Development and Validation of LC-MS/MS Method for the Determination of 1-Methyl-4-Nitrosopiperazine (MNP) in Multicomponent Products with Rifampicin—Analytical Challenges and Degradation Studies
Abstract
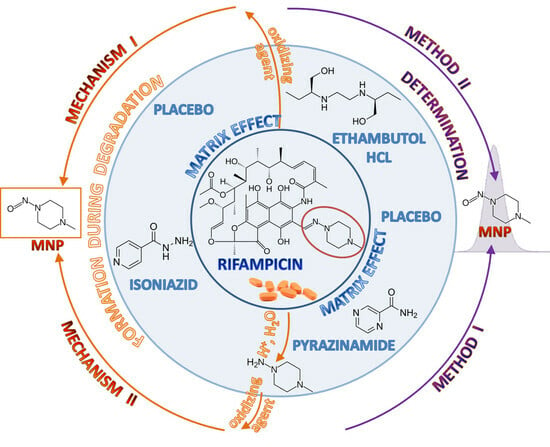
:1. Introduction
2. Results
2.1. Method Development
2.2. Validation Results
2.3. Stability and Stress Studies
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Equipment and Methods
4.3. Standard Solution Preparation
4.4. Sample Solution Preparation
4.5. Blank Preparation
4.6. Validation of the LC-MS/MS Method
4.7. Stress Studies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Potential Dangers of Nitrosamines in Rifampin and Rifapentine. Available online: https://www.adamsonlab.com/potential-dangers-of-nitrosamines-in-rifampin-and-rifapentine/ (accessed on 13 May 2021).
- Grobbelaar, M.; Louw, G.E.; Sampson, S.L.; van Helden, P.D.; Donald, P.R.; Warren, R.M. Evolution of Rifampicin Treatment for Tuberculosis. Infect. Genet. Evol. 2019, 74, 103937. [Google Scholar] [CrossRef] [PubMed]
- Beloor Suresh, A.; Rosani, A.; Wadhwa, R. Rifampin; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Alfarisi, O.; Alghamdi, W.A.; Al-Shaer, M.H.; Dooley, K.E.; Peloquin, C.A. Rifampin vs. Rifapentine: What Is the Preferred Rifamycin for Tuberculosis? Expert Rev. Clin. Pharmacol. 2017, 10, 1027–1036. [Google Scholar] [CrossRef] [PubMed]
- Bharate, S.S. Critical Analysis of Drug Product Recalls Due to Nitrosamine Impurities. J. Med. Chem. 2021, 64, 2923–2936. [Google Scholar] [CrossRef] [PubMed]
- Committee for Medicinal Products for Human Use. ICH M7(R2) Guideline on Assessment and Control of DNA Reactive (Mutagenic) Impurities in Pharmaceuticals to Limit Potential Carcinogenic Risk; European Medicines Agency: Amsterdam, The Netherlands, 2023; Available online: https://www.ema.europa.eu/en/ich-m7-assessment-control-dna-reactive-mutagenic-impurities-pharmaceuticals-limit-potential (accessed on 29 October 2023).
