Enhanced Adsorption of Textile Dyes by a Novel Sulfonated Activated Carbon Derived from Pomegranate Peel Waste: Isotherm, Kinetic and Thermodynamic Study
Abstract
:1. Introduction
2. Results
2.1. Characterization
2.2. Adsorption Study
2.2.1. Test Adsorption of Various Dyes
2.2.2. Effect of pH on Adsorption of CV Dye
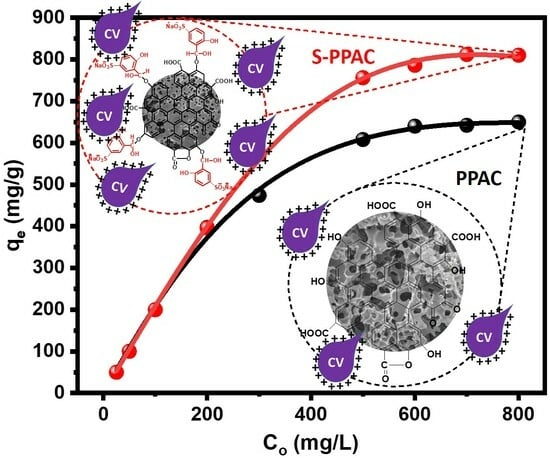
2.2.3. Effect of Initial CV Dye Concentration and Isotherm Study
2.2.4. Effect of Contact Time and Kinetic Study
2.2.5. Thermodynamic Study and Proposed Adsorption Mechanism
2.2.6. Reusability Study
3. Materials and Methods
3.1. Materials
3.2. Preparation of Activated Carbon and Sulfonated Activated Carbon
3.3. Characterization
3.4. Adsorption Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Aquim, P.M.; Hansen, É.; Gutterres, M. Water reuse: An alternative to minimize the environmental impact on the leather industry. J. Environ. Manag. 2019, 230, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Partyka, M.L.; Bond, R.F. Wastewater reuse for irrigation of produce: A review of research, regulations, and risks. Sci. Total Environ. 2022, 828, 154385. [Google Scholar] [CrossRef] [PubMed]
- Thamer, B.M.; Al-aizari, F.A.; Abdo, H.S. Boosting anionic dyes removal performance of polyacrylonitrile nanofibers by incorporating nitrogen-rich conjugated polymer. Colloids Surfaces A Physicochem. Eng. Asp. 2023, 677, 132361. [Google Scholar] [CrossRef]
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Zhang, Y.; Yu, D.G.; Tran, C.H.; Wang, M.; Varyambath, A.; Kim, J.; Kim, I. Efficient Synthesis of Folate-Conjugated Hollow Polymeric Capsules for Accurate Drug Delivery to Cancer Cells. Biomacromolecules 2021, 22, 732–742. [Google Scholar] [CrossRef]
- Tkaczyk, A.; Mitrowska, K.; Posyniak, A. Synthetic organic dyes as contaminants of the aquatic environment and their implications for ecosystems: A review. Sci. Total Environ. 2020, 717, 137222. [Google Scholar] [CrossRef] [PubMed]
- Thamer, B.M.; Al-aizari, F.A.; Hameed, M.M.A. Zero-valent Ni/NiO core-shell nanosheets supported on graphene for highly efficient dye adsorption: Isotherm, kinetic and thermodynamic study. Chem. Eng. Res. Des. 2023, 197, 656–668. [Google Scholar] [CrossRef]
- Katheresan, V.; Kansedo, J.; Lau, S.Y. Efficiency of various recent wastewater dye removal methods: A review. J. Environ. Chem. Eng. 2018, 6, 4676–4697. [Google Scholar] [CrossRef]
- Ouyang, W.; Chen, T.; Shi, Y.; Tong, L.; Chen, Y.; Wang, W.; Yang, J.; Xue, J. Physico-chemical processes. Water Environ. Res. 2019, 91, 1350–1377. [Google Scholar] [CrossRef]
- Rashid, R.; Shafiq, I.; Akhter, P.; Iqbal, M.J.; Hussain, M. A state-of-the-art review on wastewater treatment techniques: The effectiveness of adsorption method. Environ. Sci. Pollut. Res. 2021, 28, 9050–9066. [Google Scholar] [CrossRef]
- Somashekara, D.; Mulky, L. Sequestration of Contaminants from Wastewater: A Review of Adsorption Processes. ChemBioEng Rev. 2023, 10, 491–509. [Google Scholar] [CrossRef]
