Room-Temperature Synthesis of Carbon-Nanotube-Interconnected Amorphous NiFe-Layered Double Hydroxides for Boosting Oxygen Evolution Reaction
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Structural Characterizations
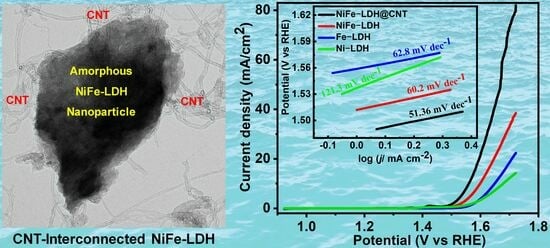
2.2. Oxygen Evolution Activity
3. Materials and Methods
3.1. Materials
3.2. Synthesis
3.2.1. Synthesis of Ni-LDH
3.2.2. Synthesis of Fe-LDH
3.2.3. Synthesis of NiFe-LDH
3.2.4. Synthesis of NiFe-LDH@CNT
3.3. Structural Characterizations
3.4. Electrochemical Characterizations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Chu, S.; Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 2012, 488, 294–303. [Google Scholar] [CrossRef]
- Xu, X.; Su, C.; Shao, Z. Fundamental Understanding and Application of Ba0.5Sr0.5Co0.8Fe0.2O3−δ Perovskite in energy storage and conversion: Past, present, and future. Energy Fuels 2021, 35, 13585–13609. [Google Scholar] [CrossRef]
- Musazade, E.; Voloshin, R.; Brady, N.; Mondal, J.; Atashova, S.; Zharmukhamedov, S.K.; Huseynova, I.; Ramakrishna, S.; Najafpour, M.M.; Sheng, J.R.; et al. Biohybrid solar cells: Fundamentals, progress, and challenges. J. Photochem. Photobiol. C-Photochem. Rev. 2018, 35, 134–156. [Google Scholar] [CrossRef]
- Rodionova, M.V.; Poudyal, R.S.; Tiwari, I.; Voloshin, R.A.; Zharmukhamedov, S.K.; Nam, H.G.; Zayadan, B.K.; Bruce, B.D.; Hou, H.J.M.; Allakhverdiev, S.I. Biofuel production: Challenges and opportunities. Int. J. Hydrogen Energy 2017, 42, 8450–8461. [Google Scholar] [CrossRef]
- Wang, B.; Srinivas, K.; Wang, X.; Su, Z.; Yu, B.; Zhang, X.; Liu, Y.; Ma, F.; Yang, D.; Chen, Y. Self-assembled CoSe2-FeSe2 heteronanoparticles along the carbon nanotube network for boosted oxygen evolution reaction. Nanoscale 2021, 13, 9651–9658. [Google Scholar] [CrossRef]
- Xu, X.; Pan, Y.; Ge, L.; Chen, Y.; Mao, X.; Guan, D.; Li, M.; Zhong, Y.; Hu, Z.; Peterson, V.K.; et al. High-performance perovskite composite electrocatalysts enabled by controllable interface engineering. Small 2021, 17, 2101573. [Google Scholar] [CrossRef]
- Karmakar, A.; Karthick, K.; Kumaravel, S.; Sankar, S.S.; Kundu, S. Enabling and inducing oxygen vacancies in cobalt iron layer double hydroxide via selenization as orecatalysts for electrocatalytic hydrogen and oxygen evolution reactions. Inorg. Chem. 2021, 60, 2023–2036. [Google Scholar] [CrossRef]
- Najafpour, M.M.; Allakhverdiev, S.I. Manganese compounds as water oxidizing catalysts for hydrogen production via water splitting: From manganese complexes to nano-sized manganese oxides. Int. J. Hydrogen Energy 2012, 37, 8753–8764. [Google Scholar] [CrossRef]
- Lyu, F.L.; Wang, Q.F.; Choi, S.M.; Yin, Y.D. Noble-metal-free electrocatalysts for oxygen evolution. Small 2019, 15, 17. [Google Scholar] [CrossRef]
- Saha, S.; Ganguli, A.K. FeCoNi alloy as noble metal-free electrocatalyst for oxygen evolution reaction (OER). Chem. Sel. 2017, 2, 1630–1636. [Google Scholar] [CrossRef]
- Tan, D.M.; Xiong, H.; Zhang, T.; Fan, X.L.; Wang, J.J.; Xu, F. Recent progress in noble-metal-free electrocatalysts for alkaline oxygen evolution reaction. Front. Chem. 2022, 10, 10. [Google Scholar] [CrossRef]
- Evans, D.G.; Slade, R.C.T. Structural aspects of layered double hydroxides. In Layered Double Hydroxides; Duan, X., Evans, D.G., Eds.; Springer: Berlin, Germany, 2006; Volume 119, pp. 1–87. [Google Scholar]
- Qin, X.; Teng, J.; Guo, W.Y.; Wang, L.; Xiao, S.N.; Xu, Q.J.; Min, Y.L.; Fan, J.C. Magnetic field enhancing OER electrocatalysis of NiFe layered double hydroxide. Catal. Lett. 2023, 153, 673–681. [Google Scholar] [CrossRef]
- Xu, X.; Zhong, Z.; Yan, X.M.; Kang, L.T.; Yao, J.N. Cobalt layered double hydroxide nanosheets synthesized in water-methanol solution as oxygen evolution electrocatalysts. J. Mater. Chem. A 2018, 6, 5999–6006. [Google Scholar] [CrossRef]
