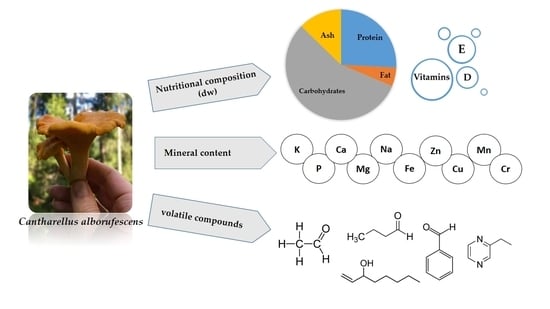
Nutritional Composition and Odor-Contributing Volatile Compounds of the Edible Mushroom Cantharellus alborufescens
Abstract
:1. Introduction
2. Results and Discussion
2.1. Proximate Analysis
2.2. Fatty Acid Composition
2.3. Amino Acid Composition
2.4. Vitamin Content
2.5. Mineral Content
2.6. Volatile Compound Profile
3. Materials and Methods
3.1. Mushroom Samples
3.2. Proximate Analysis
3.3. Fatty Acid Composition
3.4. Amino Acid Composition
3.5. Vitamin Content
3.6. Mineral Content
Daily mushroom consumption (g/day)/Average body weight (kg)
3.7. Analysis of Volatile Compound Profile
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Valverde, M.E.; Hernández-Pérez, T.; Paredes-López, O. Edible mushrooms: Improving human health and promoting quality life. Int. J. Microbiol. 2015, 2015, 376387. [Google Scholar] [CrossRef]
- Xun, W.; Wang, G.; Zhang, Y.; Liao, G.; Ge, C. Analysis of flavor-related compounds in four edible wild mushroom soups. Microchem. J. 2020, 159, 105548. [Google Scholar] [CrossRef]
- Sun, L.-B.; Zhang, Z.-Y.; Xin, G.; Sun, B.-X.; Bao, X.-J.; Wei, Y.-Y.; Zhao, X.-M.; Xu, H.-R. Advances in umami taste and aroma of edible mushrooms. Trends Food Sci. Technol. 2020, 96, 176–187. [Google Scholar] [CrossRef]
- Olariaga, I.; Moreno, G.; Manjón, J.L.; Salcedo, I.; Hofstetter, V.; Rodríguez, D.; Buyck, B. Cantharellus (Cantharellales, Basidiomycota) revisited in Europe through a multigene phylogeny. Fungal Divers. 2017, 83, 263–292. [Google Scholar] [CrossRef]
- Beluhan, S.; Ranogajec, A. Chemical composition and non-volatile components of Croatian wild edible mushrooms. Food Chem. 2011, 124, 1076–1082. [Google Scholar] [CrossRef]
- Barros, L.; Cruz, T.; Baptista, P.; Estevinho, L.M.; Ferreira, I.C. Wild and commercial mushrooms as source of nutrients and nutraceuticals. Food Chem. Toxicol. 2008, 46, 2742–2747. [Google Scholar] [CrossRef]
- Barros, L.; Venturini, B.A.; Baptista, P.; Estevinho, L.M.; Ferreira, I.C. Chemical composition and biological properties of Portuguese wild mushrooms: A comprehensive study. J. Agric. Food Chem. 2008, 56, 3856–3862. [Google Scholar] [CrossRef]
- Fogarasi, M.; Socaci, S.A.; Dulf, F.V.; Diaconeasa, Z.M.; Fărcaș, A.C.; Tofană, M.; Semeniuc, C.A. Bioactive compounds and volatile profiles of five Transylvanian wild edible mushrooms. Molecules 2018, 23, 3272. [Google Scholar] [CrossRef]
- Ugbogu, E.A.; Emmanuel, O.; Ude, V.C.; Ijioma, S.N.; Ugbogu, O.C.; Akubugwo, E.I. Nutritional composition and toxicity profile of Cantharellus species (Purple Mushroom) in rats. Sci. Afr. 2020, 8, e00375. [Google Scholar] [CrossRef]
- Bak, K.H.; Bauer, S.; Rattner, J.; Wagner, M.; Ludewig, M. Nutritional properties, microbial and sensory quality, and formation of biogenic amines in wild-grown mushrooms (Cantharellus cibarius & Boletus edulis) from Austrian local markets. Food Chem. Adv. 2023, 2, 100193. [Google Scholar]
- Nasiry, D.; Khalatbary, A.R.; Ebrahimzadeh, M.A. Anti-inflammatory and wound-healing potential of golden chanterelle mushroom, Cantharellus cibarius (Agaricomycetes). Int. J. Med. Mushrooms 2017, 19, 893–903. [Google Scholar] [CrossRef] [PubMed]
