Study on the Dynamic Changes in Non-Volatile Metabolites of Rizhao Green Tea Based on Metabolomics
Abstract
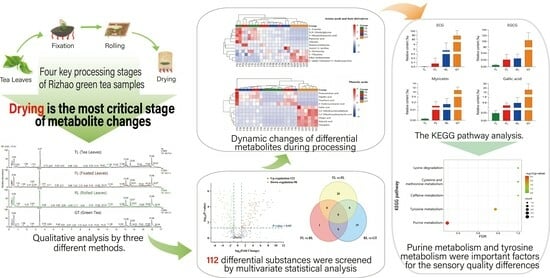
:1. Introduction
2. Results and Discussion
2.1. Non-Targeted Metabolomics Analysis of Rizhao Green Tea Samples
2.2. Multivariate Statistical Analysis
2.3. Screening of Differential Metabolites
2.4. Changes in Taste-Related Substances
| No. | Name | Taste | Relative Content (%) a | Taste Threshold (mg/L) b | DOT | |||
|---|---|---|---|---|---|---|---|---|
| TLs | FLs | RLs | GT | |||||
| 1 | Pipecolic acid | / | 10.55050 ± 0.73458 | 3.69746 ± 0.72721 | 2.96563 ± 0.26097 | 0.78934 ± 0.07104 | / | / |
| 2 | L-Norleucine | / | 1.73371 ± 0.29184 | 0.98388 ± 0.15684 | 1.24953 ± 0.21237 | 1.45375 ± 0.13938 | / | / |
| 3 | Allysine | / | 0.80972 ± 0.35580 | 0.20860 ± 0.04917 | 0.16443 ± 0.02796 | 0.02419 ± 0.00186 | / | |
| 4 | Quercetin | bitterness | 0.00010 ± 0.00002 | 0.02025 ± 0.01095 | 0.06064 ± 0.02886 | 0.39393 ± 0.05495 | / | / |
| 5 | Myricetin | bitterness | 0.00004 ± 0.00001 | 0.00112 ± 0.00092 | 0.00223 ± 0.00142 | 0.16603 ± 0.01122 | / | / |
| 6 | ECG | bitterness and astringency | 0.00016 ± 0.00004 | 0.00120 ± 0.00079 | 0.02048 ± 0.01682 | 1.35739 ± 0.08095 | 201.5 | 1.347 |
| 7 | EGCG | bitterness and astringency | 0.00013 ± 0.00002 | 0.00037 ± 0.00010 | 0.00040 ± 0.00009 | 1.59685 ± 0.19673 | 181.2 | 1.763 |
| 8 | Gallic acid | sourness and astringency | 0.00071 ± 0.00011 | 0.00737 ± 0.00222 | 0.01160 ± 0.00431 | 0.23751 ± 0.08221 | 34 | 1.397 |
| 9 | Procyanidin B2 | bitterness | 0.00018 ± 0.00007 | 0.00729 ± 0.00530 | 0.01620 ± 0.00694 | 0.10206 ± 0.00915 | / | / |
| 10 | L-Theanine | umami | 29.5388 ± 2.9869 | 19.4945 ± 4.4637 | 22.4670 ± 1.9170 | 11.8527 ± 1.3348 | 1045.2 | 2.268 |
| 11 | Methyl gallate | / | 0.0019 ± 0.0003 | 2.4876 ± 0.7255 | 2.4130 ± 0.5399 | 8.2612 ± 0.6417 | / | / |
| 12 | Salicylic acid | / | 0.0101 ± 0.0010 | 2.4634 ± 0.7006 | 2.4150 ± 0.5389 | 8.2178 ± 0.6353 | / | / |
| 13 | L-Leucine | bitterness | 13.94649 ± 2.18217 | 6.69525 ± 1.24796 | 5.63074 ± 1.01828 | 0.37763 ± 0.03533 | 1574.0 | 0.048 |
| 14 | Adenine | umami | 21.74214 ± 3.27621 | 27.66233 ± 0.88956 | 32.67142 ± 1.77501 | 1.90279 ± 0.08703 | / | / |
| 15 | Choline O-Sulfate | / | 6.24440 ± 1.31600 | 11.12400 ± 1.58627 | 8.56549 ± 0.79084 | 0.64120 ± 0.10662 | / | / |
| 16 | 1,3,5-Norcaratriene | / | 9.75277 ± 0.8181 | 20.00826 ± 2.13235 | 18.77298 ± 2.64485 | 55.61301 ± 1.75427 | / | / |
2.5. Metabolic Pathway Analysis
3. Materials and Methods
3.1. Chemicals and Instrument
3.2. Tea Processing and Sampling
3.3. Extraction of Rizhao Green Tea Metabolites
3.4. Non-Targeted Metabolomics Analysis by UHPLC-Q Exactive MS
3.5. Data Processing and Multivariate Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wong, M.; Sirisena, S.; Ng, K. Phytochemical profile of differently processed tea: A review. J. Food Sci. 2022, 87, 1925–1942. [Google Scholar] [CrossRef] [PubMed]
