | C10H14 | 134.1096 | Fruit | GC-MS | [41] |
| 267 | p-Menth-1-en-4-ol | C10H18O | 154.1358 | Fruit | GC-MS | [41] |
| 268 | Menthol | C10H20O | 156.1514 | Fruit | GC-MS | [41] |
| 269 | 1-Methyl-4-(1-methylethenyl)benzene | C10H12 | 132.0939 | Fruit | GC-MS | [41] |
| 270 | 1-Methyl-4-(1-methylethyl)-cyclohex-2-enol | C10H18O | 154.1358 | Fruit | GC-MS | [41] |
| 271 | Z-β-Ocimene | C10H16 | 136.1252 | Fruit | GC-MS | [41] |
| 272 | β-Phellandrene | C10H16 | 136.1252 | Fruit | GC-MS | [41] |
| 273 | Sabinene | C10H16 | 136.1252 | Fruit | GC-MS | [41] |
| 274 | α-Terpinene | C10H16 | 136.1252 | Fruit | GC-MS | [41] |
| 275 | β-Terpinene | C10H16 | 136.1252 | Fruit | GC-MS | [41] |
| 276 | δ-Terpinene | C10H16 | 136.1252 | Fruit | GC-MS | [41] |
| 277 | α-Terpineol | C10H18O | 154.1358 | Fruit | GC-MS | [41] |
| 278 | Ethylbenzaldehyde | C9H10O | 134.0732 | Flowers | GC-MS | [41] |
| 279 | Benzene | C6H6 | 78.0470 | Flowers | GC-MS | [41] |
| 280 | Benzyl benzoate | C14H12O2 | 212.0837 | Flowers, fruit | GC-MS | [41] |
| 281 | Ethylbenzaldehyde | C9H10O | 134.0732 | Flowers, fruit | GC-MS | [41] |
| 282 | 2-(4-Hydroxyphenyl)ethanol | C8H10O2 | 138.0681 | Flowers | GC-MS | [41] |
| 283 | Methoxybenzene | C8H10O | 122.0732 | Flowers | GC-MS | [41] |
| 284 | 2-(4-Methoxyphenyl)ethanol | C9H12O2 | 152.0837 | Flowers, fruit | GC-MS | [41] |
| 285 | Methyl 4-Methoxybenzoate | C9H10O3 | 166.0630 | Flowers | GC-MS | [41] |
| 286 | Methyl salicylate | C8H8O3 | 152.0473 | Flowers | GC-MS | [41] |
| 287 | Phenol | C6H6O | 94.0619 | Flowers | GC-MS | [41] |
| 288 | 2-Phenylethanal | C8H8O | 120.0575 | Flowers | GC-MS | [41] |
| 289 | 2-Phenylethanol | C8H10O | 122.0732 | Flowers | GC-MS | [41] |
| 290 | 2-Phenylethyl acetate | C10H12O2 | 164.0837 | Flowers | GC-MS | [41] |
| 291 | Dimethylbenzaldehyde | C9H10O | 134.0732 | Fruit | GC-MS | [41] |
| 292 | 1,2-Dimethylbenzene | C8H10 | 106.0783 | Fruit | GC-MS | [41] |
| 293 | Hex-3(Z)-enyl acetate | C8H14O2 | 142.0994 | Flowers | GC-MS | [41] |
| 294 | 3-Methylbutyl acetate | C7H14O2 | 130.0994 | Flowers | GC-MS | [41] |
| 295 | Butyl acetate | C6H12O2 | 116.0837 | Fruit | GC-MS | [41] |
| 296 | Dimethyl carbonate | C3H6O3 | 90.0317 | Fruit | GC-MS | [41] |
| 297 | Ethyl (2E)-2-butenoate | C6H10O2 | 114.0681 | Fruit | GC-MS | [41] |
| 298 | Ethyl butanoate | C6H12O2 | 116.0837 | Fruit | GC-MS | [41] |
| 299 | Ethyl decanoate | C12H24O2 | 200.1776 | Fruit | GC-MS | [41] |
| 300 | Ethyl heptanoate | C9H18O2 | 158.1307 | Fruit | GC-MS | [41] |
| 301 | Ethyl hexadecanoate | C18H36O2 | 284.2715 | Fruit | GC-MS | [41] |
| 302 | Ethyl hexadec-9-enoate | C18H34O2 | 282.2559 | Fruit | GC-MS | [41] |
| 303 | Ethyl hexanoate | C8H16O2 | 144.1150 | Flowers, fruit | GC-MS | [41] |
| 304 | Ethyl hexa-2,4-dienoate | C8H12O2 | 140.0837 | Fruit | GC-MS | [41] |