- Phillips, K.M.; Ruggio, D.M.; Horst, R.L.; Minor, B.; Simon, R.R.; Feeney, M.J.; Byrdwell, W.C.; Haytowitz, D.B. Vitamin D and sterol composition of 10 types of mushrooms from retail suppliers in the United States. J. Agric. Food Chem. 2011, 59, 7841–7853. [Google Scholar] [CrossRef] [PubMed]
- Aisala, H.; Sola, J.; Hopia, A.; Linderborg, K.M.; Sandell, M. Odor-contributing volatile compounds of wild edible Nordic mushrooms analyzed with HS–SPME–GC–MS and HS–SPME–GC–O/FID. Food Chem. 2019, 283, 566–578. [Google Scholar] [CrossRef] [PubMed]
- Pilz, D. Ecology and Management of Commercially Harvested Chanterelle Mushrooms; US Department of Agriculture, Forest Service, Pacific Northwest Research Station: Portland, OR, USA, 2003; Volume 576.
- Parad, G.A.; Ghobad-Nejhad, M.; Tabari, M.; Yousefzadeh, H.; Esmaeilzadeh, O.; Tedersoo, L.; Buyck, B. Cantharellus alborufescens and C. ferruginascens (Cantharellaceae, Basidiomycota) new to Iran. Cryptogam. Mycol. 2018, 39, 299–310. [Google Scholar] [CrossRef]
- González, A.; Nobre, C.; Simões, L.S.; Cruz, M.; Loredo, A.; Rodríguez-Jasso, R.M.; Contreras, J.; Texeira, J.; Belmares, R. Evaluation of functional and nutritional potential of a protein concentrate from Pleurotus ostreatus mushroom. Food Chem. 2021, 346, 128884. [Google Scholar] [CrossRef]
- Ao, T.; Deb, C.R. Nutritional and antioxidant potential of some wild edible mushrooms of Nagaland, India. J. Food Sci. Technol. 2019, 56, 1084–1089. [Google Scholar] [CrossRef]
- Cheung, P.C. The nutritional and health benefits of mushrooms. Nutr. Bull. 2010, 35, 292–299. [Google Scholar] [CrossRef]
- Zhou, T.; Hu, W.; Yang, Z.; Li, J.; Zeng, X. Study on nutrients, non-volatile compounds, volatile compounds and antioxidant capacity of oyster mushroom cultivated with corn distillers’ grains. LWT 2023, 183, 114967. [Google Scholar] [CrossRef]
- Thonart, P.; Bouzouita, N. Chemical composition and non-volatile components of three wild edible mushrooms collected from northwest Tunisia. Mediterr. J. Chem. 2016, 5, 434–441. [Google Scholar]
- Mleczek, M.; Szostek, M.; Siwulski, M.; Budka, A.; Kalač, P.; Budzyńska, S.; Kuczyńska-Kippen, N.; Niedzielski, P. Road traffic and abiotic parameters of underlying soils determine the mineral composition and nutritive value of the mushroom Macrolepiota procera (Scop.) Singer. Chemosphere 2022, 303, 135213. [Google Scholar] [CrossRef]
- Gałgowska, M.; Pietrzak-Fiećko, R. Evaluation of the nutritional and health values of selected Polish mushrooms considering fatty acid profiles and lipid indices. Molecules 2022, 27, 6193. [Google Scholar] [CrossRef]
- Kıvrak, İ.; Kıvrak, Ş.; Harmandar, M. Free amino acid profiling in the giant puffball mushroom (Calvatia gigantea) using UPLC–MS/MS. Food Chem. 2014, 158, 88–92. [Google Scholar] [CrossRef]
- Lee, K.J.; Yun, I.J.; Kim, K.H.; Lim, S.H.; Ham, H.J.; Eum, W.S.; Joo, J.H. Amino acid and fatty acid compositions of Agrocybe chaxingu, an edible mushroom. J. Food Compos. Anal. 2011, 24, 175–178. [Google Scholar] [CrossRef]
- Yang, J.-H.; Lin, H.-C.; Mau, J.-L. Non-volatile taste components of several commercial mushrooms. Food Chem. 2001, 72, 465–471. [Google Scholar] [CrossRef]
- Mau, J.-L.; Lin, H.-C.; Chen, C.-C. Non-volatile components of several medicinal mushrooms. Food Res. Int. 2001, 34, 521–526. [Google Scholar] [CrossRef]