- Song, W.; Zhang, M.; Huang, X.; Chen, B.; Ding, Y.; Zhang, Y.; Yu, D.G.; Kim, I. Smart l-borneol-loaded hierarchical hollow polymer nanospheres with antipollution and antibacterial capabilities. Mater. Today Chem. 2022, 26, 101252. [Google Scholar] [CrossRef]
- Thamer, B.M.; Shaker, A.A.; Abdul Hameed, M.M.; Al-Enizi, A.M. Highly selective and reusable nanoadsorbent based on expansive clay-incorporated polymeric nanofibers for cationic dye adsorption in single and binary systems. J. Water Process Eng. 2023, 54, 103918. [Google Scholar] [CrossRef]
- Altaleb, H.A.; Thamer, B.M.; Abdulhameed, M.M.; El-Hamshary, H.; Mohammady, S.Z.; Al-Enizi, A.M. Efficient electrospun terpolymer nanofibers for the removal of cationic dyes from polluted waters: A non-linear isotherm and kinetic study. J. Environ. Chem. Eng. 2021, 9, 105361. [Google Scholar] [CrossRef]
- Wong, S.; Ngadi, N.; Inuwa, I.M.; Hassan, O. Recent advances in applications of activated carbon from biowaste for wastewater treatment: A short review. J. Clean. Prod. 2018, 175, 361–375. [Google Scholar] [CrossRef]
- Yahya, M.A.; Al-Qodah, Z.; Ngah, C.W.Z. Agricultural bio-waste materials as potential sustainable precursors used for activated carbon production: A review. Renew. Sustain. Energy Rev. 2015, 46, 218–235. [Google Scholar] [CrossRef]
- Heidarinejad, Z.; Dehghani, M.H.; Heidari, M.; Javedan, G.; Ali, I.; Sillanpää, M. Methods for preparation and activation of activated carbon: A review. Environ. Chem. Lett. 2020, 18, 393–415. [Google Scholar] [CrossRef]
- Alfatah, T.; Mistar, E.M.; Supardan, M.D. Porous structure and adsorptive properties of activated carbon derived from Bambusa vulgaris striata by two-stage KOH/NaOH mixture activation for Hg2+ removal. J. Water Process Eng. 2021, 43, 102294. [Google Scholar] [CrossRef]
- Yu, M.; Li, J.; Wang, L. KOH-activated carbon aerogels derived from sodium carboxymethyl cellulose for high-performance supercapacitors and dye adsorption. Chem. Eng. J. 2017, 310, 300–306. [Google Scholar] [CrossRef]
- Patra, C.; Gupta, R.; Bedadeep, D.; Narayanasamy, S. Surface treated acid-activated carbon for adsorption of anionic azo dyes from single and binary adsorptive systems: A detail insight. Environ. Pollut. 2020, 266, 115102. [Google Scholar] [CrossRef]
- Soffian, M.S.; Abdul Halim, F.Z.; Aziz, F.; Rahman, M.A.; Mohamed Amin, M.A.; Awang Chee, D.N. Carbon-based material derived from biomass waste for wastewater treatment. Environ. Adv. 2022, 9, 100259. [Google Scholar] [CrossRef]
- Sultana, M.; Rownok, M.H.; Sabrin, M.; Rahaman, M.H.; Alam, S.M.N. A review on experimental chemically modified activated carbon to enhance dye and heavy metals adsorption. Clean. Eng. Technol. 2022, 6, 100382. [Google Scholar] [CrossRef]
- Ribeirovieira, R.; Rios, A.; Alves, D.E.; Dalmázio, I.; Fernando, S.; Bento, V.; Donnici, C.L.; Lago, R.M. Tailoring activated carbon by surface chemical modification with O, S, and N containing molecules. Mater. Res. 2003, 6, 129–135. [Google Scholar] [CrossRef]