| 305 | Ethyl hex-2-enoate | C8H14O2 | 142.0994 | Fruit | GC-MS | [41] |
| 306 | Ethyl hex-3-enoate | C8H14O2 | 142.0994 | Fruit | GC-MS | [41] |
| 307 | Ethyl 2-methylbutanoate | C7H14O2 | 130.0994 | Fruit | GC-MS | [41] |
| 308 | Ethyl 3-methylbutanoate | C7H14O2 | 130.0994 | Fruit | GC-MS | [41] |
| 309 | Ethyl 2-methylpropanoate | C6H12O2 | 116.0837 | Fruit | GC-MS | [41] |
| 310 | Ethyl octanoate | C10H20O2 | 172.1463 | Fruit | GC-MS | [41] |
| 311 | Ethyl (4Z)-oct-4-enoate | C10H18O2 | 170.1307 | Fruit | GC-MS | [41] |
| 312 | Ethyl oleate | C20H38O2 | 310.2872 | Fruit | GC-MS | [41] |
| 313 | Ethyl pentanoate | C7H14O2 | 130.0994 | Fruit | GC-MS | [41] |
| 314 | Ethyl propanoate | C5H10O2 | 102.0681 | Fruit | GC-MS | [41] |
| 315 | Hexadecyl acetate | C18H36O2 | 284.2715 | Fruit | GC-MS | [41] |
| 316 | Methyl butanoate | C5H10O2 | 102.0681 | Fruit | GC-MS | [41] |
| 317 | 1-Methylethyl tetradecanoate | C17H34O2 | 270.2559 | Fruit | GC-MS | [41] |
| 318 | Methyl hexadecanoate | C17H34O2 | 270.2559 | Fruit | GC-MS | [41] |
| 319 | Methyl linoleate | C19H34O2 | 294.2559 | Fruit | GC-MS | [41] |
| 320 | Methyl octadecanoate | C19H38O2 | 298.2872 | Fruit | GC-MS | [41] |
| 321 | Methyl oleate | C19H36O2 | 296.2715 | Fruit | GC-MS | [41] |
| 322 | Methyl prop-2-enoate | C4H6O2 | 86.0368 | Fruit | GC-MS | [41] |
| 323 | Propyl butanoate | C7H14O2 | 130.0994 | Fruit | GC-MS | [41] |
| 324 | 2-Methylbutanal | C5H10O | 86.0732 | Flowers | GC-MS | [41] |
| 325 | 3-Methylbut-2-enal | C5H8O | 84.0575 | Flowers | GC-MS | [41] |
| 326 | 2-Methylpropanal | C4H8O | 72.0575 | Flowers | GC-MS | [41] |
| 327 | Undecanal | C11H22O | 170.1671 | Flowers | GC-MS | [41] |
| 328 | Acetaldehyde | C2H4O | 44.0262 | Flowers, fruit | GC-MS | [41] |
| 329 | Decanal | C10H20O | 156.1514 | Flowers, fruit | GC-MS | [41] |
| 330 | (2E,4E)-2,4-Heptadienal | C7H10O | 110.0732 | Fruit | GC-MS | [41] |
| 331 | Heptanal | C7H14O | 114.1045 | Flowers, fruit | GC-MS | [41] |
| 332 | (2Z)-2-Heptenal | C7H12O | 112.0888 | Fruit | GC-MS | [41] |
| 333 | Hexanal | C6H12O | 100.0888 | Flowers, fruit | GC-MS | [41,46] |
| 334 | (2E)-2-Hexenal | C6H10O | 98.0732 | Fruit | GC-MS | [41] |
| 335 | (2Z)-2-Hexenal | C6H10O | 98.0732 | Fruit | GC-MS | [41] |
| 336 | (3E)-3-Hexenal | C6H10O | 98.0732 | Fruit | GC-MS | [41] |
| 337 | (3Z)-3-Hexenal | C6H10O | 98.0732 | Fruit | GC-MS | [41] |
| 338 | 3-Methylbutanal | C5H10O | 86.0732 | Flowers, fruit | GC-MS | [41] |
| 339 | 2-Methylpentenal | C6H10O | 98.0732 | Fruit | GC-MS | [41] |
| 340 | (2E,6Z)-Nona-2,6-dienal | C9H14O | 138.1045 | Fruit | GC-MS | [41] |
| 341 | Nonanal | C9H18O | 142.1358 | Flowers, fruit | GC-MS | [41] |
| 342 | (2E)-Non-2-enal | C9H16O | 140.1201 | Fruit | GC-MS | [41] |
