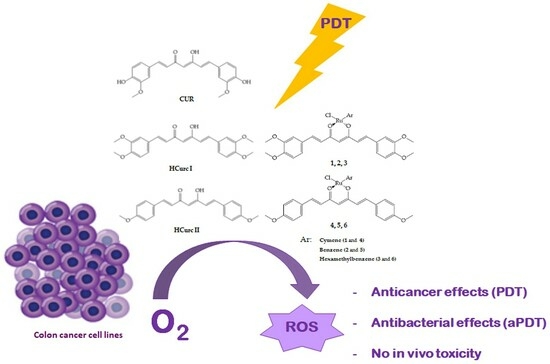
Ruthenium(II)-Arene Curcuminoid Complexes as Photosensitizer Agents for Antineoplastic and Antimicrobial Photodynamic Therapy: In Vitro and In Vivo Insights
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Photodynamic Characteristics and Stability
2.3. Studies on Cancer Cells
2.3.1. Effects on Cell Viability
2.3.2. ROS Production
2.3.3. Cell Death Induction
2.4. In Vivo Toxicity on G. mellonella Larvae
2.5. Antimicrobial Activity of Curcumin and Curcumin Derivatives
3. Materials and Methods
3.1. Reagents and Chemicals
3.2. Ruthenium(II)-Arene Derivatives Characterization
3.2.1. Lipophilicity Evaluation
3.2.2. Photobleaching Assay
3.2.3. Singlet Oxygen Generation
3.3. Studies in Cancer Cell Lines
3.3.1. Cancer Cell Lines and In Vitro Culture Conditions
3.3.2. Effects on Cell Viability
3.3.3. Flow Cytometric Analysis
3.3.4. Assessment of Autophagy
3.4. Toxicity Test on G. mellonella Larvae
3.5. Studies in Microorganisms
3.5.1. Microorganisms and Culture Conditions
3.5.2. Microbiological Assays
3.5.3. Binding Assay
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Larue, L.; Myrzakhmetov, B.; Ben-Mihoub, A.; Moussaron, A.; Thomas, N.; Arnoux, P.; Baros, F.; Vanderesse, R.; Acherar, S.; Frochot, C. Pharmaceuticals Fighting Hypoxia to Improve PDT. Pharmaceuticals 2019, 12, 163. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, L.; Li, F.; Sheng, J.; Xu, C.; Li, D.; Yu, H.; Liu, W. Combination of Chemotherapy and Photodynamic Therapy with Oxygen Self-Supply in the Form of Mutual Assistance for Cancer Therapy. Int. J. Nanomed. 2021, 16, 3679–3694. [Google Scholar] [CrossRef]
- Kubrak, T.P.; Kołodziej, P.; Sawicki, J.; Mazur, A.; Koziorowska, K.; Aebisher, D. Molecules Some Natural Photosensitizers and Their Medicinal Properties for Use in Photodynamic Therapy. Molecules 2022, 27, 1192. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.-Y.; Zhang, L.-L.; Ding, J.; Lu, J.-J. Anticancer Drug Discovery from Chinese Medicinal Herbs. Chin. Med. 2018, 13, 35. [Google Scholar] [CrossRef]
- Mansoori, B.; Mohammadi, A.; Amin Doustvandi, M.; Mohammadnejad, F.; Kamari, F.; Gjerstorff, M.F.; Baradaran, B.; Hamblin, M.R. Photodynamic Therapy for Cancer: Role of Natural Products. Photodiagn. Photodyn. Ther. 2019, 26, 395–404. [Google Scholar] [CrossRef]
- Koon, H.K.; Leung, A.W.N.; Yue, K.K.M.; Mak, N.K. Photodynamic Effect of Curcumin on NPC/CNE2 Cells. J. Environ. Pathol. Toxicol. Oncol. 2006, 25, 205–215. [Google Scholar] [CrossRef]
- Lin, H.-Y.; Lin, J.-N.; Ma, J.-W.; Yang, N.-S.; Ho, C.-T.; Kuo, S.-C.; Way, T.-D. Demethoxycurcumin Induces Autophagic and Apoptotic Responses on Breast Cancer Cells in Photodynamic Therapy. J. Funct. Foods 2015, 12, 439–449. [Google Scholar] [CrossRef]