- Iannicelli-Zubiani, E.M.; Gallo Stampino, P.; Cristiani, C.; Dotelli, G. Enhanced lanthanum adsorption by amine modified activated carbon. Chem. Eng. J. 2018, 341, 75–82. [Google Scholar] [CrossRef]
- Goswami, M.; Phukan, P. Enhanced adsorption of cationic dyes using sulfonic acid modified activated carbon. J. Environ. Chem. Eng. 2017, 5, 3508–3517. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting Physisorption Data for Gas/Solid Systems with Special Reference to the Determination of Surface Area and Porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Bégin, S.J.; Scotland, K.M.; Pede, P.R.; Vreugdenhil, A.J. Polyacrylamide Grafted Activated Carbon by Surface-Initiated AGET ATRP for the Flocculation of MFT. Macromol. Chem. Phys. 2023, 224, 2300223. [Google Scholar] [CrossRef]
- Pognon, G.; Brousse, T.; Bélanger, D. Effect of molecular grafting on the pore size distribution and the double layer capacitance of activated carbon for electrochemical double layer capacitors. Carbon 2011, 49, 1340–1348. [Google Scholar] [CrossRef]
- Chen, Y.; Zi, F.; Hu, X.; Yu, H.; Nie, Y.; Yang, P.; Cheng, H.; Wang, Q.; Qin, X.; Chen, S.; et al. Grafting of organic sulfur-containing functional groups on activated carbon for gold(I) adsorption from thiosulfate solution. Hydrometallurgy 2019, 185, 102–110. [Google Scholar] [CrossRef]
- Shafeeyan, M.S.; Daud, W.M.A.W.; Houshmand, A.; Shamiri, A. A review on surface modification of activated carbon for carbon dioxide adsorption. J. Anal. Appl. Pyrolysis 2010, 89, 143–151. [Google Scholar] [CrossRef]
- Crini, G.; Peindy, H.N.; Gimbert, F.; Robert, C. Removal of C.I. Basic Green 4 (Malachite Green) from aqueous solutions by adsorption using cyclodextrin-based adsorbent: Kinetic and equilibrium studies. Sep. Purif. Technol. 2007, 53, 97–110. [Google Scholar] [CrossRef]
- Fakioğlu, M.; Kalpaklı, Y. Mechanism and behavior of caffeine sorption: Affecting factors. RSC Adv. 2022, 12, 26504–26513. [Google Scholar] [CrossRef] [PubMed]
- Thamer, B.M.; Aldalbahi, A.; Meera Moydeen, A.; Rahaman, M.; El-Newehy, M.H. Modified Electrospun Polymeric Nanofibers and Their Nanocomposites as Nanoadsorbents for Toxic Dye Removal from Contaminated Waters: A Review. Polymers 2020, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Chahinez, H.O.; Abdelkader, O.; Leila, Y.; Tran, H.N. One-stage preparation of palm petiole-derived biochar: Characterization and application for adsorption of crystal violet dye in water. Environ. Technol. Innov. 2020, 19, 100872. [Google Scholar] [CrossRef]
- Doke, K.M.; Yusufi, M.; Joseph, R.D.; Khan, E.M. Comparative Adsorption of Crystal Violet and Congo Red onto ZnCl2 Activated Carbon. J. Dispers. Sci. Technol. 2016, 37, 1671–1681. [Google Scholar] [CrossRef]
- Liu, L.; Lin, Y.; Liu, Y.; Zhu, H.; He, Q. Removal of methylene blue from aqueous solutions by sewage sludge based granular activated carbon: Adsorption equilibrium, kinetics, and thermodynamics. J. Chem. Eng. Data 2013, 58, 2248–2253. [Google Scholar] [CrossRef]