- Koch, W.; Kukula-Koch, W.; Komsta, Ł.; Marzec, Z.; Szwerc, W.; Głowniak, K. Green Tea Quality Evaluation Based on Its Catechins and Metals Composition in Combination with Chemometric Analysis. Molecules 2018, 23, 1689. [Google Scholar] [CrossRef]
- Sun, A.; Li, C.; Lv, S.; Gao, J.; Xia, Y.; Geng, Y. Study on the Effects of Processes on Aroma Compounds in Rizhao Green Tea Based on the Gas Chromatography-Ion Mobility Spectrometry. J. Food Process. Preserv. 2023, 2023, 3046129. [Google Scholar] [CrossRef]
- Zhang, L.; Ho, C.T.; Zhou, J.; Santos, J.S.; Armstrong, L.; Granato, D. Chemistry and Biological Activities of Processed Camellia sinensis Teas: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1474–1495. [Google Scholar] [CrossRef]
- Li, W.; Gao, H.; Fan, Q.; Wang, J.; Tang, X. Comparison of the Quality and Aroma Components of Rizhao Green Tea harvested in Summer Dried under Different Roasting Conditions. Mod. Food Sci. Technol. 2020, 36, 255–262. [Google Scholar]
- Xu, Y. Study on Influencing Factors of Consumers’ Purchase Behavior of Rizhao Tea. Ph.D. Thesis, Shandong Agricultural University, Tai’an, China, 2022. [Google Scholar]
- Deng, S.; Zhang, G.; Aluko, O.O.; Mo, Z.; Mao, J.; Zhang, H.; Liu, X.; Ma, M.; Wang, Q.; Liu, H. Bitter and astringent substances in green tea: Composition, human perception mechanisms, evaluation methods and factors influencing their formation. Food Res. Int. 2022, 157, 111262. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Yeo, A.S.L.; Low, M.Y.; Zhou, W. Identifying key non-volatile compounds in ready-to-drink green tea and their impact on taste profile. Food Chem. 2014, 155, 9–16. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, T.; Dai, Y.; Fang, S.; Liu, Y.; Ran, Q.; Li, Q.; Sheng, Q.; Pan, K. Research Progress of Tea Taste based on Molecular Sensory Science. Sci. Technol. Food Ind. 2021, 42, 337–343. [Google Scholar]
- Chen, Y.; Zhang, Y.; Xing, N. Change Rule of Main Flavor Substance in the Process of Semi-Mechanical and Semi-Manual Xinyang Maojian. Food Sci. Technol. 2020, 45, 61–65. [Google Scholar]
- Wang, H.; Cao, X.; Yuan, Z.; Guo, G. Untargeted metabolomics coupled with chemometrics approach for Xinyang Maojian green tea with cultivar, elevation and processing variations. Food Chem. 2021, 352, 129359. [Google Scholar] [CrossRef]
- Wang, P.; Gu, M.; Shao, S.; Chen, X.; Hou, B.; Ye, N.; Zhang, X. Changes in Non-Volatile and Volatile Metabolites Associated with Heterosis in Tea Plants (Camellia sinensis). J. Agric. Food Chem. 2022, 70, 3067–3078. [Google Scholar] [CrossRef]
- Yang, J.; Zhou, H.; Liu, Y.; Wang, H.; Xu, Y.; Huang, J.; Lei, P. Chemical constituents of green teas processed from albino tea cultivars with white and yellow shoots. Food Chem. Mol. Sci. 2022, 5, 100143. [Google Scholar] [CrossRef]