- Comini, L.R.; Fernandez, I.M.; Vittar, N.B.R.; Núñez Montoya, S.C.; Cabrera, J.L.; Rivarola, V.A. Photodynamic Activity of Anthraquinones Isolated from Heterophyllaea Pustulata Hook f. (Rubiaceae) on MCF-7c3 Breast Cancer Cells. Phytomedicine 2011, 18, 1093–1095. [Google Scholar] [CrossRef]
- Dujic, J.; Kippenberger, S.; Ramirez-Bosca, A.; Diaz-Alperi, J.; Bereiter-Hahn, J.; Kaufmann, R.; Bernd, A.; Hofmann, M. Curcumin in Combination with Visible Light Inhibits Tumor Growth in a Xenograft Tumor Model. Int. J. Cancer 2009, 124, 1422–1428. [Google Scholar] [CrossRef]
- Xie, L.; Ji, X.; Zhang, Q.; Wei, Y. Curcumin Combined with Photodynamic Therapy, Promising Therapies for the Treatment of Cancer. Biomed. Pharmacother. 2022, 146, 112567. [Google Scholar] [CrossRef] [PubMed]
- Comini, L.R.; Núñez Montoya, S.C.; Páez, P.L.; Argüello, G.A.; Albesa, I.; Cabrera, J.L. Antibacterial Activity of Anthraquinone Derivatives from Heterophyllaea pustulata (Rubiaceae). J. Photochem. Photobiol. B Biol. 2011, 102, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Dovigo, L.N.; Pavarina, A.C.; Ribeiro, A.P.D.; Brunetti, I.L.; Costa, C.A.D.S.; Jacomassi, D.P.; Bagnato, V.S.; Kurachi, C. Investigation of the Photodynamic Effects of Curcumin against Candida albicans. Photochem. Photobiol. 2011, 87, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Soria-Lozano, P.; Gilaberte, Y.; Paz-Cristobal, M.; Pérez-Artiaga, L.; Lampaya-Pérez, V.; Aporta, J.; Pérez-Laguna, V.; García-Luque, I.; Revillo, M.; Rezusta, A. In Vitro Effect Photodynamic Therapy with Differents Photosensitizers on Cariogenic Microorganisms. BMC Microbiol. 2015, 15, 187. [Google Scholar] [CrossRef]
- Sobotta, L.; Skupin-Mrugalska, P.; Piskorz, J.; Mielcarek, J. Non-Porphyrinoid Photosensitizers Mediated Photodynamic Inactivation against Bacteria. Dye Pigment. 2019, 163, 337–355. [Google Scholar] [CrossRef]
- Kah, G.; Chandran, R.; Abrahamse, H. Curcumin a Natural Phenol and Its Therapeutic Role in Cancer and Photodynamic Therapy: A Review. Pharmaceutics 2023, 15, 639. [Google Scholar] [CrossRef]
- Sohn, S.-I.; Priya, A.; Balasubramaniam, B.; Muthuramalingam, P.; Sivasankar, C.; Selvaraj, A.; Valliammai, A.; Jothi, R.; Pandian, S. Biomedical Applications and Bioavailability of Curcumin—An Updated Overview. Pharmaceutics 2021, 13, 2102. [Google Scholar] [CrossRef]
- Li, J.; Chen, T. Transition Metal Complexes as Photosensitizers for Integrated Cancer Theranostic Applications. Coord. Chem. Rev. 2020, 418, 213355. [Google Scholar] [CrossRef]
- Alves, S.R.; Calori, I.R.; Tedesco, A.C. Photosensitizer-Based Metal-Organic Frameworks for Highly Effective Photodynamic Therapy. Mater. Sci. Eng. C 2021, 131, 112514. [Google Scholar] [CrossRef]
- Zeng, Y.; Liao, D.; Kong, X.; Huang, Q.; Zhong, M.; Liu, J.; Nezamzadeh-Ejhieh, A.; Pan, Y.; Song, H. Current Status and Prospect of ZIF-Based Materials for Breast Cancer Treatment. Colloids Surf. B Biointerfaces 2023, 232, 113612. [Google Scholar] [CrossRef]