- Foroutan, R.; Peighambardoust, S.J.; Peighambardoust, S.H.; Pateiro, M.; Lorenzo, J.M. Adsorption of Crystal Violet Dye Using Activated Carbon of Lemon Wood and Activated Carbon/Fe3O4 Magnetic Nanocomposite from Aqueous Solutions: A Kinetic, Equilibrium and Thermodynamic Study. Molecules 2021, 26, 2241. [Google Scholar] [CrossRef]
- Ji, Q.; Li, H. High surface area activated carbon derived from chitin for efficient adsorption of Crystal Violet. Diam. Relat. Mater. 2021, 118, 108516. [Google Scholar] [CrossRef]
- Liang, Y.D.; He, Y.J.; Wang, T.T.; Lei, L.H. Adsorptive removal of gentian violet from aqueous solution using CoFe2O4/activated carbon magnetic composite. J. Water Process Eng. 2019, 27, 77–88. [Google Scholar] [CrossRef]
- Mohanty, K.; Naidu, J.T.; Meikap, B.C.; Biswas, M.N. Removal of crystal violet from wastewater by activated carbons prepared from rice husk. Ind. Eng. Chem. Res. 2006, 45, 5165–5171. [Google Scholar] [CrossRef]
- AbdEl-Salam, A.H.; Ewais, H.A.; Basaleh, A.S. Silver nanoparticles immobilised on the activated carbon as efficient adsorbent for removal of crystal violet dye from aqueous solutions. A kinetic study. J. Mol. Liq. 2017, 248, 833–841. [Google Scholar] [CrossRef]
- Aljeboree, A.M.; Alkaim, A.F.; Al-Dujaili, A.H. Adsorption isotherm, kinetic modeling and thermodynamics of crystal violet dye on coconut husk-based activated carbon. Desalin. Water Treat. 2015, 53, 3656–3667. [Google Scholar] [CrossRef]
- Çavuşoğlu, F.C.; Akan, S.; Arı, E.A.; Çetinkaya, E.; Çolak, E.; Daştan, G.N.; Deniz, S.; Erdem, D.; Köksal, M.; Korkmaz, S.; et al. Preparation of magnetic activated carbon-chitosan nanocomposite for crystal violet adsorption. Korean J. Chem. Eng. 2019, 36, 1915–1921. [Google Scholar] [CrossRef]
- Aldalbahi, A.; Thamer, B.M.; Rahaman, M.; El-Newehy, M.H. Self-Nitrogen-Doped Nanoporous Carbons Derived from Poly(1,5-diaminonaphthalene) for the Removal of Toxic Dye Pollutants from Wastewater: Non-Linear Isotherm and Kinetic Analysis. Polymers 2020, 12, 2563. [Google Scholar] [CrossRef] [PubMed]
- Yusuff, A.S.; Ajayi, O.A.; Popoola, L.T. Application of Taguchi design approach to parametric optimization of adsorption of crystal violet dye by activated carbon from poultry litter. Sci. Afr. 2021, 13, e00850. [Google Scholar] [CrossRef]
- Raji, Y.; Nadi, A.; Mechnou, I.; Saadouni, M.; Cherkaoui, O.; Zyade, S. High adsorption capacities of crystal violet dye by low-cost activated carbon prepared from Moroccan Moringa oleifera wastes: Characterization, adsorption and mechanism study. Diam. Relat. Mater. 2023, 135, 109834. [Google Scholar] [CrossRef]
- Aysu, T.; Küçük, M.M. Removal of crystal violet and methylene blue from aqueous solutions by activated carbon prepared from Ferula orientalis. Int. J. Environ. Sci. Technol. 2015, 12, 2273–2284. [Google Scholar] [CrossRef]