| 343 | Octanal | C8H16O | 128.1201 | Flowers, fruit | GC-MS | [41] |
| 344 | (2E)-Oct-2-enal | C8H14O | 126.1045 | Fruit | GC-MS | [41] |
| 345 | Propanal | C3H6O | 58.0419 | Fruit | GC-MS | [41] |
| 346 | Acetone | C3H6O | 58.0419 | Flowers, fruit | GC-MS | [41] |
| 347 | Butan-2-one | C4H8O | 72.0575 | Flowers, fruit | GC-MS | [41] |
| 348 | Butane-2,3-dione | C4H6O2 | 86.0368 | Flowers | GC-MS | [41] |
| 349 | 3-Hydroxybutan-2-one | C4H8O2 | 88.0524 | Flowers, fruit | GC-MS | [41] |
| 350 | 7,8-Dehydro-β-ionone | C13H22O | 194.1671 | Flowers | GC-MS | [41] |
| 351 | β-Ionone | C13H20O | 192.1514 | Flowers | GC-MS | [41] |
| 352 | Jasmone | C11H16O | 164.1201 | Flowers | GC-MS | [41] |
| 353 | 2-Methylpentan-3-one | C6H12O | 100.0888 | Flowers | GC-MS | [41] |
| 354 | 4-Methylpentan-2-one | C6H12O | 100.0888 | Flowers | GC-MS | [41] |
| 355 | Octan-3-one | C8H6O | 128.1201 | Flowers | GC-MS | [41] |
| 356 | Pentadecan-2-one | C15H30O | 226.2297 | Flowers | GC-MS | [41] |
| 357 | Cyclopentanone | C5H8O | 84.0575 | Fruit | GC-MS | [41] |
| 358 | 4-Hydroxy-4-methylpentan-2-one | C6H12O2 | 116.0837 | Fruit | GC-MS | [41] |
| 359 | 4-Methylpent-3-en-2-one | C6H10O | 98.0732 | Fruit | GC-MS | [41] |
| 360 | Octan-2,3-dione | C8H14O2 | 142.0994 | Fruit | GC-MS | [41] |
| 361 | Penten-3-one | C5H8O | 84.0575 | Fruit | GC-MS | [41] |
| 362 | (3E)-3-Penten-2-one | C5H8O | 84.0575 | Fruit | GC-MS | [41] |
| 363 | Butanol | C4H10O | 74.0732 | Flowers | GC-MS | [41] |
| 364 | Butan-2-ol | C4H10O | 74.0732 | Flowers | GC-MS | [41] |
| 365 | 2-Ethylhexanol | C8H18O | 130.1358 | Flowers | GC-MS | [41] |
| 366 | Hexadecanol | C16H34O | 242.2610 | Flowers, fruit | GC-MS | [41] |
| 367 | Methanol | CH4O | 32.0262 | Flowers | GC-MS | [41] |
| 368 | 1-Methoxypropan-2-ol | C4H10O2 | 90.0681 | Flowers | GC-MS | [41] |
| 369 | 2-Methylbutanol | C5H12O | 88.0888 | Flowers | GC-MS | [41] |
| 370 | 3-Methylbutanol | C5H12O | 88.0888 | Flowers, fruit | GC-MS | [41] |
| 371 | 3-Methylbut-2-enol | C5H10O | 86.0732 | Flowers | GC-MS | [41] |
| 372 | 3-Methylbut-3-enol | C5H10O | 86.0732 | Flowers | GC-MS | [41] |
| 373 | 2-Methylbut-3-en-2-ol | C5H10O | 86.0732 | Flowers | GC-MS | [41] |
| 374 | 2-Methylpropanol | C4H10O | 74.0732 | Flowers, fruit | GC-MS | [41] |
| 375 | Nonanol | C9H20O | 144.1514 | Flowers, fruit | GC-MS | [41] |
| 376 | Pentanol | C5H12O | 88.0888 | Flowers, fruit | GC-MS | [41] |
| 377 | Pentan-2-ol | C5H12O | 88.0888 | Flowers | GC-MS | [41] |
| 378 | Pentan-3-ol | C5H12O | 88.0888 | Flowers | GC-MS | [41] |
| 379 | Penten-3-ol | C5H10O | 86.0732 | Flowers, fruit | GC-MS | [41] |
| 380 | Propanol | C3H8O | 60.0575 | Flowers, fruit | GC-MS | [41] |
| 381 | Octanol | C8H18O | 130.1358 | Flowers | GC-MS | [41] |
| 382 | Octan-4-ol | C8H18O | 130.1358 | Flowers | GC-MS | [41] |