- Mattila, P.; Lampi, A.-M.; Ronkainen, R.; Toivo, J.; Piironen, V. Sterol and vitamin D2 contents in some wild and cultivated mushrooms. Food Chem. 2002, 76, 293–298. [Google Scholar] [CrossRef]
- Teichmann, A.; Dutta, P.C.; Staffas, A.; Jägerstad, M. Sterol and vitamin D2 concentrations in cultivated and wild grown mushrooms: Effects of UV irradiation. LWT-Food Sci. Technol. 2007, 40, 815–822. [Google Scholar] [CrossRef]
- Engin, K.N. Alpha-tocopherol: Looking beyond an antioxidant. Mol. Vis. 2009, 15, 855. [Google Scholar] [PubMed]
- Vulin, M.; Magušić, L.; Metzger, A.-M.; Muller, A.; Drenjančević, I.; Jukić, I.; Šijanović, S.; Lukić, M.; Stanojević, L.; Davidović Cvetko, E. Sodium-to-potassium ratio as an indicator of diet quality in healthy pregnant women. Nutrients 2022, 14, 5052. [Google Scholar] [CrossRef] [PubMed]
- Melgar, M.; Alonso, J.; García, M. Total contents of arsenic and associated health risks in edible mushrooms, mushroom supplements and growth substrates from Galicia (NW Spain). Food Chem. Toxicol. 2014, 73, 44–50. [Google Scholar] [CrossRef]
- Grzybek, J.; Janczy, B. Quantitative estimatation of lead, cadmium. and nickel contents by means of atomic absorption spectroscopy in fruitbodies of some macromycetes in Poland. Acta Mycol. 1990, 26, 17–23. [Google Scholar] [CrossRef]
- Kokkoris, V.; Massas, I.; Polemis, E.; Koutrotsios, G.; Zervakis, G.I. Accumulation of heavy metals by wild edible mushrooms with respect to soil substrates in the Athens metropolitan area (Greece). Sci. Total Environ. 2019, 685, 280–296. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, H.H.; Claver, I.P.; Zhu, K.X.; Peng, W.; Zhou, H.M. Effect of different cooking methods on the flavour constituents of mushroom (Agaricus bisporus (Lange) Sing) soup. Int. J. Food Sci. Technol. 2011, 46, 1100–1108. [Google Scholar] [CrossRef]
- Liu, Q.; Bau, T.; Jin, R.; Cui, X.; Zhang, Y.; Kong, W. Comparison of different drying techniques for shiitake mushroom (Lentinus edodes): Changes in volatile compounds, taste properties, and texture qualities. LWT 2022, 164, 113651. [Google Scholar] [CrossRef]
- Aquilina, G.; Azimonti, G.; Bampidis, V.; de Lourdes Bastos, M.; Bories, G.; Chesson, A.; Cocconcelli, P.S.; Flachowsky, G.; Gropp, J.; Kolar, B.; et al. Safety and efficacy of pyrazine derivatives including saturated ones belonging to chemical group 24 when used as flavourings for all animal species. EFSA J. 2017, 15, e04671. [Google Scholar]
- Ismail, F.M.; Levitsky, D.O.; Dembitsky, V.M. Aziridine alkaloids as potential therapeutic agents. Eur. J. Med. Chem. 2009, 44, 3373–3387. [Google Scholar] [CrossRef] [PubMed]
- Bajgar, J.; Kassa, J.; Fusek, J.; Kuca, K.; Jun, D. Other toxic chemicals as potential chemical warfare agents. In Handbook of Toxicology of Chemical Warfare Agents; Elsevier: Amsterdam, The Netherlands, 2015; pp. 337–345. [Google Scholar]
- Cunniff, P. Official Methods of Analysis of AOAC International; Association of Official Analytical Chemists: Washington, DC, USA, 1995. [Google Scholar]
- ISO 12966-1; 2014-Animal and Vegetable Fats and Oils—Gas Chromatography of Fatty Acid Methyl Esters—Part 1: Guidelines on Modern Gas Chromatography of Fatty Acid Methyl Esters. International Organization for Standardization: Geneva, Switzerland, 2014.
