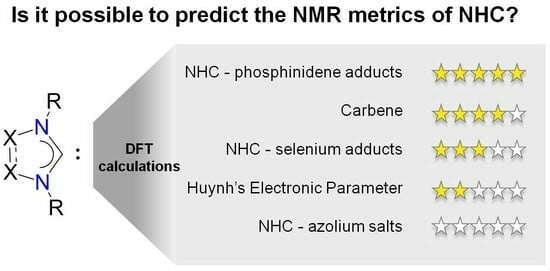
NMR “Finger Prints” of N-Heterocyclic Carbenes, DFT Analysis: Scopes and Limitations
Abstract
:1. Introduction
2. Results and Discussion
2.1. 77Se-NMR Shifts of NHC–Derived Selenoureas
2.1.1. General Overview
2.1.2. Solvent Effects
2.1.3. The Quality of the Basis Sets
2.1.4. Variation of Functional Parameters
2.1.5. Full-Relativistic Correction to the 77Se NMR Shift
2.1.6. Some Final Remarks on 77Se NMR Shift Calculations of NHC–Selenoureas
2.2. 31P NMR Shifts of NHC–Phosphinidene Adducts
2.3. 13C NMR Shifts of NHC–Phosphinidene Adducts
2.4. 13C Shifts of NHCs
2.5. 13C Shifts of NHC–Azolium Salts
2.6. Huynh’s Electronic Parameter (HEP)
2.7. Minimization of Systematical Errors—Empirical Linear Scaling Procedure
2.8. Practical Aspects
3. Materials and Methods
Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, C.A.; Narouz, M.R.; Lummis, P.A.; Singh, I.; Nazemi, A.; Li, C.H.; Crudden, C.M. N-heterocyclic carbenes in materials chemistry. Chem. Rev. 2019, 119, 4986–5056. [Google Scholar] [CrossRef]
- Wang, Y.; Chang, J.P.; Xu, R.; Bai, S.; Wang, D.; Yang, G.P.; Sun, L.Y.; Peng, L.; Han, Y.F. N-Heterocyclic carbenes and their precursors in functionalised porous materials. Chem. Soc. Rev. 2021, 50, 13559–13586. [Google Scholar] [CrossRef]
- Breitwieser, K.; Bahmann, H.; Weiss, R.; Munz, D. Gauging Radical Stabilization with Carbenes. Angew. Chem. Int. Ed. 2022, 61, e202206390. [Google Scholar] [CrossRef]
- De Fremont, P.; Marion, N.; Nolan, S.P. Carbenes: Synthesis, properties, and organometallic chemistry. Coord. Chem. Rev. 2009, 253, 862–892. [Google Scholar] [CrossRef]
- Roos, L.; Malan, F.P.; Landman, M. Naphthalimide-NHC complexes: Synthesis and properties in catalytic, biological and photophysical applications. Coord. Chem. Rev. 2021, 449, 214201. [Google Scholar] [CrossRef]
- Zhao, S.; Yang, Z.; Jiang, G.; Huang, S.; Bian, M.; Lu, Y.; Liu, W. An overview of anticancer platinum N-heterocyclic carbene complexes. Coord. Chem. Rev. 2021, 449, 214217. [Google Scholar] [CrossRef]
- Shen, H.; Tian, G.; Xu, Z.; Wang, L.; Wu, Q.; Zhang, Y.; Teo, B.K.; Zheng, N. N-heterocyclic carbene coordinated metal nanoparticles and nanoclusters. Coord. Chem. Rev. 2022, 458, 214425. [Google Scholar] [CrossRef]
- He, Y.; Huang, Z.; Wu, K.; Ma, J.; Zhou, Y.G.; Yu, Z. Recent advances in transition-metal-catalyzed carbene insertion to C–H bonds. Chem. Soc. Rev. 2022, 51, 2759–2852. [Google Scholar] [CrossRef]
- Sau, S.C.; Hota, P.K.; Mandal, S.K.; Soleilhavoup, M.; Bertrand, G. Stable abnormal N-heterocyclic carbenes and their applications. Chem. Soc. Rev. 2020, 49, 1233–1252. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.J.; Nolan, S.P. Quantifying and understanding the electronic properties of N-heterocyclic carbenes. Chem. Soc. Rev. 2013, 42, 6723–6753. [Google Scholar] [CrossRef] [PubMed]
- Huynh, H.V. Electronic properties of N-heterocyclic carbenes and their experimental determination. Chem. Rev. 2018, 118, 9457–9492. [Google Scholar] [CrossRef]
- Mathew, J.; Suresh, C.H. Use of molecular electrostatic potential at the carbene carbon as a simple and efficient electronic parameter of N-heterocyclic carbenes. Inorg. Chem. 2010, 49, 4665–4669. [Google Scholar] [CrossRef]