| 383 | Oct-1-en-3-ol | C8H16O | 128.1201 | Flowers | GC-MS | [41] |
| 384 | Decanol | C10H22O | 158.1671 | Fruit | GC-MS | [41] |
| 385 | Dodecanol | C12H26O | 186.1984 | Fruit | GC-MS | [41] |
| 386 | Heptanol | C7H16O | 116.1201 | Fruit | GC-MS | [41] |
| 387 | (2E)-2-Hexen-1-ol | C6H12O | 100.0888 | Fruit | GC-MS | [41] |
| 388 | (2Z)-2-Hexen-1-ol | C6H12O | 100.0888 | Fruit | GC-MS | [41] |
| 389 | (3Z)-3-Hexen-1-ol | C6H12O | 100.0888 | Fruit | GC-MS | [41] |
| 390 | Octanol | C8H18O | 130.1358 | Fruit | GC-MS | [41] |
| 391 | Oct-1-en-3-ol | C8H16O | 128.1201 | Fruit | GC-MS | [41] |
| 392 | (2E)-2-Penten-1-ol | C5H10O | 86.0732 | Fruit | GC-MS | [41] |
| 393 | Dodecanoic acid | C12H24O2 | 200.1776 | Flowers | GC-MS | [41] |
| 394 | Heptanoic acid | C7H14O2 | 130.0994 | Flowers | GC-MS | [41] |
| 395 | Hexanoic acid | C6H12O2 | 116.0837 | Flowers | GC-MS | [41] |
| 396 | 3-Methylbutanoic acid | C5H10O2 | 102.0681 | Flowers | GC-MS | [41] |
| 397 | Nonanoic acid | C9H18O2 | 158.1307 | Flowers | GC-MS | [41] |
| 398 | Octanoic acid | C8H16O2 | 144.1150 | Flowers | GC-MS | [41] |
| 399 | Butanoic acid | C4H8O2 | 88.0524 | Fruit | GC-MS | [41] |
| 400 | Heptacosane | C27H56 | 380.4382 | Flowers | GC-MS | [41,46] |
| 401 | Hexacosane | C18H54 | 366.4226 | Flowers | GC-MS | [41,46] |
| 402 | Hexadecane | C16H34 | 226.2661 | Flowers, fruit | GC-MS | [41] |
| 403 | Hexa-1,4-diene | C6H10 | 82.0783 | Flowers | GC-MS | [41] |
| 404 | (2Z,4Z)-2,4-Hexadiene | C6H10 | 82.0783 | Flowers | GC-MS | [41] |
| 405 | 3-Methylcyclopentene | C6H10 | 82.0783 | Flowers | GC-MS | [41] |
| 406 | 3-Methylpenta-1,3-diene | C6H10 | 82.0783 | Flowers | GC-MS | [41] |
| 407 | Nonacosane | C29H60 | 408.4695 | Flowers | GC-MS | [41,46] |
| 408 | Nonadecane | C19H40 | 268.3130 | Flowers, fruit | GC-MS | [41,46] |
| 409 | Nonane | C9H20 | 128.1565 | Flowers | GC-MS | [41] |
| 410 | Octane | C8H18 | 114.1409 | Flowers | GC-MS | [41] |
| 411 | Pentacosane | C25H52 | 352.4069 | Flowers | GC-MS | [41,46] |
| 412 | Pentadecane | C15H32 | 212.2504 | Flowers, fruit | GC-MS | [41] |
| 413 | Tricosane | C23H48 | 324.3756 | Flowers | GC-MS | [41,46] |
| 414 | 2,6-Dimethyldecane | C12H26 | 170.2035 | Fruit | GC-MS | [41] |
| 415 | 2-Methylpenta-1,3-diene | C6H10 | 82.0783 | Fruit | GC-MS | [41] |
| 416 | 2-Methoxy-2-methylpropane | C5H12O | 88.0888 | Fruit | GC-MS | [41] |
| 417 | Octadecane | C18H38 | 254.2974 | Fruit | GC-MS | [41,46] |
| 418 | Tridecane | C13H28 | 184.2191 | Fruit | GC-MS | [41] |
| 419 | Bis(1-methylethyl)disulphide | C6H14S2 | 150.0537 | Flowers, fruit | GC-MS | [41] |
| 420 | Carbon disulphide | CS2 | 75.9441 | Flowers | GC-MS | [41] |
| 421 | Dimethyl disulphide | C2H6S2 | 93.9911 | Flowers | GC-MS | [41] |
| 422 | Butanenitrile | C4H7N | 69.0578 | Flowers | GC-MS | [41] |