- Alessio, E.; Messori, L. NAMI-A and KP1019/1339, Two Iconic Ruthenium Anticancer Drug Candidates Face-to-Face: A Case Story in Medicinal Inorganic Chemistry. Molecules 2019, 24, 1995. [Google Scholar] [CrossRef]
- Leijen, S.; Burgers, S.A.; Baas, P.; Pluim, D.; Tibben, M.; Van Werkhoven, E.; Alessio, E.; Sava, G.; Beijnen, J.H.; Schellens, J.H.M. Phase I/II Study with Ruthenium Compound NAMI-A and Gemcitabine in Patients with Non-Small Cell Lung Cancer after First Line Therapy. Investig. New Drugs 2015, 33, 201–214. [Google Scholar] [CrossRef]
- Monro, S.; Colón, K.L.; Yin, H.; Roque, J.; Konda, P.; Gujar, S.; Thummel, R.P.; Lilge, L.; Cameron, C.G.; McFarland, S.A. Transition Metal Complexes and Photodynamic Therapy from a Tumor-Centered Approach: Challenges, Opportunities, and Highlights from the Development of TLD1433. Chem. Rev. 2019, 119, 797–828. [Google Scholar] [CrossRef]
- Loftus, L.M.; Al-Afyouni, K.F.; Turro, C. New RuII Scaffold for Photoinduced Ligand Release with Red Light in the Photodynamic Therapy (PDT) Window. Chem.-A Eur. J. 2018, 24, 11550–11553. [Google Scholar] [CrossRef]
- Le Gall, T.; Lemercier, G.; Chevreux, S.; Tücking, K.-S.; Ravel, J.; Thétiot, F.; Jonas, U.; Schönherr, H.; Montier, T. Ruthenium(II) Polypyridyl Complexes as Photosensitizers for Antibacterial Photodynamic Therapy: A Structure–Activity Study on Clinical Bacterial Strains. ChemMedChem 2018, 13, 2229–2239. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, C.Y.; Nam, T.-G. Ruthenium Complexes as Anticancer Agents: A Brief History and Perspectives. Drug Des. Devel. Ther. 2020, 14, 5375–5392. [Google Scholar] [CrossRef]
- Di Nicola, C.; Marchetti, F.; Pettinari, R.; Tombesi, A.; Pettinari, C.; Grappasonni, I.; Dyson, P.J.; Scuri, S. Tethering (Arene)Ru(II) Acylpyrazolones Decorated with Long Aliphatic Chains to Polystyrene Surfaces Provides Potent Antibacterial Plastics. Materials 2020, 13, 526. [Google Scholar] [CrossRef]
- Harding, C.R.; Schroeder, G.N.; Collins, J.W.; Frankel, G. Use of Galleria mellonella as a Model Organism to Study Legionella pneumophila Infection. J. Vis. Exp. 2013, 81, e50964. [Google Scholar]
- Serrano, I.; Verdial, C.; Tavares, L.; Oliveira, M. The Virtuous Galleria mellonella Model for Scientific Experimentation. Antibiotics 2023, 12, 505. [Google Scholar] [CrossRef]
- Ménard, G.; Rouillon, A.; Cattoir, V.; Donnio, P.-Y. Galleria mellonella as a Suitable Model of Bacterial Infection: Past, Present and Future. Front. Cell. Infect. Microbiol. 2021, 11, 782733. [Google Scholar] [CrossRef]
- Champion, O.L.; Wagley, S.; Titball, R.W. Galleria mellonella as a Model Host for Microbiological and Toxin Research. Virulence 2016, 7, 840–845. [Google Scholar] [CrossRef] [PubMed]
- Allegra, E.; Titball, R.W.; Carter, J.; Champion, O.L. Galleria mellonella Larvae Allow the Discrimination of Toxic and Non-Toxic Chemicals. Chemosphere 2018, 198, 469–472. [Google Scholar] [CrossRef]