- Akkaraju, H.; Tatia, R.; Mane, S.S.; Khade, A.B.; Dengale, S.J. A Comprehensive Review of Sources of Nitrosamine Contamination of Pharmaceutical Substances and Products. Regul. Toxicol. Pharmacol. 2023, 139, 105355. [Google Scholar] [CrossRef]
- Cioc, R.C.; Joyce, C.; Mayr, M.; Bream, R.N. Formation of N-Nitrosamine Drug Substance Related Impurities in Medicines: A Regulatory Perspective on Risk Factors and Mitigation Strategies. Org. Process. Res. Dev. 2023, 27, 1736–1750. [Google Scholar] [CrossRef]
- Sedlo, I.; Kolonić, T.; Tomić, S. Presence of Nitrosamine Impurities in Medicinal Products. Arch. Ind. Hyg. Toxicol. 2021, 72, 1–5. [Google Scholar] [CrossRef]
- Tuesuwan, B.; Vongsutilers, V. Nitrosamine Contamination in Pharmaceuticals: Threat, Impact, and Control. J. Pharm. Sci. 2021, 110, 3118–3128. [Google Scholar] [CrossRef]
- Pettersson, S.; Pérez-Nueno, V.I.; Ros-Blanco, L.; de La Bellacasa, R.P.; Rabal, M.O.; Batllori, X.; Clotet, B.; Clotet-Codina, I.; Armand-Ugón, M.; Esté, J.; et al. Discovery of Novel Non-Cyclam Polynitrogenated CXCR4 Coreceptor Inhibitors. ChemMedChem 2008, 3, 1549–1557. [Google Scholar] [CrossRef]
- Charushnikov, K.A.; Sulimov, S.G.; Petrov, A.Y.; Nesterova, E.M.; Kuteliya, A.V.; Feklistova, V.F.; Vetrova, V.A. Method of Preparing 1-Nitroso-4-Methylpiperazine RU2095355C1. 2017. Available online: https://patents.google.com/patent/RU2095355C1/en (accessed on 29 October 2023).
- Tao, X.; Tian, Y.; Liu, W.H.; Yao, S.; Yin, L. Trace Level Quantification of 4-Methyl-1-Nitrosopiperazin in Rifampicin Capsules by LC-MS/MS. Front. Chem. 2022, 10, 834124. [Google Scholar] [CrossRef]
- Keefer, L.K.; Fodor, C.H. Facile Hydrogen Isotope Exchange as Evidence for an .Alpha.-Nitrosamino Carbanion. J. Am. Chem. Soc. 1970, 92, 5747–5748. [Google Scholar] [CrossRef]
- Zeiger, E.; Legator, M.S.; Lijinsky, W. Mutagenicity of N-Nitrosopiperazines for Salmonella Typhimurium in the Host-Mediated Assay. Cancer Res. 1972, 32, 1598–1599. [Google Scholar] [PubMed]
- Hoffmann, D.; Raineri, R.; Hecht, S.S.; Maronpot, R.; Wynder, E.L. A Study of Tobacco Carcinogenesis. XIV. Effects of N′-Nitrosonornicotine and N′-Nitrosonanabasine in Rats 2. JNCI J. Natl. Cancer Inst. 1975, 55, 977–981. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency Appendix 1 to Questions and Answers for Marketing Authorisation Holders/Applicants on the CHMP Opinion for the Article 5(3) of Regulation (EC) No 726/2004 Referral on Nitrosamine Impurities in Human Medicinal Products; Acceptable Intakes Established for N-Nitrosamines. Available online: https://www.ema.europa.eu/en/documents/other/appendix-1-acceptable-intakes-established-n-nitrosamines_.pdf (accessed on 24 October 2023).
- Paglialunga, S.; van Haarst, A. The Impact of N-Nitrosamine Impurities on Clinical Drug Development. J. Pharm. Sci. 2023, 112, 1183–1191. [Google Scholar] [CrossRef]
- FDA. Laboratory Analysis of Rifampin/Rifapentine Products. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/laboratory-analysis-rifampinrifapentine-products (accessed on 29 August 2023).
- Scherf-Clavel, O.; Kinzig, M.; Besa, A.; Schreiber, A.; Bidmon, C.; Abdel-Tawab, M.; Wohlfart, J.; Sörgel, F.; Holzgrabe, U. The Contamination of Valsartan and Other Sartans, Part 2: Untargeted Screening Reveals Contamination with Amides Additionally to Known Nitrosamine Impurities. J. Pharm. Biomed. Anal. 2019, 172, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Marzan, T.A.; Ye, W.; Sommers, C.D.; Rodriguez, J.D.; Keire, D.A. A Cautionary Tale: Quantitative LC-HRMS Analytical Procedures for the Analysis of N-Nitrosodimethylamine in Metformin. AAPS J. 2020, 22, 89. [Google Scholar] [CrossRef] [PubMed]
- FDA. Liquid Chromatography-High Resolution Mass Spectrometry (LC-ESI-HRMS) Method for the Determination of MNP in Rifampin and CPNP in Rifapentine Drug Substance and Drug Product. Available online: https://www.fda.gov/media/142092/download (accessed on 29 August 2023).