- Tonner, R.; Frenking, G. Tolman’s electronic parameters for divalent carbon(0) compounds. Organometallics 2009, 28, 3901–3905. [Google Scholar] [CrossRef]
- Gusev, D.G. Electronic and steric parameters of 76 N-heterocyclic carbenes in Ni(CO)3(NHC). Organometallics 2009, 28, 6458–6461. [Google Scholar] [CrossRef]
- Huynh, H.V.; Han, Y.; Jothibasu, R.; Yang, J.A. 13C NMR spectroscopic determination of ligand donor strengths using N-heterocyclic carbene complexes of palladium(II). Organometallics 2009, 28, 5395–5404. [Google Scholar] [CrossRef]
- Teng, Q.; Huynh, H.V. A unified ligand electronic parameter based on 13C NMR spectroscopy of N-heterocyclic carbene complexes. Dalton Trans. 2017, 46, 614–627. [Google Scholar] [CrossRef] [PubMed]
- Teng, Q.; Huynh, H.V. Determining the electron-donating properties of bidentate ligands by 13C NMR spectroscopy. Inorg. Chem. 2014, 53, 10964–10973. [Google Scholar] [CrossRef]
- Verlinden, K.; Buhl, H.; Frank, W.; Ganter, C. Determining the ligand properties of N-heterocyclic carbenes from 77Se NMR parameters. Eur. J. Inorg. Chem. 2015, 2015, 2416–2425. [Google Scholar] [CrossRef]
- Liske, A.; Verlinden, K.; Buhl, H.; Schaper, K.; Ganter, C. Determining the π-acceptor properties of N-heterocyclic carbenes by measuring the 77Se NMR chemical shifts of their selenium adducts. Organometallics 2013, 32, 5269–5272. [Google Scholar] [CrossRef]
- Vummaleti, S.V.; Nelson, D.J.; Poater, A.; Gómez-Suárez, A.; Cordes, D.B.; Slawin, A.M.; Nolan, S.P.; Cavallo, L. What can NMR spectroscopy of selenoureas and phosphinidenes teach us about the π-accepting abilities of N-heterocyclic carbenes? Chem. Sci. 2015, 6, 1895–1904. [Google Scholar] [CrossRef]
- Back, O.; Henry-Ellinger, M.; Martin, C.D.; Martin, D.; Bertrand, G. 31P NMR Chemical Shifts of Carbene–Phosphinidene Adducts as an Indicator of the π-Accepting Properties of Carbenes. Angew. Chem. Int. Ed. 2013, 52, 2939–2943. [Google Scholar] [CrossRef]
- Lodewyk, M.W.; Siebert, M.R.; Tantillo, D.J. Computational prediction of 1H and 13C chemical shifts: A useful tool for natural product, mechanistic, and synthetic organic chemistry. Chem. Rev. 2012, 112, 1839–1862. [Google Scholar] [CrossRef] [PubMed]
- Flaig, D.; Maurer, M.; Hanni, M.; Braunger, K.; Kick, L.; Thubauville, M.; Ochsenfeld, C. Benchmarking hydrogen and carbon NMR chemical shifts at HF, DFT, and MP2 levels. J. Chem. Theory Comput. 2014, 10, 572–578. [Google Scholar] [CrossRef]
- Semenov, V.A.; Krivdin, L.B. DFT computational schemes for 1H and 13C NMR chemical shifts of natural products, exemplified by strychnine. Magn. Reson. Chem. 2020, 58, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Iron, M.A. Evaluation of the Factors Impacting the Accuracy of 13C NMR Chemical Shift Predictions using Density Functional Theory—The Advantage of Long-Range Corrected Functionals. J. Chem. Theory Comput. 2017, 13, 5798–5819. [Google Scholar] [CrossRef]
- Potmischil, F.; Hillebrand, M.; Kalchhauser, H. Hydroacridines: Part 33. An experimental and DFT study of the 13C NMR chemical shifts of the nitrosamines derived from the six stereoisomers of tetradecahydroacridine. Magn. Reson. Chem. 2020, 58, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Blanco, F.; Alkorta, I.; Elguero, J. Statistical analysis of 13C and 15N NMR chemical shifts from GIAO/B3LYP/6-311++ G** calculated absolute shieldings. Magn. Reson. Chem. 2007, 45, 797–800. [Google Scholar] [CrossRef]