- Malacarne, M.C.; Mastore, M.; Gariboldi, M.B.; Brivio, M.F.; Caruso, E. Preliminary Toxicity Evaluation of a Porphyrin Photosensitizer in an Alternative Preclinical Model. Int. J. Mol. Sci. 2023, 24, 3131. [Google Scholar] [CrossRef]
- Caruso, F.; Pettinari, R.; Rossi, M.; Monti, E.; Gariboldi, M.B.; Marchetti, F.; Pettinari, C.; Caruso, A.; Ramani, M.V.; Subbaraju, G.V. The in Vitro Antitumor Activity of Arene-Ruthenium(II) Curcuminoid Complexes Improves When Decreasing Curcumin Polarity. J. Inorg. Biochem. 2016, 162, 44–51. [Google Scholar] [CrossRef]
- Banerjee, S.; Chakravarty, A.R. Metal Complexes of Curcumin for Cellular Imaging, Targeting, and Photoinduced Anticancer Activity. Acc. Chem. Res. 2015, 48, 2075–2083. [Google Scholar] [CrossRef] [PubMed]
- Nardo, L.; Andreoni, A.; Bondani, M.; Másson, M.; Haukvik, T.; Tønnesen, H.H. Studies on Curcumin and Curcuminoids. XLVI. Photophysical Properties of Dimethoxycurcumin and Bis-Dehydroxycurcumin. J. Fluoresc. 2012, 22, 597–608. [Google Scholar] [CrossRef]
- Dias, L.D.; Blanco, K.C.; Mfouo-Tynga, I.S.; Inada, N.M.; Bagnato, V.S. Curcumin as a Photosensitizer: From Molecular Structure to Recent Advances in Antimicrobial Photodynamic Therapy. J. Photochem. Photobiol. C Photochem. Rev. 2020, 45, 100384. [Google Scholar] [CrossRef]
- Castillo, M.L.R.D.; López-Tobar, E.; Sanchez-Cortes, S.; Flores, G.; Blanch, G.P. Stabilization of Curcumin against Photodegradation by Encapsulation in Gamma-Cyclodextrin: A Study Based on Chromatographic and Spectroscopic (Raman and UV-Visible) Data. Vib. Spectrosc. 2015, 81, 106–111. [Google Scholar] [CrossRef]
- Kazantzis, K.T.; Koutsonikoli, K.; Mavroidi, B.; Zachariadis, M.; Alexiou, P.; Pelecanou, M.; Politopoulos, K.; Alexandratou, E.; Sagnou, M. Curcumin Derivatives as Photosensitizers in Photodynamic Therapy: Photophysical Properties and: In Vitro Studies with Prostate Cancer Cells. Photochem. Photobiol. Sci. 2020, 19, 193–206. [Google Scholar] [CrossRef]
- Pereira, D.M.; Valentão, P.; Pereira, J.A.; Andrade, P.B. Phenolics: From Chemistry to Biology. Molecules 2009, 14, 2202–2211. [Google Scholar] [CrossRef]
- Lo Cascio, F.; Marzullo, P.; Kayed, R.; Palumbo Piccionello, A. Curcumin as Scaffold for Drug Discovery against Neurodegenerative Diseases. Biomedicines 2021, 9, 173. [Google Scholar] [CrossRef]
- Caruso, E.; Malacarne, M.C.; Banfi, S.; Gariboldi, M.B.; Orlandi, V.T. Cationic Diarylporphyrins: In Vitro Versatile Anticancer and Antibacterial Photosensitizers. J. Photochem. Photobiol. B Biol. 2019, 197, 111548. [Google Scholar] [CrossRef]
- Acedo, P.; Zawacka-Pankau, J. P53 Family Members—Important Messengers in Cell Death Signaling in Photodynamic Therapy of Cancer? Photochem. Photobiol. Sci. 2015, 14, 1390–1396. [Google Scholar] [CrossRef]