- Souleymen, R.; Wang, Z.T.; Qiao, C.; Naveed, M.; Cao, C.B. Microwave-assisted synthesis of graphene-like cobalt sulfide freestanding sheets as an efficient bifunctional electrocatalyst for overall water splitting. J. Mater. Chem. A 2018, 6, 7592–7607. [Google Scholar] [CrossRef]
- Gang, C.A.; Chen, J.Y.; Chen, Q.H.; Chen, Y.T. Heterostructure of ultrafine FeOOH nanodots supported on CoAl-layered double hydroxide nanosheets as highly efficient electrocatalyst for water oxidation. J. Colloid Interface Sci. 2021, 600, 594–601. [Google Scholar] [CrossRef]
- Ye, Y.; Shan, Y.; Zhu, H.; Chen, K.; Yu, X. Controllable formation of amorphous structure to improve the oxygen evolution reaction performance of a CoNi LDH. Rsc Adv. 2023, 13, 2467–2475. [Google Scholar] [CrossRef]
- Lu, X.Y.; Xue, H.R.; Gong, H.; Bai, M.J.; Tang, D.M.; Ma, R.Z.; Sasaki, T. 2D layered double hydroxide nanosheets and their derivatives toward efficient oxygen evolution reaction. Nano-Micro Lett. 2020, 12, 32. [Google Scholar] [CrossRef]
- Liu, Z.B.; Yu, C.; Han, X.T.; Yang, J.; Zhao, C.T.; Huang, H.W.; Qiu, J.S. CoMn layered double hydroxides/carbon nanotubes architectures as high-performance electrocatalysts for the oxygen evolution reaction. ChemElectroChem 2016, 3, 906–912. [Google Scholar] [CrossRef]
- Wu, L.; Yu, L.; Zhang, F.; Wang, D.; Luo, D.; Song, S.; Yuan, C.; Karim, A.; Chen, S.; Ren, Z. Facile synthesis of nanoparticle-stacked tungsten-doped nickel iron layered double hydroxide nanosheets for boosting oxygen evolution reaction. J. Mater. Chem. A 2020, 8, 8096–8103. [Google Scholar] [CrossRef]
- Yan, L.; Xu, Z.Y.; Liu, X.A.; Mahmood, S.; Shen, J.L.; Ning, J.Q.; Li, S.; Zhong, Y.J.; Hu, Y. Integrating trifunctional Co@NC-CNTs@NiFe-LDH electrocatalysts with arrays of porous triangle carbon plates for high-power-density rechargeable Zn-air batteries and self-powered water splitting. Chem. Eng. J. 2022, 446, 11. [Google Scholar] [CrossRef]
- Huang, L.; Wu, H.; Liu, H.; Zhang, Y. Phosphorous doped cobalt-iron sulfide/carbon nanotube as active and robust electrocatalysts for water splitting. Electrochim. Acta 2019, 318, 892–900. [Google Scholar] [CrossRef]
- Diard, J.P.; Le Gorrec, B.; Montella, C. Diffusion layer approximation under transient conditions. J. Electroanal. Chem. 2005, 584, 182–191. [Google Scholar] [CrossRef]
- Wang, C.; Jin, L.; Shang, H.; Xu, H.; Shiraishi, Y.; Du, Y. Advances in engineering RuO2 electrocatalysts towards oxygen evolution reaction. Chin. Chem. Lett. 2021, 32, 2108–2116. [Google Scholar] [CrossRef]
- Fang, Y.-H.; Liu, Z.-P. Mechanism and Tafel Lines of Electro-Oxidation of Water to Oxygen on RuO2(110). J. Am. Chem. Soc. 2010, 132, 18214–18222. [Google Scholar] [CrossRef]
- Fletcher, S. Butler-Volmer meets microscopic reversibility. Curr. Opin. Electrochem. 2023, 37, 101199. [Google Scholar] [CrossRef]
- Srinivas, K.; Chen, Y.; Su, Z.; Yu, B.; Karpuraranjith, M.; Ma, F.; Wang, X.; Zhang, W.; Yang, D. Heterostructural CoFe2O4/CoO nanoparticles-embedded carbon nanotubes network for boosted overall water-splitting performance. Electrochim. Acta 2022, 404, 139745. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Qu, Q.; Li, X.; Srinivas, K.; Chen, Y.; Zhu, M. Room-Temperature Synthesis of Carbon-Nanotube-Interconnected Amorphous NiFe-Layered Double Hydroxides for Boosting Oxygen Evolution Reaction. Molecules 2023, 28, 7289. https://doi.org/10.3390/molecules28217289
Chen Z, Qu Q, Li X, Srinivas K, Chen Y, Zhu M. Room-Temperature Synthesis of Carbon-Nanotube-Interconnected Amorphous NiFe-Layered Double Hydroxides for Boosting Oxygen Evolution Reaction. Molecules. 2023; 28(21):7289. https://doi.org/10.3390/molecules28217289
Chicago/Turabian StyleChen, Zhuo, Qiang Qu, Xinsheng Li, Katam Srinivas, Yuanfu Chen, and Mingqiang Zhu. 2023. "Room-Temperature Synthesis of Carbon-Nanotube-Interconnected Amorphous NiFe-Layered Double Hydroxides for Boosting Oxygen Evolution Reaction" Molecules 28, no. 21: 7289. https://doi.org/10.3390/molecules28217289