- Wang, S.; Boyjoo, Y.; Choueib, A.; Zhu, Z.H. Removal of dyes from aqueous solution using fly ash and red mud. Water Res. 2005, 39, 129–138. [Google Scholar] [CrossRef]
- Senthilkumaar, S.; Kalaamani, P.; Subburaam, C.V. Liquid phase adsorption of Crystal violet onto activated carbons derived from male flowers of coconut tree. J. Hazard. Mater. 2006, 136, 800–808. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Over the adsorption in solution. Z. Phys. Chem. 1906, 57, 385–471. [Google Scholar]
- Temkin, M.; Pyzhev, V. Kinetics of ammonia synthesis on promoted iron catalys. Acta Phys. Chim USSR 1940, 12, 327–356. [Google Scholar]
- Lagergren, S. About the theory of so-called adsorption of soluble substances. Kongliga Sven. Vetensk.-Akad. Handl. 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; Wase, D.A.J.; Forster, C.F. Kinetic Studies of Competitive Heavy Metal Adsorption by Sphagnum Moss Peat. Environ. Technol. 1996, 17, 71–77. [Google Scholar] [CrossRef]
- Chien, S.H.; Clayton, W.R. Application of Elovich Equation to the Kinetics of Phosphate Release and Sorption in Soils. Soil Sci. Soc. Am. J. 1980, 44, 265. [Google Scholar] [CrossRef]











| Sample | SBET (m2/g) | Smicro | VT cm3/g | Vmicro cm3/g | Vnon-micro cm3/g | Vmicro/VT (%) | Dp (nm) | APS (nm) |
|---|---|---|---|---|---|---|---|---|
| PPAC | 1180.63 | 890.74 | 0.6644 | 0.4706 | 0.1938 | 70.83 | 2.25 | 5.08 |
| S-PPAC | 740.75 | 555.84 | 0.4210 | 0.2929 | 0.1281 | 69.57 | 2.27 | 8.10 |
| Model | PPAC | S-PPAC | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 298 K | 303 K | 308 K | 313 K | 318 K | 298 K | 303 K | 308 K | 313 K | 318 K | |
| qe,exp | 578 | 604 | 626 | 640 | 650 | 706 | 720 | 754 | 790 | 810 |
| Langmuir | ||||||||||
| qmax (mg/g) | 552.79 | 570.79 | 584.36 | 600.1 | 606.7 | 667.84 | 689.75 | 731.59 | 764.22 | 785.53 |
| KL (L/mg) | 0.4432 | 0.5081 | 1.36 | 1.25 | 1.21 | 1.37 | 1.24 | 0.5026 | 0.4357 | 0.3980 |
| R2 | 0.8786 | 0.8815 | 0.8816 | 0.8845 | 0.8854 | 0.9181 | 0.9230 | 0.9328 | 0.9350 | 0.9362 |
| SE | 41.08 | 41.88 | 42.22 | 43.01 | 43.45 | 40.89 | 41.35 | 42.72 | 44.49 | 45.78 |
| Freundlich | ||||||||||
| KF (mg/g)/(mg/L)n | 325.87 | 328.37 | 361.49 | 359.81 | 358.42 | 412.94 | 420.40 | 399.32 | 394.16 | 391.52 |
| 1/n | 0.093 | 0.098 | 0.087 | 0.094 | 0.097 | 0.090 | 0.092 | 0.11 | 0.121 | 0.128 |
| R2 | 0.8923 | 0.8990 | 0.9028 | 0.9075 | 0.9094 | 0.9299 | 0.9334 | 0.9372 | 0.9428 | 0.9456 |
| SE | 87.76 | 85.15 | 79.84 | 79.71 | 79.56 | 68.56 | 67.62 | 69.66 | 68.33 | 67.84 |
| Temkin | ||||||||||
| β (J/mol) | 43.25 | 46.24 | 40.98 | 44.61 | 46.21 | 49.95 | 53.29 | 66.57 | 75.22 | 80.85 |
| AT (L/mg) | 1229.2 | 844.72 | 6571.13 | 3039.90 | 2227.0 | 3169.4 | 2096.8 | 253.97 | 115.44 | 75.91 |
| bT = RT/β | 57.28 | 54.47 | 62.48 | 58.33 | 57.21 | 49.60 | 47.27 | 38.47 | 34.60 | 32.70 |
| R2 | 0.8914 | 0.8975 | 0.9004 | 0.9050 | 0.9066 | 0.9220 | 0.9265 | 0.9318 | 0.9383 | 0.9416 |
| Adsorbent | Source of AC | Activation | BET (m2/g) | qmax (mg/g) | Ref. |