- Latypov, S.K.; Polyancev, F.M.; Yakhvarov, D.G.; Sinyashin, O.G. Quantum chemical calculations of 31P NMR chemical shifts: Scopes and limitations. Phys. Chem. Chem. Phys. 2015, 17, 6976–6987. [Google Scholar] [CrossRef]
- Latypov, S.K.; Kondrashova, S.A.; Polyancev, F.M.; Sinyashin, O.G. Quantum chemical calculations of 31P NMR chemical shifts in nickel complexes: Scope and limitations. Organometallics 2020, 39, 1413–1422. [Google Scholar] [CrossRef]
- Kondrashova, S.A.; Polyancev, F.M.; Ganushevich, Y.S.; Latypov, S.K. DFT approach for predicting 13C NMR shifts of atoms directly coordinated to nickel. Organometallics 2021, 40, 1614–1625. [Google Scholar] [CrossRef]
- Payard, P.A.; Perego, L.A.; Grimaud, L.; Ciofini, I. A DFT protocol for the prediction of 31P NMR chemical shifts of phosphine ligands in first-row transition-metal complexes. Organometallics 2020, 39, 3121–3130. [Google Scholar] [CrossRef]
- Kondrashova, S.A.; Polyancev, F.M.; Latypov, S.K. DFT Calculations of 31P NMR Chemical Shifts in Palladium Complexes. Molecules 2022, 27, 2668. [Google Scholar] [CrossRef]
- Kondrashova, S.A.; Latypov, S.K. DFT Approach for Predicting 13C NMR Shifts of Atoms Directly Coordinated to Pd. Organometallics 2023, 42, 1951–1962. [Google Scholar] [CrossRef]
- Rusakov, Y.Y.; Rusakova, I.L.; Krivdin, L.B. MP2 calculation of 77Se NMR chemical shifts taking into account relativistic corrections. Magn. Reson. Chem. 2015, 53, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Rusakov, Y.Y.; Krivdin, L.B. Four-component relativistic DFT calculations of 77Se NMR chemical shifts: A gateway to a reliable computational scheme for the medium-sized organoselenium molecules. J. Comput. Chem. 2015, 36, 1756–1762. [Google Scholar] [CrossRef] [PubMed]
- Rusakova, I.L.; Rusakov, Y.Y. Quantum chemical calculations of 77Se and 125Te nuclear magnetic resonance spectral parameters and their structural applications. Magn. Reson. Chem. 2021, 59, 359–407. [Google Scholar] [CrossRef]
- Bayse, C.A. Considerations for reliable calculation of 77Se chemical shifts. J. Chem. Theory Comput. 2005, 1, 1119–1127. [Google Scholar] [CrossRef]
- Gordon, C.P.; Raynaud, C.; Andersen, R.A.; Copéret, C.; Eisenstein, O. Carbon-13 NMR Chemical Shift: A Descriptor for Electronic Structure and Reactivity of Organometallic Compounds. Acc. Chem. Res. 2019, 52, 2278–2289. [Google Scholar] [CrossRef] [PubMed]
- Junor, G.P.; Lorkowski, J.; Weinstein, C.M.; Jazzar, R.; Pietraszuk, C.; Bertrand, G. The Influence of C(sp3)H–Selenium Interactions on the 77Se NMR Quantification of the π-Accepting Properties of Carbenes. Angew. Chem. 2020, 132, 22212–22217. [Google Scholar] [CrossRef]
- Miertus, S.; Scrocco, E.; Tomasi, J. Electrostatic interaction of a solute with a continuum. A direct utilization of AB initio molecular potentials for the prevision of solvent effects. Chem. Phys. 1981, 55, 117–129. [Google Scholar] [CrossRef]
- Paschoal, D.; Guerra, C.F.; de Oliveira, M.A.L.; Ramalho, T.C.; Dos Santos, H.F. Predicting Pt-195 NMR chemical shift using new relativistic all-electron basis set. J. Comput. Chem. 2016, 37, 2360–2373. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, B.P.; Altarawy, D.; Didier, B.; Gibson, T.D.; Windus, T.L. New basis set exchange: An open, up-to-date resource for the molecular sciences community. J. Chem. Inf. Model. 2019, 59, 4814–4820. [Google Scholar] [CrossRef]