- ISO 12966-2: 2017; Animal and Vegetable Fats and Oils—Gas Chromatography of Fatty Acid Methyl Esters—Part 2: Preparation of Methyl Esters of Fatty Acids. International Organization for Standardization: Geneva, Switzerland, 2017.
- DIN EN 12821:2009; Foodstuffs-Determination of Vitamin D by High Performance Liquid Chromatography—Measurement of Cholecalciferol (D3) or Ergocalciferol (D2). European Union: Luxembourg, 2009.
- DIN EN 12822: 2012; Foodstuffs—Determination of Vitamin E by High Performance Liquid Chromatography—Measurement of α-, β-, γ- and δ-Tocopherols. European Union: Luxembourg, 2012.
- Sami, A.S.; Suat, E.; Alkis, I.; Karakus, Y.; Guler, S. The role of trace element, mineral, vitamin and total antioxidant status in women with habitual abortion. J. Matern. -Fetal Neonatal Med. 2021, 34, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Nag, R.; Cummins, E. Human health risk assessment of lead (Pb) through the environmental-food pathway. Sci. Total Environ. 2022, 810, 151168. [Google Scholar] [CrossRef]
- Liu, S.; Fu, Y.; Shi, M.; Wang, H.; Guo, J. Pollution level and risk assessment of lead, cadmium, mercury, and arsenic in edible mushrooms from Jilin Province, China. J. Food Sci. 2021, 86, 3374–3383. [Google Scholar] [CrossRef]
- Širić, I.; Kumar, P.; Eid, E.M.; Bachheti, A.; Kos, I.; Bedeković, D.; Mioč, B.; Humar, M. Occurrence and health risk assessment of cadmium accumulation in three Tricholoma mushroom species collected from wild habitats of Central and Coastal Croatia. J. Fungi 2022, 8, 685. [Google Scholar] [CrossRef]
- Rangel-Vargas, E.; Rodriguez, J.A.; Domínguez, R.; Lorenzo, J.M.; Sosa, M.E.; Andrés, S.C.; Rosmini, M.; Pérez-Alvarez, J.A.; Teixeira, A.; Santos, E.M. Edible mushrooms as a natural source of food ingredient/additive replacer. Foods 2021, 10, 2687. [Google Scholar] [CrossRef] [PubMed]



| Sample Code | 1F | 2F | 3F | |
|---|---|---|---|---|
| Content (mean ± SD) | ||||
| Crude protein% | 22.7 ± 0.0 | 24.9 ± 0.6 | 29.7 ± 0.0 | |
| Total fat% | 6.08 ± 0.07 | 4.50 ± 0.01 | 6.00 ± 0.06 | |
| Total carbohydrates% | 57.6 ± 0.0 | 58.0 ± 0.6 | 52.0 ± 0.0 | |
| Fiber% | 11.5 ± 0.0 | 11.3 ± 0.0 | 11.2 ± 0.0 | |
| Ash% | 13.5 ± 0.0 | 12.4 ± 0.0 | 12.1 ± 0.0 | |
| Total energy (kcal) | 376 ± 1 | 372 ± 1 | 381 ± 1 | |