- Ullrich, L.; Casty, B.; André, A.; Hühn, T.; Steinhaus, M.; Chetschik, I. Decoding the Fine Flavor Properties of Dark Chocolates. J. Agric. Food Chem. 2022, 70, 13730–13740. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, L.; Zheng, F.; Zhang, F.; Shen, C.; Gao, X.; Sun, B.; Huang, M.; Li, H.; Chen, F. Determination and comparison of flavor (retronasal) threshold values of 19 flavor compounds in Baijiu. J. Food Sci. 2021, 86, 2061–2074. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Y.Y.; Liu, Z.; Zhang, Y.M.; Lu, N.; Hai, G.Q.; Shao, S.Z.; Zheng, Q.X.; Zhang, X.; Fu, H.Y.; et al. Differentiating Westlake Longjing tea from the first- and second-grade producing regions using ultra high performance liquid chromatography with quadrupole time-of-flight mass spectrometry-based untargeted metabolomics in combination with chemometrics. J. Sep. Sci. 2020, 43, 2794–2803. [Google Scholar] [CrossRef]
- Ni, T.; Xu, S.; Wei, Y.; Li, T.; Jin, G.; Deng, W.W.; Ning, J. Understanding the promotion of withering treatment on quality of postharvest tea leaves using UHPLC-orbitrap-MS metabolomics integrated with TMT-Based proteomics. LWT 2021, 147, 111614. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, G. Metabolomic analysis reveals the quality characteristics of Yi Gong tea leaves at different harvesting periods. J. Food Biochem. 2022, 46, e14478. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Huang, J.A.; Zhang, S.G.; Liu, Z.H. Research Progress of Chemical Components Deciding the Green Tea Taste. J. Tea Commun. 2018, 45, 9–13. [Google Scholar]
- Cao, Q.Q.; Fu, Y.Q.; Liu, Y.Y.; Qin, Y.; Chen, J.X.; Yin, J.F.; Xu, Y.Q. A targeted and nontargeted metabolomics study on the oral processing of epicatechins from green tea. Food Chem. 2022, 378, 132129. [Google Scholar] [CrossRef]
- Liu, F.; Tu, Z.; Chen, L.; Lin, J.; Zhu, H.; Ye, Y. Analysis of metabolites in green tea during the roasting process using non-targeted metabolomics. J. Sci. Food Agric. 2023, 103, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, Z.; Guo, G. The change pathern of the main taste substances during the traditional processing technologies of Xinyang Maojian. Food Sci. Technol. 2019, 44, 74–78. [Google Scholar]
- Chen, S.; Liu, H.; Zhao, X.; Li, X.; Shan, W.; Wang, X.; Wang, S.; Yu, W.; Yang, Z.; Yu, X. Non-targeted metabolomics analysis reveals dynamic changes of volatile and non-volatile metabolites during oolong tea manufacture. Food Res. Int. 2020, 128, 108778. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, C.; Jiao, Z.; Ruan, J.; Liu, M.Y. Integration of Metabolomics and Transcriptomics Reveal the Mechanism Underlying Accumulation of Flavonols in Albino Tea Leaves. Molecules 2022, 27, 5792. [Google Scholar] [CrossRef]
- Zhou, B.; Wang, Z.; Yin, P.; Ma, B.; Ma, C.; Xu, C.; Wang, J.; Wang, Z.; Yin, D.; Xia, T. Impact of prolonged withering on phenolic compounds and antioxidant capability in white tea using LC-MS-based metabolomics and HPLC analysis: Comparison with green tea. Food Chem. 2022, 368, 130855. [Google Scholar] [CrossRef]