- Teale, A.M.; Lutnæs, O.B.; Helgaker, T.; Tozer, D.J.; Gauss, J. Benchmarking density-functional theory calculations of NMR shielding constants and spin–rotation constants using accurate coupled-cluster calculations. J. Chem. Phys. 2013, 138, 024111. [Google Scholar] [CrossRef]
- Schreckenbach, G. The Fe57 nuclear magnetic resonance shielding in ferrocene revisited. A density-functional study of orbital energies, shielding mechanisms, and the influence of the exchange-correlation functional. J. Chem. Phys. 1999, 110, 11936–11949. [Google Scholar] [CrossRef]
- Komorovský, S.; Repiský, M.; Malkina, O.L.; Malkin, V.G.; Malkin Ondík, I.; Kaupp, M. A fully relativistic method for calculation of nuclear magnetic shielding tensors with a restricted magnetically balanced basis in the framework of the matrix Dirac–Kohn–Sham equation. J. Chem. Phys. 2008, 128, 104101. [Google Scholar] [CrossRef] [PubMed]
- Dyall, K.G. Relativistic and nonrelativistic finite nucleus optimized double zeta basis sets for the 4p, 5p and 6p elements. Theor. Chem. Acc. 1998, 99, 366–371. [Google Scholar]
- Rodrigues, R.R.; Dorsey, C.L.; Arceneaux, C.A.; Hudnall, T.W. Phosphaalkene vs. phosphinidene: The nature of the P–C bond in carbonyl-decorated carbene→PPh adducts. Chem. Commun. 2014, 50, 162–164. [Google Scholar] [CrossRef] [PubMed]
- Bax, A.; Summers, M.F. Proton and carbon-13 assignments from sensitivity-enhanced detection of heteronuclear multiple-bond connectivity by 2D multiple quantum NMR. J. Am. Chem. Soc. 1986, 108, 2093–2094. [Google Scholar] [CrossRef]
- Pierens, G.K. 1H and 13C NMR scaling factors for the calculation of chemical shifts in commonly used solvents using density functional theory. J. Comput. Chem. 2014, 35, 1388–1394. [Google Scholar] [CrossRef]
- Konstantinov, I.A.; Broadbelt, L.J. Regression formulas for density functional theory calculated 1H and 13C NMR chemical shifts in toluene-d8. J. Phys. Chem. A 2011, 115, 12364–12372. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-consistent equations including exchange and correlation effects. Phys. Rev. 1965, 140, A1133. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision A.03; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Hehre, W.J.; Ditchfield, R.; Pople, J.A. Self-Consistent Molecular Orbital Methods. XII. Further Extensions of Gaussian-Type Basis Sets for Use in Molecular Orbital Studies of Organic Molecules. J. Chem. Phys. 1972, 56, 2257–2261. [Google Scholar] [CrossRef]
- Clark, T.; Chandrasekhar, J.; Spitznagel, G.W.; Schleyer, P.V.R. Efficient diffuse function-augmented basis sets for anion calculations. III. The 3-21+G basis set for first-row elements, Li–F. J. Comput. Chem. 1983, 4, 294–301. [Google Scholar] [CrossRef]
- Francl, M.M.; Pietro, W.J.; Hehre, W.J.; Binkley, J.S.; Gordon, M.S.; DeFrees, D.J.; Pople, J.A. Self-consistent molecular orbital methods. XXIII. A polarization-type basis set for second-row elements. J. Chem. Phys. 1982, 77, 3654–3665. [Google Scholar] [CrossRef]