| 423 | Methenamine | C6H12N4 | 140.1062 | Flowers | GC-MS | [41] |
| 424 | 2-Methylbutanenitrile | C5H9N | 83.0735 | Flowers | GC-MS | [41] |
| 425 | Tetrahydrofuran | C4H8O | 72.0575 | Flowers, fruit | GC-MS | [41] |
| 426 | Ethyl 2-furancarboxylate | C7H8O3 | 140.0473 | Fruit | GC-MS | [41] |
| 427 | 2-Furancarboxaldehyde | C5H4O2 | 96.0211 | Fruit | GC-MS | [41] |
| 428 | 4-Methoxy-2,5-dimethyl-3(2H)-furanone | C7H10O3 | 142.0630 | Fruit | GC-MS | [41] |
| 429 | 2-Methylfuran | C5H6O | 82.0419 | Fruit | GC-MS | [41] |
| 430 | 5-Methyl-2-furfural | C6H6O2 | 110.0368 | Fruit | GC-MS | [41] |
| 431 | Methyl 2-furoate | C6H6O3 | 126.0317 | Fruit | GC-MS | [41] |
| 432 | n-Docosane | C21H44 | 296.3443 | Stems | IR, EI-MS | [38,46] |
| 433 | Benzeneethanol | C8H10O | 122.0732 | Fruit | GC-MS | [46] |
| 434 | Benzoic acid ethyl ester | C9H10O2 | 150.0681 | Fruit | GC-MS | [46] |
| 435 | 1-Eicosanol | C20H42O | 298.3236 | Fruit | GC-MS | [46] |
| 436 | Neophytadiene | C20H38 | 278.2974 | Fruit | GC-MS | [46] |
| 437 | Cyclotetradecane | C14H28 | 196.2191 | Fruit | GC-MS | [46] |
| 438 | Isopropyl palmitate | C19H38O2 | 298.2872 | Fruit | GC-MS | [46] |
| 439 | 1-Octadecene | C18H36 | 252.2817 | Fruit | GC-MS | [46] |
| 440 | Heneicosane | C21H44 | 296.3443 | Fruit | GC-MS | [46] |
| 441 | Decylcyclohexane | C16H32 | 224.2504 | Fruit | GC-MS | [46] |
| 442 | Hexadecanamide | C16H33NO | 255.2562 | Fruit | GC-MS | [46] |
| 443 | 1-Naphthalenamine, N-phenyl- | C16H13N | 219.1048 | Fruit | GC-MS | [46] |
| 444 | 9-Octadecenamide | C18H35NO | 281.2719 | Fruit | GC-MS | [46] |
| 445 | Octadecanamide | C18H37NO | 283.2875 | Fruit | GC-MS | [46] |
| 446 | Tetracosane | C24H50 | 338.3913 | Fruit | GC-MS | [46] |
| 447 | Linoleic acid butyl ester | C22H40O2 | 336.3028 | Fruit | GC-MS | [46] |
| 448 | Octacosane | C28H58 | 394.4539 | Fruit | GC-MS | [46] |
| 449 | Schizandrin | C24H32O7 | 432.2148 | Fruit | GC-MS | [46] |
| 450 | Octadecane | C18H38 | 254.2974 | Fruit | GC-MS | [46] |
| 451 | Triacontane | C30H62 | 422.4852 | Fruit | GC-MS | [46] |
| 452 | β-Tocopherol | C28H48O2 | 416.3654 | Fruit | GC-MS | [46] |
| 453 | Hentriacontane | C31H64 | 436.5008 | Fruit | GC-MS | [46] |
| 454 | Hexacosanol | C26H54O | 382.4175 | Fruit | GC-MS | [46] |
| 455 | Tritriacontane | C33H68 | 464.5321 | Fruit | GC-MS | [46] |
| 456 | Cholest-5-en-3-ol | C27H46O | 386.3549 | Fruit | GC-MS | [46] |
| 457 | Campesterol | C28H48O | 400.3705 | Fruit | GC-MS | [46] |
| 458 | Stigmasta-5,22-dien-3-ol | C29H48O | 412.3705 | Fruit | GC-MS | [46] |
| 459 | γ-Sitosterol | C29H50O | 414.3862 | Fruit | GC-MS | [46] |
| 460 | β-Amyrin | C30H50O | 426.3862 | Fruit | GC-MS | [46] |
| 461 | α-Amyrin | C30H50O | 426.3862 | Fruit | GC-MS | [46] |
| 462 | Stigmast-7-en-3-ol | C29H50O | 414.3862 | Fruit | GC-MS | [46] |