- Zawacka-Pankau, J.; Krachulec, J.; Grulkowski, I.; Bielawski, K.P.; Selivanova, G. The P53-Mediated Cytotoxicity of Photodynamic Therapy of Cancer: Recent Advances. Toxicol. Appl. Pharmacol. 2008, 232, 487–497. [Google Scholar] [CrossRef]
- Abrantes, A.B.D.P.; Dias, G.C.; Souza-Pinto, N.C.; Baptista, M.S. P53-Dependent and P53-Independent Responses of Cells Challenged by Photosensitization. Photochem. Photobiol. 2019, 95, 355–363. [Google Scholar] [CrossRef]
- Fisher, A.M.R.; Ferrario, A.; Rucker, N.; Zhang, S.; Gomer, C.J. Photodynamic Therapy Sensitivity Is Not Altered in Human Tumor Cells after Abrogation of P53 Function. Cancer Res. 1999, 59, 331–335. [Google Scholar]
- Giorgi, C.; Bonora, M.; Missiroli, S.; Poletti, F.; Ramirez, F.G.; Morciano, G.; Morganti, C.; Pandolfi, P.P.; Mammano, F.; Pinton, P. Intravital Imaging Reveals P53-Dependent Cancer Cell Death Induced by Phototherapy via Calcium Signaling. Oncotarget 2015, 6, 1435–1445. [Google Scholar] [CrossRef]
- Fisher, A.M.R.; Rucker, N.; Wong, S.; Gomer, C.J. Differential Photosensitivity in Wild-Type and Mutant P53 Human Colon Carcinoma Cell Lines. J. Photochem. Photobiol. B Biol. 1998, 42, 104–107. [Google Scholar] [CrossRef]
- Carlsen, L.; El-Deiry, W.S. Differential P53-Mediated Cellular Responses to Dna-Damag-Ing Therapeutic Agents. Int. J. Mol. Sci. 2021, 22, 11828. [Google Scholar] [CrossRef]
- Baptista, M.S.; Cadet, J.; Di Mascio, P.; Ghogare, A.A.; Greer, A.; Hamblin, M.R.; Lorente, C.; Nunez, S.C.; Ribeiro, M.S.; Thomas, A.H.; et al. Type I and Type II Photosensitized Oxidation Reactions: Guidelines and Mechanistic Pathways. Photochem. Photobiol. 2017, 93, 912–919. [Google Scholar] [CrossRef]
- Mahalingam, S.M.; Ordaz, J.D.; Low, P.S. Targeting of a Photosensitizer to the Mitochondrion Enhances the Potency of Photodynamic Therapy. ACS Omega 2018, 3, 6066–6074. [Google Scholar] [CrossRef]
- Zhou, Z.; Song, J.; Nie, L.; Chen, X. Reactive Oxygen Species Generating Systems Meeting Challenges of Photodynamic Cancer Therapy. Chem. Soc. Rev. 2016, 45, 6597–6626. [Google Scholar] [CrossRef]
- Mishchenko, T.; Balalaeva, I.; Gorokhova, A.; Vedunova, M.; Krysko, D.V. Which Cell Death Modality Wins the Contest for Photodynamic Therapy of Cancer? Cell Death Dis. 2022, 13, 455. [Google Scholar] [CrossRef] [PubMed]
- Kessel, D. Apoptosis, Paraptosis and Autophagy: Death and Survival Pathways Associated with Photodynamic Therapy. Photochem. Photobiol. 2019, 95, 119–125. [Google Scholar] [CrossRef]
- Caruso, E.; Cerbara, M.; Malacarne, M.C.; Marras, E.; Monti, E.; Gariboldi, M.B. Synthesis and Photodynamic Activity of Novel Non-Symmetrical Diaryl Porphyrins against Cancer Cell Lines. J. Photochem. Photobiol. B Biol. 2019, 195, 39–50. [Google Scholar] [CrossRef]