- Wichitnithad, W.; Sudtanon, O.; Srisunak, P.; Cheewatanakornkool, K.; Nantaphol, S.; Rojsitthisak, P. Development of a Sensitive Headspace Gas Chromatography–Mass Spectrometry Method for the Simultaneous Determination of Nitrosamines in Losartan Active Pharmaceutical Ingredients. ACS Omega 2021, 6, 11048–11058. [Google Scholar] [CrossRef]
- Giménez-Campillo, C.; Pastor-Belda, M.; Campillo, N.; Hernández-Córdoba, M.; Viñas, P. Development of a New Methodology for the Determination of N-Nitrosamines Impurities in Ranitidine Pharmaceuticals Using Microextraction and Gas Chromatography-Mass Spectrometry. Talanta 2021, 223, 121659. [Google Scholar] [CrossRef]
- Schülé, A.; Ates, C.; Palacio, M.; Stofferis, J.; Delatinne, J.-P.; Martin, B.; Lloyd, S. Monitoring and Control of Genotoxic Impurity Acetamide in the Synthesis of Zaurategrast Sulfate. Org. Process. Res. Dev. 2010, 14, 1008–1014. [Google Scholar] [CrossRef]
- Kecili, R.; Billing, J.; Leeman, M.; Nivhede, D.; Sellergren, B.; Rees, A.; Yilmaz, E. Selective Scavenging of the Genotoxic Impurity Methyl P-Toluenesulfonate from Pharmaceutical Formulations. Sep. Purif. Technol. 2013, 103, 173–179. [Google Scholar] [CrossRef]
- Székely, G.; Bandarra, J.; Heggie, W.; Ferreira, F.C.; Sellergren, B. Design, Preparation and Characterization of Novel Molecularly Imprinted Polymers for Removal of Potentially Genotoxic 1,3-Diisopropylurea from API Solutions. Sep. Purif. Technol. 2012, 86, 190–198. [Google Scholar] [CrossRef]
- Witkowska, A.B.; Giebułtowicz, J.; Dąbrowska, M.; Stolarczyk, E.U. Development of a Sensitive Screening Method for Simultaneous Determination of Nine Genotoxic Nitrosamines in Active Pharmaceutical Ingredients by GC-MS. Int. J. Mol. Sci. 2022, 23, 12125. [Google Scholar] [CrossRef] [PubMed]
- de Souza, G.F.P.; Araujo Vieira Matos, M.F.; de Castro Aglio, T.; Salles, A.G.; Rath, S. A Comprehensive LC-UHPLC-MS/MS Method for the Monitoring of N-Nitrosamines in Lipophilic Drugs: A Case Study with Rifampicin. J. Pharm. Biomed. Anal. 2023, 236, 115685. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.; Liu, Y.; Xie, Y.; Hou, W.; Zhang, L.; Zhang, Z. Simultaneous Determination of 13 Nitrosamine Impurities in Biological Medicines Using Salting-out Liquid-Liquid Extraction Coupled with Liquid Chromatography Tandem Mass Spectrometry. J. Pharm. Biomed. Anal. 2022, 218, 114867. [Google Scholar] [CrossRef] [PubMed]
- Kalauz, A.; Tiringer, K.V.; Horváth, V.; Kapui, I. Simultaneous Determination of Low Molecular Weight Nitrosamines in Pharmaceutical Products by Fast Gas Chromatography Mass Spectrometry. J. Chromatogr. A 2023, 1708, 464323. [Google Scholar] [CrossRef]
- Schmidtsdorff, S.; Neumann, J.; Schmidt, A.H.; Parr, M.K. Analytical Lifecycle Management for Comprehensive and Universal Nitrosamine Analysis in Various Pharmaceutical Formulations by Supercritical Fluid Chromatography. J. Pharm. Biomed. Anal. 2021, 197, 113960. [Google Scholar] [CrossRef]
- International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. ICH Topic Q 1 A (R2) Stability Testing of New Drug Substances and Products Step 5 Note for Guidance on Stability Testing: Stability Testing of New Drug Substances and Products; European Medicines Agency: Amsterdam, The Netherlands, 2003; Available online: https://www.ema.europa.eu/en/ich-q1a-r2-stability-testing-new-drug-substances-drug-products-scientific-guideline (accessed on 29 October 2023).