| 463 | 9,19-Cyclolanostan-3-ol,24-methylene | C31H52O | 440.4018 | Fruit | GC-MS | [46] |
| 464 | 9,19-Cyclolanostan-3-ol,acetate | C32H54O2 | 470.4124 | Fruit | GC-MS | [46] |
| 465 | D:A-Friedooleanan-3-one | C30H50O | 426.3862 | Fruit | GC-MS | [46] |
| No. | Name | Formula | Exact Theoretical Molecular Weight | Source | Characterization Method | Refs. |
|---|---|---|---|---|---|---|
| 466 | β-Sitosterol | C29H50O | 414.3862 | Roots | IR, EI-MS, 1H-NMR | [17] |
| 467 | Daucosterol | C35H60O6 | 576.4390 | Stems | TLC, IR, 13C-NMR | [19] |
| 468 | Ergosterol-4,6,8 (14), 22-tetraene-3-one | C28H39O2 | 407.2950 | Roots | HPLC-DAD-ESI-MS | [18] |
| 469 | Aconitine | C34H47NO11 | 645.3149 | Fruit | HPLC-MS | [51] |
| 470 | Berberine | C20H18NO4 | 336.1230 | Fruit | HPLC-MS | [51] |
| 471 | Corydaline | C34H47NO11 | 369.1940 | Fruit | HPLC-MS | [51] |
| 472 | Tetrahydropalmatine | C34H47NO11 | 355.1784 | Fruit | HPLC-MS | [51] |
| 473 | Hypaconitine | C33H45NO10 | 615.3043 | Fruit | HPLC-MS | [51] |
| 474 | Physostigmine | C34H47NO11 | 275.1634 | Fruit | HPLC-MS | [51] |
| 475 | Atropine | C17H23NO3 | 289.1678 | Fruit | HPLC-MS | [51] |
| 476 | Actinidine | C10H13N | 147.1048 | Fruit | HPLC-MS | [51] |
| 477 | 5-Hydroxy-6-methoxy-7-O-β-D-glucopyranosyloxy-coumarin | C16H18O10 | 370.0900 | Roots | HPLC-DAD-ESI-MS | [18] |
| 478 | Bis(2-ethylhexyl) phthalate | C24H38O4 | 390.2770 | Roots | HPLC-DAD-ESI-MS | [18] |
| 479 | Argutosides E | C24H24O12 | 504.1268 | Leaves | IR, ESI-TOF-MS, 1H-NMR, 13C-NMR, HMBC | [29] |
| 480 | Eculetin 7-O-(6′-O-trans-coumaroyl)-β-glucopyranoside | C24H24O11 | 488.1319 | Leaves | IR, ESI-TOF-MS, 1H-NMR, 13C-NMR | [29] |
| 481 | Umbelliferone 7-O-(6′-O-trans-coumaroyl)-β-glucopyranoside | C24H24O10 | 472.1369 | Leaves | IR, ESI-TOF-MS, 1H-NMR, 13C-NMR | [29] |
| 482 | Esculetin | C9H6O4 | 178.0266 | Leaves | IR, ESI-TOF-MS, 1H-NMR, 13C-NMR | [29] |
| 483 | 7,8-Dihydroxycoumarin | C9H6O4 | 178.0266 | Leaves | IR, ESI-TOF-MS, 1H-NMR, 13C-NMR | [29] |
| 484 | Umbelliferone | C9H6O3 | 162.0317 | Leaves | IR, ESI-TOF-MS, 1H-NMR, 13C-NMR | [29] |
| 485 | Aspartic acid | C4H7NO4 | 133.0375 | Fruit, seeds | Amino acid analyzer | [53] |
| 486 | Threonine | C4H9NO3 | 119.0582 | Fruit, seeds | Amino acid analyzer | [53] |
| 487 | Serine | C3H7NO3 | 105.0426 | Fruit, seeds | Amino acid analyzer | [53] |
| 488 | Glutamate | C5H9NO4 | 147.0532 | Fruit, seeds | Amino acid analyzer | [53] |
| 489 | Glycine | C2H5NO2 | 75.0320 | Seeds | Amino acid analyzer | [53] |
| 490 | Alanine | C3H7NO2 | 89.0744 | Seeds | Amino acid analyzer | [53] |
| 491 | Cystine | C6H12N2O4S2 | 240.0238 | Fruit, seeds | Amino acid analyzer | [53] |
| 492 | Valine | C5H11NO2 | 117.0790 | Fruit | Amino acid analyzer | [53] |