|---|---|---|---|---|---|
| AC/Fe3O4 | Lemon wood | pyrolysis | 38.69 | 35.3 | [37] |
| AC | Rice husk | H2SO4 | 681 | 64.87 | [40] |
| CoFe2O4/AC | Commercial | - | 109.9 | 184.2 | [39] |
| AC | Date palm petioles | pyrolysis | 640 | 209 | [34] |
| Ag NPs/AC | Commercial | pyrolysis | - | 87.2 | [41] |
| AC | Coconut husk | H2SO4 | - | 418 | [42] |
| AC-Fe3O4-Chitosan | Commercial | HNO3 | - | 505.87 | [43] |
| AC | P(1,5-DANPh) | KOH | 1679 | 487.53 | [44] |
| AC | Poultry litter | ZnCl2 | 148.05 | 70.32 | [45] |
| AC | Moringa oleifera | H3PO4 | 1394 | 469.55 | [46] |
| AC | Ferula orientalis | ZnCl2 | 1476 | 769.23 | [47] |
| AC | Chitin | KOH | 2186.3 | 420 | [38] |
| PPAC | Pomegranate peel waste | KOH | 1180.63 | 606.7 | This study |
| S-PPAC | 740.75 | 785.53 |
| Adsorbent | ||
|---|---|---|
| Applied Model | PPAC | S-PPAC |
| qt,exep | 204 | 242 |
| PFO | ||
| qe,cal | 182.59 ± 4.68 | 222.48 ± 3.39 |
| k1 | 0.0447 ± 0.0094 | 0.0575 ± 0.0094 |
| R2 | 0.99691 | 0.99818 |
| χ2 | 228.81801 | 133.62 |
| PSO | ||
| qt, cal | 194.37 ± 4.28 | 232.12 ± 2.63 |
| K2 | 0.00037 ± 0.00008 | 0.00054 ± 0.00008 |
| R2 | 0.99858 | 0.99938 |
| χ2 | 105.40 | 45.36 |
| Elovich | ||
| α | 713.24 ± 238.51 | 42,698.53 ± 10,238.40 |
| β | 0.05055 ± 0.00219 | 0.06015 ± 0.00122 |
| R2 | 0.99978 | 0.99996 |
| χ2 | 16.27 | 2.66 |
| Intraparticle diffusion | ||
| Kip(1) | 8.37 | 6.25 |
| C1 | 92.70 | 149.41 |
| Kip(2) | 3.68 | 2.35 |
| C2 | 120.36 | 185.40 |
| Kip(3) | 0.8250 | 0.45558 |
| C3 | 180.16 | 226.25 |
| Adsorbent | Temperature (K) | Van’t Hoff Equation | KC | ΔGο (KJ/mol) | ΔHο (KJ/mol) | ΔSο (J/mol K) |
|---|---|---|---|---|---|---|
| PPAC | 298 | y = −872.32x + 4.77 R2 = 0.9822 | 6.33333 | −4.57 | 7.25 | 39.66 |
| 303 | 6.57143 | −4.74 | ||||
| 308 | 6.95522 | −4.97 | ||||
| 313 | 7.375 | −5.20 | ||||
| 318 | 7.52381 | −5.34 | ||||
| S-PPAC | 298 | y = −2526.12x + 9.78 R2 = 0.9933 | 3.71429 | −3.25 | 21.00 | 81.28 |
| 303 | 4.13497 | −3.56 | ||||
| 308 | 4.80272 | −4.02 | ||||
| 313 | 5.63359 | −4.50 | ||||
| 318 | 6.19672 | −4.82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thamer, B.M.; Al-aizari, F.A.; Abdo, H.S. Enhanced Adsorption of Textile Dyes by a Novel Sulfonated Activated Carbon Derived from Pomegranate Peel Waste: Isotherm, Kinetic and Thermodynamic Study. Molecules 2023, 28, 7712. https://doi.org/10.3390/molecules28237712
Thamer BM, Al-aizari FA, Abdo HS. Enhanced Adsorption of Textile Dyes by a Novel Sulfonated Activated Carbon Derived from Pomegranate Peel Waste: Isotherm, Kinetic and Thermodynamic Study. Molecules. 2023; 28(23):7712. https://doi.org/10.3390/molecules28237712
Chicago/Turabian StyleThamer, Badr M., Faiz A. Al-aizari, and Hany S. Abdo. 2023. "Enhanced Adsorption of Textile Dyes by a Novel Sulfonated Activated Carbon Derived from Pomegranate Peel Waste: Isotherm, Kinetic and Thermodynamic Study" Molecules 28, no. 23: 7712. https://doi.org/10.3390/molecules28237712