| Saturated Fatty Acids | Concentrations (g/100 g fat) | Unsaturated Fatty Acids | Concentrations (g/100 g fat) |
|---|---|---|---|
| C10:0 | 0.60 ±0.01 | C14:1 | 0.06 ± 0.03 |
| C12:0 | 0.45 ± 0.03 | C16:1 | 0.22 ± 0.01 |
| C14:0 | 0.66 ± 0.01 | C18:1n9c | 28.6 ± 2.0 |
| C16:0 | 18.2 ± 1.2 | C18:2n6c | 42.0 ± 2.5 |
| C18:0 | 7.70 ± 0.50 | C18:3n6 | 0.03 ± 0.02 |
| C20:0 | 0.26 ± 0.03 | C18:3n3 | 0.38 ± 0.01 |
| C22:0 | 0.24 ± 0.03 | C22:2 | 0.54 ± 0.03 |
| Total SFA | 28.1 ± 2.0 | Total UFA | 91.9 ± 2.6 |
| Essential Amino Acids | Percentage | Non-Essential Amino Acids | Percentage |
|---|---|---|---|
| His | 0.28 ± 0.02 | Glu | 3.80 ± 0.10 |
| Ile | 0.51 ± 0.04 | Gly | 0.10 ± 0.02 |
| Lys | 0.91 ± 0.10 | Arg | 0.80 ± 0.02 |
| Leu | 1.20 ± 0.05 | Ala | 1.00 ± 0.05 |
| Phe | 0.68 ± 0.05 | Tyr | 0.73 ± 0.03 |
| Met | 0.21 ± 0.01 | Pro | 2.0 ± 0.1 |
| Thr | 0.36 ± 0.03 | Asp | 1.0 ± 0.2 |
| Val | 0.61 ± 0.02 | Ser | 2.30 ± 0.04 |
| Elements | Concentration in C. alborufescens | FDA DVs (mg) | C. alborufescens DVs (for 30 g of dw) |
|---|---|---|---|
| Na | 665 ± 6 | 2300 | 19.9 |
| K | >10,000 | 4700 | >300 |
| Fe | 531 ± 5 | 18.0 | 15.9 |
| Ca | 4560 ± 10 | 1300 | 137 |
| P | 5860 ± 20 | 1250 | 176 |
| Mg | 1210 ± 10 | 420 | 36.2 |
| Mn | 25.3 ± 0.2 | 2.3 | 0.7 |
| Cu | 38.1 ± 0.3 | 0.9 | 1.1 |
| Zn | 82.0 ± 0.4 | 11 | 2.4 |
| Cr | 2.20 ± 0.02 | 0.03 | 0.06 |
| Elements | Concentration in C. alborufescens | Daily Intake (DI) | Health Risk Index (HRI) |
|---|---|---|---|
| Pb | 4.09 ± 0.04 | 1.75 | 0.43 |
| Cd | 0.80 ± 0.01 | 0.34 | 0.69 |
| As | 2.40 ± 0.02 | 1.03 | 3.45 |
| Hg | 0.80 ± 0.01 | 0.30 | 1.14 |
| Compound Name | tR 1 [min] | ToC 2 [%] | WfC 3 [%] | RI 4 | Prob 5 | SI 6 | ID 7 |
|---|---|---|---|---|---|---|---|
| Water | 1.73 | 48.29 | - | 541 | 86.12 | 818 | NIST |
| Acetaldehyde | 1.94 | 7.73 | 16.69 | 547 | 94.67 | 940 | NIST |
| Propanal | 3.06 | 0.05 | 0.11 | 577 | 70.32 | 811 | NIST |
| Acetone | 3.15 | 12.80 | 27.63 | 580 | 87.94 | 900 | NIST |
| 2-Methylpropanal | 4.40 | 1.83 | 3.95 | 613 | 77.89 | 890 | NIST |
| n-Butanal | 5.37 | 2.54 | 5.48 | 639 | 71.17 | 865 | NIST |
| 2-Butanone | 5.60 | 0.27 | 0.58 | 645 | 75.95 | 856 | NIST |
| 3-Methylbutanal | 7.37 | 4.14 | 8.94 | 693 | 82.11 | 893 | NIST |
| 2-Methylbutanal | 7.63 | 2.76 | 5.96 | 700 | 32.43 | 830 | NIST |