| 493 | Methionine | C5H11O2NS | 149.0510 | Fruit | Amino acid analyzer | [53] |
| 494 | isoleucine | C6H13NO2 | 131.0946 | Fruit | Amino acid analyzer | [53] |
| 495 | Leucine | C6H13NO2 | 131.0946 | Fruit | Amino acid analyzer | [53] |
| 496 | Tyrosine | C9H11NO3 | 181.0739 | Fruit | Amino acid analyzer | [53] |
| 497 | Phenylalanine | C9H11NO2 | 165.0790 | Fruit | Amino acid analyzer | [53] |
| 498 | Lysine | C6H14N2O2 | 146.1055 | Fruit | Amino acid analyzer | [53] |
| 499 | Histidine | C6H9N3O2 | 155.0695 | Fruit | Amino acid analyzer | [53] |
| 500 | Arginine | C6H14N4O2 | 147.1117 | Fruit | Amino acid analyzer | [53] |
| 501 | Proline | C5H9NO2 | 115.0633 | Seeds | Amino acid analyzer | [53] |
| 502 | Inositol | C6H12O6 | 180.0634 | Roots | 1H-NMR | [54] |
| 503 | Sucrose | C12H22O11 | 342.1162 | Sprouts | HPLC | [39] |
| 504 | Glucose | C6H12O6 | 180.0634 | Sprouts | HPLC | [39] |
| 505 | Fructose | C6H12O6 | 180.0634 | Sprouts | HPLC | [39] |
| 506 | Maltose | C12H22O11 | 342.1162 | Sprouts | HPLC | [39] |
| 507 | Xylose | C5H10O5 | 150.0528 | Sprouts | HPLC | [39] |
| 508 | Emodin | C15H10O5 | 270.0528 | Roots | HPLC | [52] |
| 509 | Chrysophanol | C15H10O4 | 254.0579 | Roots | HPLC | [52] |
| 510 | 3-Hydroxy-1-(4-O-β-D-glucopyranosyl-3-methoxyphenyl) propan-1-one | C16H22O9 | 358.1264 | Fruit | ESI-MS, 1H-NMR, 13C-NMR | [21] |
| No. | Name | Formula | Exact Theoretical Molecular Weight | Source | Characterization Method | Refs. |
|---|---|---|---|---|---|---|
| 511 | Calcium | Ca | 39.9626 | Fruit, leaves, branches, stems, roots, velamina, fibers | FPD, ICP, AAS, AFS | [55] |
| 512 | Kalium | K | 38.9637 | Fruit, leaves, branches, stems, roots, velamina, fibers | FPD, ICP, AAS, AFS | [55] |
| 513 | Magnesium | Mg | 23.9850 | Fruit, leaves, branches, stems, roots, velamina, fibers | FPD, ICP, AAS, AFS | [55] |
| 514 | Phosphorus | P | 30.9738 | Fruit, leaves, branches, stems, roots, velamina, fibers | FPD, ICP, AAS, AFS | [55] |
| 515 | Sodium | Na | 22.9898 | Fruit, leaves, branches, stems, roots, velamina, fibers | FPD, ICP, AAS, AFS | [55] |
| 516 | Aluminium | Al | 26.9815 | Fruit, leaves, branches, stems, roots, velamina, fibers | FPD, ICP, AAS, AFS | [55] |
| 517 | Ferrum | Fe | 55.9349 | Fruit, leaves, branches, stems, roots, velamina, fibers | FPD, ICP, AAS, AFS | [55] |
| 518 | Barium | Ba | 137.9052 | Fruit, leaves, branches, stems, roots, velamina, fibers | FPD, ICP, AAS, AFS | [55] |
| 519 | Strontium | Sr | 87.9056 | Fruit, leaves, branches, stems, roots, velamina, fibers | FPD, ICP, AAS, AFS | [55] |
| 520 | Manganese | Mn | 54.9380 | Fruit, leaves, branches, stems, roots, velamina, fibers | FPD, ICP, AAS, AFS | [55] |
| 521 | Lithium | Li | 7.0160 | Fruit, leaves, branches, stems, roots, velamina, fibers | FPD, ICP, AAS, AFS | [55] |