- Xu, D.D.; Lam, H.M.; Hoeven, R.; Xu, C.B.; Leung, A.W.N.; Cho, W.C.S. Photodynamic Therapy Induced Cell Death of Hormone Insensitive Prostate Cancer PC-3 Cells with Autophagic Characteristics. Photodiagn. Photodyn. Ther. 2013, 10, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Xu, W.; Wu, H.; Wang, X.; Gong, Q.; Liu, C.; Liu, J.; Zhou, L. Photodynamic Therapy Induces Autophagy-Mediated Cell Death in Human Colorectal Cancer Cells via Activation of the ROS/JNK Signaling Pathway. Cell Death Dis. 2020, 11, 938. [Google Scholar] [CrossRef]
- Wei, M.-F.; Chen, M.-W.; Chen, K.-C.; Lou, P.-J.; Lin, S.Y.-F.; Hung, S.-C.; Hsiao, M.; Yao, C.-J.; Shieh, M.-J. Autophagy Promotes Resistance to Photodynamic Therapy-Induced Apoptosis Selectively in Colorectal Cancer Stem-like Cells. Autophagy 2014, 10, 1179–1192. [Google Scholar] [CrossRef]
- Reiners, J.J., Jr.; Agostinis, P.; Berg, K.; Oleinick, N.L.; Kessel, D. Assessing Autophagy in the Context of Photodynamic Therapy. Autophagy 2010, 6, 7–18. [Google Scholar] [CrossRef]
- Tanida, I.; Ueno, T.; Kominami, E. LC3 Conjugation System in Mammalian Autophagy. Int. J. Biochem. Cell Biol. 2004, 36, 2503–2518. [Google Scholar] [CrossRef] [PubMed]
- Mrakovcic, M.; Fröhlich, L.F. P53-Mediated Molecular Control of Autophagy in Tumor Cells. Biomolecules 2018, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Park, M.N.; Rahman, M.D.H.; Rashid, M.M.; Islam, R.; Uddin, M.J.; Hannan, M.A.; Kim, B. P53 Modulation of Autophagy Signaling in Cancer Therapies: Perspectives Mechanism and Therapeutic Targets. Front. Cell Dev. Biol. 2022, 10, 761080. [Google Scholar] [CrossRef]
- Ozturk, S. Demonstration of the Efficacy of Curcumin on Carbapenem-Resistant Pseudomonas aeruginosa with Galleria mellonella Larvae Model. Arch. Microbiol. 2022, 204, 524. [Google Scholar] [CrossRef] [PubMed]
- Marques Meccatti, V.; De Souza Moura, L.; Guerra Pinto, J.; Ferreira-Strixino, J.; Abu Hasna, A.; Alves Figueiredo-Godoi, L.M.; Campos Junqueira, J.; Marcucci, M.C.; Paula Ramos, L.D.; Carvalho, C.A.T.; et al. Curcuma longa L. Extract and Photodynamic Therapy Are Effective against Candida spp. and Do Not Show Toxicity In Vivo. Int. J. Dent. 2022, 2022, 5837864. [Google Scholar] [CrossRef] [PubMed]
- Sanches, C.V.G.; Sardi, J.D.C.O.; Terada, R.S.S.; Lazarini, J.G.; Freires, I.A.; Polaquini, C.R.; Torrezan, G.S.; Regasini, L.O.; Fujimaki, M.; Rosalen, P.L. Diacetylcurcumin: A New Photosensitizer for Antimicrobial Photodynamic Therapy in Streptococcus Mutans Biofilms. Biofouling 2019, 35, 340–349. [Google Scholar] [CrossRef]
- Li, J.; Qin, M.; Liu, C.; Ma, W.; Zeng, X.; Ji, Y. Antimicrobial Photodynamic Therapy against Multidrug-Resistant Acinetobacter Baumannii Clinical Isolates Mediated by Aloe-Emodin: An in Vitro Study. Photodiagn. Photodyn. Ther. 2020, 29, 101632. [Google Scholar] [CrossRef]
- García, I.; Ballesta, S.; Gilaberte, Y.; Rezusta, A.; Pascual, Á. Antimicrobial Photodynamic Activity of Hypericin against Methicillin-Susceptible and Resistant Staphylococcus aureus Biofilms. Future Microbiol. 2015, 10, 347–356. [Google Scholar] [CrossRef]