- Blessy, M.; Patel, R.D.; Prajapati, P.N.; Agrawal, Y.K. Development of Forced Degradation and Stability Indicating Studies of Drugs—A Review. J. Pharm. Anal. 2014, 4, 159–165. [Google Scholar] [CrossRef]
- Gallo, G.G.; Radaelli, P. Rifampin. In Analytical Profiles of Drug Substances and Excipients; Academic Press: Cambridge, MA, USA, 1976; Volume 5, pp. 467–513. [Google Scholar] [CrossRef]
- Alves, R.; Reis, T.V.d.S.; Silva, L.C.C.d.; Storpírtis, S.; Mercuri, L.P.; Matos, J.d.R. Thermal Behavior and Decomposition Kinetics of Rifampicin Polymorphs under Isothermal and Non-Isothermal Conditions. Braz. J. Pharm. Sci. 2010, 46, 343–351. [Google Scholar] [CrossRef]
- Swamy, N.; Basavaiah, K.; Vamsikrishna, P. Stability-Indicating HPLC Determination of Rifampicin in Bulk Drug and Dosage Form. Pharm. Chem. J. 2019, 53, 580–588. [Google Scholar] [CrossRef]
- Mwila, C.; Walker, R.B. Improved Stability of Rifampicin in the Presence of Gastric-resistant Isoniazid Microspheres in Acidic Media. Pharmaceutics 2020, 12, 234. [Google Scholar] [CrossRef]
- Beard, J.C.; Swager, T.M. An Organic Chemist’s Guide to N-Nitrosamines: Their Structure, Reactivity, and Role as Contaminants. J. Org. Chem. 2021, 86, 2037–2057. [Google Scholar] [CrossRef]
- Bayne, A.-C.V.; Misic, Z.; Stemmler, R.T.; Wittner, M.; Frerichs, M.; Bird, J.K.; Besheer, A. N-Nitrosamine Mitigation with Nitrite Scavengers in Oral Pharmaceutical Drug Products. J. Pharm. Sci. 2023, 112, 1794–1800. [Google Scholar] [CrossRef] [PubMed]
- Moser, J.; Ashworth, I.W.; Harris, L.; Hillier, M.C.; Nanda, K.K.; Scrivens, G. N-Nitrosamine Formation in Pharmaceutical Solid Drug Products: Experimental Observations. J. Pharm. Sci. 2023, 112, 1255–1267. [Google Scholar] [CrossRef] [PubMed]
- Horne, S.; Vera, M.D.; Nagavelli, L.R.; Sayeed, V.A.; Heckman, L.; Johnson, D.; Berger, D.; Yip, Y.Y.; Krahn, C.L.; Sizukusa, L.O.; et al. Regulatory Experiences with Root Causes and Risk Factors for Nitrosamine Impurities in Pharmaceuticals. J. Pharm. Sci. 2023, 112, 1166–1182. [Google Scholar] [CrossRef] [PubMed]
- Association of Official Analytical Chemists Appendix F: Guidelines for Standard Method Performance Requirements. Available online: https://www.aoac.org/wp-content/uploads/2019/08/app_f.pdf (accessed on 24 October 2023).