| 522 | Zinc | Zn | 63.9291 | Fruit, leaves, branches, stems, roots, velamina, fibers | FPD, ICP, AAS, AFS | [55] |
| 523 | Boron | B | 11.0093 | Fruit, leaves, branches, stems, roots, velamina, fibers | FPD, ICP, AAS, AFS | [55] |
| 524 | Cuprum | Cu | 62.9296 | Fruit, leaves, branches, stems, roots, velamina, fibers | FPD, ICP, AAS, AFS | [55] |
| 525 | Titanium | Ti | 47.9479 | Fruit, leaves, branches, stems, roots, velamina, fibers | FPD, ICP, AAS, AFS | [55] |
| 526 | Molybdenum | Mo | 97.9054 | Fruit, leaves, branches, stems, roots, velamina, fibers | FPD, ICP, AAS, AFS | [55] |
| 527 | Lead | Pb | 207.9766 | Fruit, leaves, branches, stems, roots, velamina, fibers | FPD, ICP, AAS, AFS | [55] |
| 528 | Chromium | Cr | 51.9405 | Fruit, leaves, branches, stems, roots, velamina, fibers | FPD, ICP, AAS, AFS | [55] |
| 529 | Nickel | Ni | 57.9353 | Fruit, leaves, branches, stems, roots, velamina, fibers | FPD, ICP, AAS, AFS | [55] |
| 530 | Indium | In | 114.9039 | Fruit, leaves, branches, stems, roots, velamina, fibers | FPD, ICP, AAS, AFS | [55] |
| 531 | Vanadium | V | 50.9440 | Fruit, leaves, branches, stems, roots, velamina, fibers | FPD, ICP, AAS, AFS | [55] |
| 532 | Arsenic | As | 74.9216 | Fruit, leaves, branches, stems, roots, velamina, fibers | FPD, ICP, AAS, AFS | [55] |
| 533 | Zirconium | Zr | 89.9047 | Fruit, leaves, branches, stems, roots, velamina, fibers | FPD, ICP, AAS, AFS | [55] |
| 534 | Cobalt | Co | 58.9332 | Fruit, leaves, branches, stems, roots, velamina, fibers | FPD, ICP, AAS, AFS | [55] |
| 535 | Cadmium | Cd | 113.9034 | Fruit, leaves, branches, stems, roots, velamina, fibers | FPD, ICP, AAS, AFS | [55] |
| 536 | Mercury | Hg | 201.9706 | Fruit, leaves, branches, stems, roots, velamina, fibers | FPD, ICP, AAS, AFS | [55] |
| 537 | Beryllium | Be | 9.0122 | Fruit, leaves, branches, stems, roots, velamina, fibers | FPD, ICP, AAS, AFS | [55] |
| 538 | Selenium | Se | 79.9165 | Fruit, leaves, branches, stems, roots, velamina, fibers | FPD, ICP, AAS, AFS | [55] |
| 539 | Yttrium | Y | 88.9058 | Fruit, leaves, branches, stems, roots, velamina, fibers | FPD, ICP, AAS, AFS | [55] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Teng, K.; Zang, H. Actinidia arguta (Sieb. et Zucc.) Planch. ex Miq.: A Review of Phytochemistry and Pharmacology. Molecules 2023, 28, 7820. https://doi.org/10.3390/molecules28237820
Zhang H, Teng K, Zang H. Actinidia arguta (Sieb. et Zucc.) Planch. ex Miq.: A Review of Phytochemistry and Pharmacology. Molecules. 2023; 28(23):7820. https://doi.org/10.3390/molecules28237820
Chicago/Turabian StyleZhang, Haifeng, Kun Teng, and Hao Zang. 2023. "Actinidia arguta (Sieb. et Zucc.) Planch. ex Miq.: A Review of Phytochemistry and Pharmacology" Molecules 28, no. 23: 7820. https://doi.org/10.3390/molecules28237820