- Wang, L.; Luo, Y.; Wu, Y.; Liu, Y.; Wu, Z. Fermentation and complex enzyme hydrolysis for improving the total soluble phenolic contents, flavonoid aglycones contents and bio-activities of guava leaves tea. Food Chem. 2018, 264, 189–198. [Google Scholar] [CrossRef]
- Peng, A. Study on Taste Characteristics of Strong Feeling, Astringency, Umami and Sweetness of Famous Green Teas based on Sensory Omics. Ph.D. Thesis, Zhejiang University, Hangzhou, China, 2021. [Google Scholar]
- Wang, Y.; Kan, Z.; Thompson, H.J.; Ling, T.; Ho, C.T.; Li, D.; Wan, X. Impact of Six Typical Processing Methods on the Chemical Composition of Tea Leaves Using a Single Camellia sinensis Cultivar, Longjing 43. J. Agric. Food Chem. 2019, 67, 5423–5436. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ran, W.; He, C.; Zhou, J.; Chen, Y.; Yu, Z.; Ni, D. Effects of different tea tree varieties on the color, aroma, and taste of Chinese Enshi green tea. Food Chem. X 2022, 14, 100289. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Gao, Y. Taste-related Compounds in Tea Infusion and the Regulation. Food Res. Dev. 2022, 43, 189–197. [Google Scholar]
- Scharbert, S.; Hofmann, T. Molecular Definition of Black Tea Taste by Means of Quantitative Studies, Taste Reconstitution, and Omission Experiments. J. Agric. Food Chem. 2005, 53, 5377–5384. [Google Scholar] [CrossRef]
- Dai, W.; Xie, D.; Lu, M.; Li, P.; Lv, H.; Yang, C.; Peng, Q.; Zhu, Y.; Guo, L.; Zhang, Y.; et al. Characterization of white tea metabolome: Comparison against green and black tea by a nontargeted metabolomics approach. Food Res. Int. 2017, 96, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Mu, W.; Zhang, T.; Jiang, B. An overview of biological production of L-theanine. Biotechnol. Adv. 2015, 33, 335–342. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, W.; Liu, X.H.; Yang, Z.; Gaur, R.; Wang, J.J.; Ke, J.P.; Bao, G.H. Detection and quantification of flavoalkaloids in different tea cultivars and during tea processing using UPLC-TOF-MS/MS. Food Chem. 2021, 339, 127864. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Dai, W.; Lu, M.; Yan, Y.; Zhang, Y.; Chen, D.; Wu, W.; Gao, J.; Dong, M.; Lin, Z. New insights into the influences of baking and storage on the nonvolatile compounds in oolong tea: A nontargeted and targeted metabolomics study. Food Chem. 2022, 375, 131872. [Google Scholar] [CrossRef] [PubMed]
- Bo, J.; Gong, L.; Ye, X.; Lu, Z.; Li, J.; Luo, W.; Li, N.; Xiao, L. Dynamic Changes of Main Quality Components in Jinhua White Tea Processing. Mod. Food Sci. Technol. 2022, 38, 306–314. [Google Scholar]
- Zhang, Y. The Effect of the Interaction of Taste Nucleotide with EGCG and Its Protein Complex on Taste. Ph.D. Thesis, Shandong Agricultural University, Tai’an, China, 2021. [Google Scholar]
- Koshiishi, C.; Crozier, A.; Ashihara, H. Profiles of Purine and Pyrimidine Nucleotides in Fresh and Manufactured Tea Leaves. J. Agric. Food Chem. 2001, 49, 4378–4382. [Google Scholar] [CrossRef]