- Paschoal, M.A.; Tonon, C.C.; Spolidório, D.M.P.; Bagnato, V.S.; Giusti, J.S.M.; Santos-Pinto, L. Photodynamic Potential of Curcumin and Blue LED against Streptococcus mutans in a Planktonic Culture. Photodiagn. Photodyn. Ther. 2013, 10, 313–319. [Google Scholar] [CrossRef]
- Zhou, F.; Lin, S.; Zhang, J.; Kong, Z.; Tan, B.K.; Hamzah, S.S.; Hu, J. Enhancement of Photodynamic Bactericidal Activity of Curcumin against Pseudomonas aeruginosa Using Polymyxin B. Photodiagn. Photodyn. Ther. 2022, 37, 102677. [Google Scholar] [CrossRef]
- Orlandi, V.T.; Caruso, E.; Tettamanti, G.; Banfi, S.; Barbieri, P. Photoinduced Antibacterial Activity of Two Dicationic 5,15-Diarylporphyrins. J. Photochem. Photobiol. B Biol. 2013, 127, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Sperandio, F.F.; Huang, Y.-Y.; Hamblin, M.R. Antimicrobial Photodynamic Therapy to Kill Gram-Negative Bacteria. Recent Pat. Antiinfect. Drug Discov. 2013, 8, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, J.; Rahban, D.; Aghamiri, S.; Teymouri, A.; Bahador, A. Photosensitizers in Antibacterial Photodynamic Therapy: An Overview. Laser Ther. 2018, 27, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Dahl, T.A.; McGowan, W.M.; Shand, M.A.; Srinivasan, V.S. Photokilling of Bacteria by the Natural Dye Curcumin. Arch. Microbiol. 1989, 151, 183–185. [Google Scholar] [CrossRef]
- Ballestri, M.; Caruso, E.; Guerrini, A.; Ferroni, C.; Banfi, S.; Gariboldi, M.; Monti, E.; Sotgiu, G.; Varchi, G. Core–Shell Poly-Methyl Methacrylate Nanoparticles Covalently Functionalized with a Non-Symmetric Porphyrin for Anticancer Photodynamic Therapy. J. Photochem. Photobiol. B Biol. 2018, 186, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Radunz, S.; Wedepohl, S.; Röhr, M.; Calderón, M.; Tschiche, H.R.; Resch-Genger, U. PH-Activatable Singlet Oxygen-Generating Boron-Dipyrromethenes (BODIPYs) for Photodynamic Therapy and Bioimaging. J. Med. Chem. 2020, 63, 1699–1708. [Google Scholar] [CrossRef]
- Orlandi, V.T.; Martegani, E.; Trivellin, N.; Bolognese, F.; Caruso, E. Photo-Inactivation of Staphylococcus Aureus by Diaryl-Porphyrins. Antibiotics 2023, 12, 228. [Google Scholar] [CrossRef]
- Yang, Q.-Q.; Farha, A.K.; Kim, G.; Gul, K.; Gan, R.-Y.; Corke, H. Antimicrobial and Anticancer Applications and Related Mechanisms of Curcumin-Mediated Photodynamic Treatments. Trends Food Sci. Technol. 2020, 97, 341–354. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Jameei, A.; Karande, A.A.; Chakravarty, A.R. BODIPY-Attached Zinc(II) Complexes of Curcumin Drug for Visible Light Assisted Photo-Sensitization, Cellular Imaging and Targeted PDT. Eur. J. Med. Chem. 2021, 220, 113438. [Google Scholar] [CrossRef]











| Compound | LogP | 1O2 * |
|---|---|---|
| CUR | 2.67 | 0.19 |