| Acceptance Criteria | Results for MNP |
|---|---|
| Range: LOQ-120% [ng/mL] | 0.51–48.62 |
| Linearity | |
| R2 ≥ 0.990 | 0.9997 |
| a | 0.0499 |
| b | 0.0096 |
| Sa | 0.0003 |
| Sb | 0.0074 |
| Sxy | 0.0152 |
| Accuracy | |
| Recovery 60–115% | 100.38 |
| RSD ≤ 21% | 8.17 |
| LOD [ng/mL] | 0.51 |
| LOQ [ng/mL] | 1.52 |
| Degradation Conditions | MNP Content [ppm] |
|---|---|
| Control sample (before degradation) | 7.07 |
| Alkaline hydrolysis (0.1 M NaOH, 1 h, RT) | 7.26 |
| Oxidative degradation (3% H2O2, 1 h, RT) | 2920 |
| Thermal stress (80 °C, 24 h, RT) | 8.62 |
| Stress Conditions | 1-Amino-4-Methyl-Piperazine [ppm] | MNP [ppm] |
|---|---|---|
| API | ||
| control | 0.841 | 3.146 |
| 0.1 HCl, 1 h, 25 °C | 3.020 | 3.648 |
| 0.1 HCl, 1 h, 25 °C + 3% H2O2 | 2.821 | 2601 |
| 4-component rifampicin product | ||
| control | 10.186 | 3.871 |
| 0.1 HCl, 1 h, 25 °C | 20.922 | 3.616 |
| 0.1 HCl, 1 h, 25 °C + 3% H2O2 | 10.494 | 654 |
| LC Parameters | |||
| Mobile phase A | 10 mM ammoniu formate pH = 9 | ||
| Mobile phase B | Methanol | ||
| Gradient elution program: | Time, min | Eluent A, % | Eluent B, % |
| 0 | 90 | 10 | |
| 2.0 | 90 | 10 | |
| 5.0 | 5 | 95 | |
| 7.0 | 5 | 95 | |
| 7.2 | 90 | 10 | |
| 10.0 | 90 | 10 | |
| Column temperature | 35 °C | ||
| Autosampler temperature | 10 °C | ||
| Flow rate | 0.3 mL/min | ||
| Injection volume | 5 µL | ||
| MS parameters | |||
| Gas temperature | 350 °C | ||
| Gas flow | 11 L/min | ||
| Nebulizer | 50 psi | ||
| Sheath gas temperature | 350 °C | ||
| Sheath gas flow | 12 L/min | ||
| Capillary | 3000 V | ||
| Nozzle Voltage | 500 V | ||
| Fragmentor | 40 | ||
| Analyte Name | RT (min) | Precursor Ion | Fragmentor (V) | Product Ion 1 | CE 1 (V) | Product Ion 2 | CE 2 (V) |
|---|---|---|---|---|---|---|---|
| MNP | 3.985 | 130.1 | 40 | 100.2 | 10 | 58.2 | 18 |
| MNP-d4 | 3.944 | 134.1 | 70 | 104.1 | 8 | 58.1 | 24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Witkowska, A.B.; Wołczyńska, A.; Lis-Cieplak, A.; Stolarczyk, E.U. Development and Validation of LC-MS/MS Method for the Determination of 1-Methyl-4-Nitrosopiperazine (MNP) in Multicomponent Products with Rifampicin—Analytical Challenges and Degradation Studies. Molecules 2023, 28, 7405. https://doi.org/10.3390/molecules28217405
Witkowska AB, Wołczyńska A, Lis-Cieplak A, Stolarczyk EU. Development and Validation of LC-MS/MS Method for the Determination of 1-Methyl-4-Nitrosopiperazine (MNP) in Multicomponent Products with Rifampicin—Analytical Challenges and Degradation Studies. Molecules. 2023; 28(21):7405. https://doi.org/10.3390/molecules28217405
Chicago/Turabian StyleWitkowska, Anna B., Aleksandra Wołczyńska, Agnieszka Lis-Cieplak, and Elżbieta U. Stolarczyk. 2023. "Development and Validation of LC-MS/MS Method for the Determination of 1-Methyl-4-Nitrosopiperazine (MNP) in Multicomponent Products with Rifampicin—Analytical Challenges and Degradation Studies" Molecules 28, no. 21: 7405. https://doi.org/10.3390/molecules28217405