| 1-Ethenylaziridine | 7.95 | 0.22 | 0.47 | 709 | 53.43 | 792 | NIST |
| n-Pentanal | 8.87 | 1.40 | 3.02 | 733 | 84.92 | 910 | Adams |
| (Z)-3-Hepten-1-yne | 9.01 | 0.45 | 0.97 | 737 | 12.41 | 863 | NIST |
| 2-Hexanone | 10.76 | 0.55 | 1.19 | 784 | 26.04 | 812 | NIST |
| 3-Penten-2-one | 10.86 | 0.07 | 0.15 | 787 | 45.37 | 777 | NIST |
| 1-Pentanol | 12.01 | 0.78 | 1.68 | 817 | 67.93 | 914 | NIST |
| n-Hexanal | 12.79 | 5.68 | 12.26 | 837 | 64.00 | 894 | NIST |
| Methylpyrazine | 13.48 | 0.50 | 1.08 | 855 | 66.69 | 864 | NIST |
| 3-Methylcyclopentyl acetate | 16.15 | 0.24 | 0.52 | 926 | 95.68 | 859 | NIST |
| 2-Heptanone | 16.33 | 0.43 | 0.93 | 931 | 69.82 | 864 | Adams |
| n-Heptanal | 16.64 | 0.30 | 0.65 | 939 | 67.01 | 826 | NIST |
| 2,5-Dimethylpyrazine | 16.71 | 0.24 | 0.52 | 941 | 37.45 | 819 | Adams |
| Ethylpyrazine | 16.90 | 0.11 | 0.24 | 946 | 53.37 | 789 | NIST |
| 2-Pentylfuran | 19.04 | 0.99 | 2.14 | 1005 | 88.33 | 921 | NIST |
| Benzaldehyde | 19.43 | 0.45 | 0.97 | 1017 | 33.22 | 761 | NIST |
| 1-Octen-3-ol | 19.60 | 0.73 | 1.58 | 1022 | 80.06 | 918 | NIST |
| n-Octanal | 20.29 | 0.14 | 0.30 | 1042 | 47.92 | 779 | NIST |
| 3-Octen-2-one | 21.91 | 0.26 | 0.56 | 1090 | 42.62 | 860 | NIST |
| n-Nonanal | 23.69 | 0.27 | 0.58 | 1145 | 28.25 | 790 | Adams |
| 2-Butyl-2-octenal | 29.72 | 0.17 | 0.37 | 1426 | 37.25 | 776 | NIST |
| Isobornyl acrylate | 29.81 | 0.22 | 0.47 | 1432 | 46.62 | 863 | NIST |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moghaddam, M.; Ghobad-Nejhad, M.; Stegemann, T.; Çiçek, S.S.; Zidorn, C.; Javanmard, M. Nutritional Composition and Odor-Contributing Volatile Compounds of the Edible Mushroom Cantharellus alborufescens. Molecules 2023, 28, 7516. https://doi.org/10.3390/molecules28227516
Moghaddam M, Ghobad-Nejhad M, Stegemann T, Çiçek SS, Zidorn C, Javanmard M. Nutritional Composition and Odor-Contributing Volatile Compounds of the Edible Mushroom Cantharellus alborufescens. Molecules. 2023; 28(22):7516. https://doi.org/10.3390/molecules28227516
Chicago/Turabian StyleMoghaddam, Mohaddeseh, Masoomeh Ghobad-Nejhad, Thomas Stegemann, Serhat Sezai Çiçek, Christian Zidorn, and Majid Javanmard. 2023. "Nutritional Composition and Odor-Contributing Volatile Compounds of the Edible Mushroom Cantharellus alborufescens" Molecules 28, no. 22: 7516. https://doi.org/10.3390/molecules28227516