| HCurc I | 3.55 | 0.02 |
| 1 | 2.74 | 0.08 |
| 2 | 1.6 | 0.14 |
| 3 | 2.85 | 0.03 |
| HCurc II | 4.48 | 0.01 |
| 4 | 3.21 | 0.08 |
| 5 | 2.11 | 0.11 |
| 6 | 3.39 | 0.14 |
| Time (min) | CUR | HCurc I | 1 | 2 | 3 | HCurc II | 4 | 5 | 6 |
|---|---|---|---|---|---|---|---|---|---|
| 10 | 100% | 57.1% | 100% | 100% | 100% | 31.2% | 100% | 100% | 100% |
| 20 | 100% | 25.2% | 100% | 100% | 100% | 14.7% | 100% | 100% | 100% |
| 30 | 73.6% | 0% | 76.2% | 79.7% | 81.1% | 0% | 66.7% | 62.9% | 65.3% |
| 60 | 60.9% | 0% | 68.6% | 67.5% | 80.1% | 0% | 59.9% | 57.9% | 59.6% |
| 90 | 55.4% | 0% | 63.2% | 62.1% | 76.9% | 0% | 52.6% | 57.5% | 47.3% |
| 120 | 49.6% | 0% | 61.9% | 61.1% | 71.8% | 0% | 50% | 57.5% | 44.6% |
| IC50 (μM) | HCT116 | HT29 | ||
|---|---|---|---|---|
| Dark | Light | Dark | Light | |
| CUR | 7.43 ± 1.24 | 1.05 ± 0.19 | 8.43 ± 1.31 | 3.83 ± 0.44 |
| HCurc I | 3.01 ± 0.28 | 1.06 ± 0.23 | 14.57 ± 1.09 | 1.59 ± 0.27 * |
| HCurc II | 49.14 ± 1.18 | 1.91 ± 0.14 | 25.95 ± 2.55 | 3.36 ± 0.74 |
| HCT116 | HT29 | |
|---|---|---|
| HCurc I | 2.8 | 9.1 |
| 1 | 4.6 | 5.5 |
| 2 | 2.0 | 12.5 |
| 3 | 3.6 | 8.6 |
| HCurc II | 23.5 | 7.7 |
| 4 | 4.1 | 5.4 |
| 5 | 3.1 | 7.1 |
| 6 | 2.8 | 4.7 |
| 0 J/cm2 | 10 J/cm2 | 20 J/cm2 | |
|---|---|---|---|
| CUR | >100 | 20.83 ± 5.89 | 14.58 ± 7.80 |
| HCurc I | >100 | 3.13 ± 0.00 | 3.13 ± 0.00 |
| 1 | >100 | 50 ± 0.00 | 31.25 ± 10.83 |
| 2 | >100 | 66.67 ± 23.57 | 50 ± 0.00 |
| 3 | >100 | 41.67 ± 11.79 | 29.17 ± 6.25 |
| HCurc II | >100 | 6.25 ± 4.42 | 2.60 ± 0.78 |
| 4 | 50 ± 0.00 | 22.50 ± 5.00 | 13.75 ± 3.13 |
| 5 | 100 ± 0.00 | 25 ± 0.00 | 22.5 ± 6.25 |
| 6 | 12.5 ± 0.00 | 8.33 ± 2.95 | 6.25 ± 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marras, E.; Balacchi, C.J.; Orlandi, V.; Caruso, E.; Brivio, M.F.; Bolognese, F.; Mastore, M.; Malacarne, M.C.; Rossi, M.; Caruso, F.; et al. Ruthenium(II)-Arene Curcuminoid Complexes as Photosensitizer Agents for Antineoplastic and Antimicrobial Photodynamic Therapy: In Vitro and In Vivo Insights. Molecules 2023, 28, 7537. https://doi.org/10.3390/molecules28227537
Marras E, Balacchi CJ, Orlandi V, Caruso E, Brivio MF, Bolognese F, Mastore M, Malacarne MC, Rossi M, Caruso F, et al. Ruthenium(II)-Arene Curcuminoid Complexes as Photosensitizer Agents for Antineoplastic and Antimicrobial Photodynamic Therapy: In Vitro and In Vivo Insights. Molecules. 2023; 28(22):7537. https://doi.org/10.3390/molecules28227537
Chicago/Turabian StyleMarras, Emanuela, Camilla J. Balacchi, Viviana Orlandi, Enrico Caruso, Maurizio F. Brivio, Fabrizio Bolognese, Maristella Mastore, Miryam C. Malacarne, Miriam Rossi, Francesco Caruso, and et al. 2023. "Ruthenium(II)-Arene Curcuminoid Complexes as Photosensitizer Agents for Antineoplastic and Antimicrobial Photodynamic Therapy: In Vitro and In Vivo Insights" Molecules 28, no. 22: 7537. https://doi.org/10.3390/molecules28227537