- Fan, F.Y.; Huang, C.S.; Tong, Y.L.; Guo, H.W.; Zhou, S.J.; Ye, J.H.; Gong, S.Y. Widely targeted metabolomics analysis of white peony teas with different storage time and association with sensory attributes. Food Chem. 2021, 362, 130257. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.C.; Qin, C.Y.; Liu, P.P.; Feng, L.; Ling, T.J.; Ning, J.M.; Zhang, L.; Wan, X.C. Untargeted Metabolomics Combined with Bioassay Reveals the Change in Critical Bioactive Compounds during the Processing of Qingzhuan Tea. Molecules 2021, 26, 6718. [Google Scholar] [CrossRef]
- Wang, H.; Hua, J.; Yu, Q.; Li, J.; Wang, J.; Deng, Y.; Yuan, H.; Jiang, Y. Widely targeted metabolomic analysis reveals dynamic changes in non-volatile and volatile metabolites during green tea processing. Food Chem. 2021, 363, 130131. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Liu, Y.; Cao, D.; Liu, P.; Wang, S.; Huang, Y.; Jin, X. Analysis and evaluation model for the taste quality of green tea made from various cultivars or strains. Trans. Chin. Soc. Agric. Eng. 2020, 36, 277–286. [Google Scholar]
- Wei, S.; Peng, J.; Chen, Z. Detection of Bitterness-related Substances in Youxi Bitter Tea and Correlation Analysis with Bitterness. J. Tea Sci. 2021, 41, 337–349. [Google Scholar]
- Wang, Z.; Ma, B.; Ma, C.; Zheng, C.; Zhou, B.; Guo, G.; Xia, T. Region identification of Xinyang Maojian tea using UHPLC-Q-TOF/MS-based metabolomics coupled with multivariate statistical analyses. J. Food Sci. 2021, 86, 1681–1691. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhu, C.; Zhao, S.; Zhang, S.; Wang, W.; Fu, H.; Li, X.; Zhou, C.; Chen, L.; Lin, Y.; et al. De novo transcriptome and phytochemical analyses reveal differentially expressed genes and characteristic secondary metabolites in the original oolong tea (Camellia sinensis) cultivar ‘Tieguanyin’ compared with cultivar ‘Benshan’. BMC Genom. 2019, 20, 265. [Google Scholar] [CrossRef] [PubMed]
- Wang, K. Find the Differential Metabolites of Rizhao Green Tea Based on Metabolomics Technology and Explore the Applications. Ph.D. Thesis, Shandong Normal University, Jinan, China, 2017. [Google Scholar]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, A.; Liu, G.; Sun, L.; Li, C.; Wu, Q.; Gao, J.; Xia, Y.; Geng, Y. Study on the Dynamic Changes in Non-Volatile Metabolites of Rizhao Green Tea Based on Metabolomics. Molecules 2023, 28, 7447. https://doi.org/10.3390/molecules28217447
Sun A, Liu G, Sun L, Li C, Wu Q, Gao J, Xia Y, Geng Y. Study on the Dynamic Changes in Non-Volatile Metabolites of Rizhao Green Tea Based on Metabolomics. Molecules. 2023; 28(21):7447. https://doi.org/10.3390/molecules28217447
Chicago/Turabian StyleSun, Ao, Guolong Liu, Luyan Sun, Chun Li, Qiu Wu, Jianhua Gao, Yuanzhi Xia, and Yue Geng. 2023. "Study on the Dynamic Changes in Non-Volatile Metabolites of Rizhao Green Tea Based on Metabolomics" Molecules 28, no. 21: 7447. https://doi.org/10.3390/molecules28217447