- Frisch, M.J.; Pople, J.A.; Binkley, J.S. Self-consistent molecular orbital methods 25. Supplementary functions for Gaussian basis sets. J. Chem. Phys. 1984, 80, 3265–3269. [Google Scholar] [CrossRef]
- Krishnan, R.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- McLean, A.D.; Chandler, G.S. Contracted Gaussian basis sets for molecular calculations. I. Second row atoms, Z = 11–18. J. Chem. Phys. 1980, 72, 5639–5648. [Google Scholar] [CrossRef]
- Spitznagel, G.W.; Clark, T.; von Raguй Schleyer, P.; Hehre, W.J. An evaluation of the performance of diffuse function-augmented basis sets for second row elements, Na-Cl. J. Comput. Chem. 1987, 8, 1109–1116. [Google Scholar] [CrossRef]
- Ditchfield, R.; Hehre, W.J.; Pople, J.A. Self-Consistent Molecular-Orbital Methods. IX. An Extended Gausian-Type Basis for Molecular-Orbital Studies of Organic Molecules. J. Chem. Phys. 1971, 54, 724–728. [Google Scholar] [CrossRef]
- Van Duijneveldt, F.B.; van Duijneveldt-van de Rijdt, J.G.; van Lenthe, J.H. State of the art in counterpoise theory. Chem. Rev. 1994, 94, 1873–1885. [Google Scholar] [CrossRef]
- Hansen, A.E.; Bouman, T.D. Localized orbital/local origin method for calculation and analysis of NMR shieldings. Applications to 13C shielding tensors. J. Chem. Phys. 1985, 82, 5035–5047. [Google Scholar] [CrossRef]
- Repisky, M.; Komorovsky, S.; Malkin, V.G.; Malkina, O.L.; Kaupp, M.; Ruud, K.; Bast, R.; Ekstrom, U.; Kadek, M.; Knecht, S.; et al. MAG-ReSpect, version 5.1.0; 2019. Available online: http://www.respectprogram.org (accessed on 16 November 2023).











| Nuclei | Range, ppm | Level b | Slope | Intercept | R2 | RMSE, ppm | nRMSE c, % | |
|---|---|---|---|---|---|---|---|---|
| Carbene·Se adducts | 77Se | 219.0 a | A | 1.20 | −27.16 | 0.951 | 14.7 | 6.7 |
| B | 1.15 | −0.55 | 0.969 | 11.5 | 5.3 | |||
| C | 1.29 | −6.59 | 0.975 | 10.3 | 4.7 | |||
| 1202.1 | A | 1.12 | −19.53 | 0.991 | 23.8 | 2.0 | ||
| B | 1.10 | 4.59 | 0.994 | 19.3 | 1.6 | |||
| C | 1.15 | 3.56 | 0.993 | 21.0 | 1.7 | |||
| Carbene·PPh adducts | 31P | 130.1 a | A | 1.05 | −7.99 | 0.999 | 1.5 | 1.1 |
| B | 1.05 | −1.49 | 0.999 | 1.6 | 1.2 | |||
| D | 1.07 | −2.44 | 0.998 | 1.9 | 1.5 | |||
| 187.5 | A | 1.11 | −7.96 | 0.992 | 4.8 | 2.6 | ||
| B | 1.09 | −1.61 | 0.993 | 4.3 | 2.3 | |||
| D | 1.10 | −2.79 | 0.993 | 4.3 | 2.3 | |||
| 13C | 78.7 | A | 1.13 | −17.09 | 0.985 | 2.2 | 2.8 | |
| B | 1.11 | −14.87 | 0.985 | 2.2 | 2.8 | |||
| D | 1.11 | −12.73 | 0.986 | 2.1 | 2.7 | |||
| Carbene | 13C | 145.4 | A | 1.21 | −34.84 | 0.994 | 3.0 | 2.1 |
| Carbene·H+ azolium salts | 13C | 22.0 | A | 1.09 | −12.95 | 0.890 | 3.0 | 13.5 |
| trans-[PdBr2(iPr2-bimy)L] | 13C | 24.0 | E | 0.90 | 29.49 | 0.961 | 1.6 | 6.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kondrashova, S.A.; Latypov, S.K. NMR “Finger Prints” of N-Heterocyclic Carbenes, DFT Analysis: Scopes and Limitations. Molecules 2023, 28, 7729. https://doi.org/10.3390/molecules28237729
Kondrashova SA, Latypov SK. NMR “Finger Prints” of N-Heterocyclic Carbenes, DFT Analysis: Scopes and Limitations. Molecules. 2023; 28(23):7729. https://doi.org/10.3390/molecules28237729
Chicago/Turabian StyleKondrashova, Svetlana A., and Shamil K. Latypov. 2023. "NMR “Finger Prints” of N-Heterocyclic Carbenes, DFT Analysis: Scopes and Limitations" Molecules 28, no. 23: 7729. https://doi.org/10.3390/molecules28237729