Facile Synthesis of Cu-Doped ZnO Nanoparticles for the Enhanced Photocatalytic Disinfection of Bacteria and Fungi
Abstract
:1. Introduction
2. Results and Discussion
2.1. Material Characterization
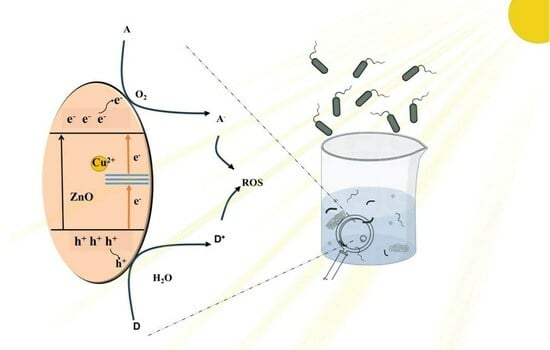
2.2. Photocatalytic Sterilization Performance
2.3. Antifungal Performance Test
2.4. Fluorescence Staining Tests
2.5. Safety Test
3. Material and Methods
3.1. Experimental Reagents
3.2. Preparation of Cu-ZnO Nanocomposites
3.3. Characterization
Electrochemical Workstation
3.4. Disinfection Test
3.5. Fluorescence Staining Tests
3.6. Safety Assessment Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Abu Elella, M.H.; Goda, E.S.; Abdallah, H.M.; Shalan, A.E.; Gamal, H.; Yoon, K.R. Innovative bactericidal adsorbents containing modified xanthan gum/montmorillonite nanocomposites for wastewater treatment. Int. J. Biol. Macromol. 2021, 167, 1113–1125. [Google Scholar] [CrossRef] [PubMed]
- Azamzam, A.A.; Rafatullah, M.; Yahya, E.B.; Ahmad, M.I.; Lalung, J.; Alharthi, S.; Alosaimi, A.M.; Hussein, M.A. Insights into solar disinfection enhancements for drinking water treatment applications. Sustainability 2021, 13, 910570. [Google Scholar] [CrossRef]
- Stec, J.; Kosikowska, U.; Mendrycka, M.; Stępień-Pyśniak, D.; Niedźwiedzka-Rystwej, P.; Bębnowska, D.; Hrynkiewicz, R.; Ziętara-Wysocka, J.; Grywalska, E. Opportunistic pathogens of recreational waters with emphasis on antimi-crobial resistance—A possible subject of human health concern. Int. J. Environ. Res. Public Health 2022, 19, 7308. [Google Scholar] [CrossRef] [PubMed]
- Bain, R.; Cronk, R.; Hossain, R.; Bonjour, S.; Onda, K.; Wright, J.; Yang, H.; Slaymaker, T.; Hunter, P.; Prüss-Ustün, A.; et al. Global assessment of exposure to faecal contamination through drinking water based on a systematic review. Trop. Med. Int. Health 2014, 19, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Raeiszadeh, M.; Adeli, B. A critical review on ultraviolet disinfection systems against COVID-19 Outbreak: Applicability, validation, and safety considerations. Acs Photonics 2020, 7, 2941–2951. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Lee, Y.J.; Koo, Y.J.; Pack, E.C.; Lim, K.M.; Choi, D.W. Migration of monomers, plastic additives, and non-intentionally added substances from food utensils made of melamine–formaldehyde resin following ultraviolet sterilization. Food Control 2021, 125, 107981. [Google Scholar] [CrossRef]
- Li, Y.; Xia, Z.; Yang, Q.; Wang, L.; Xing, Y. Review on g-C3N4-based S-scheme heterojunction photocatalysts. J. Mater. Sci. Technol. 2022, 125, 128–144. [Google Scholar] [CrossRef]
- An, W.-K.; Zheng, S.-J.; Xu, X.; Liu, L.-J.; Ren, J.-S.; Fan, L.; Yang, Z.-K.; Ren, Y.; Xu, C. Integrating benzofuran and heteroradialene into donor-acceptor covalent organic frameworks for photocatalytic construction of multi-substituted olefins. Appl. Catal. B Environ. 2022, 316, 121630. [Google Scholar] [CrossRef]
- Kumaravel, V.; Nair, K.M.; Mathew, S.; Bartlett, J.; Kennedy, J.E.; Manning, H.G.; Whelan, B.J.; Leyland, N.S.; Pillai, S.C. Antimicrobial TiO2 nanocomposite coatings for surfaces, dental and orthopaedic implants. Chem. Eng. J. 2021, 416, 129071. [Google Scholar] [CrossRef]
- Sayadi, M.H.; Ahmadpour, N.; Homaeigohar, S. Photocatalytic and antibacterial properties of Ag-CuFe2O4@ WO3 magnetic nanocomposite. Nanomaterials 2021, 11, 020298. [Google Scholar] [CrossRef]
- Sharma, S.; Dutta, V.; Raizada, P.; Thakur, V.K.; Saini, A.K.; Mittal, D.; Nguyen, V.-H.; Ahamad, T.; Nguyen, C.C.; Kim, S.Y.; et al. Synergistic photocatalytic dye mitigation and bacterial disinfection using carbon quantum dots decorated dual Z-scheme manganese indium sulfide/cuprous oxide/silver oxide heterojunction. Mater. Lett. 2022, 313, 131716. [Google Scholar] [CrossRef]
- Weldegebrieal, G.K. Synthesis method, antibacterial and photocatalytic activity of ZnO nanoparticles for azo dyes in wastewater treatment: A review. Inorg. Chem. Commun. 2020, 120, 108140. [Google Scholar] [CrossRef]
- Dhiman, P.; Rana, G.; Kumar, A.; Sharma, G.; Vo, D.V.N.; Naushad, M. ZnO-based heterostructures as photocatalysts for hydrogen generation and depollution: A review. Environ. Chem. Lett. 2022, 20, 1047–1081. [Google Scholar] [CrossRef]
- Intaphong, P.; Phuruangrat, A.; Yeebu, H.; Akhbari, K.; Sakhon, T.; Thongtem, S.; Thongtem, T. Sonochemical synthesis of Pd nanoparticle/ZnO flower photocatalyst used for methylene blue and methyl orange degradation under UV radiation. Russ. J. Inorg. Chem. 2021, 66, 2123–2133. [Google Scholar] [CrossRef]
- Sun, Y.; Wu, J.; Wang, Y.; Li, J.; Wang, N.; Harding, J.; Mo, S.; Chen, L.; Chen, P.; Fu, M.; et al. Plasma-catalytic CO2 hydrogenation over a Pd/ZnO catalyst: In situ probing of gas-phase and surface reactions. JACS Au 2022, 2, 1800–1810. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Tan, C.; He, L.; Guo, J.; Zhao, X.; Qi, K.; Yan, Y. Mn2+ doped AgInS2 photocatalyst for formaldehyde degradation and hydrogen production from water splitting by carbon tube enhancement. Chemosphere 2022, 304, 135292. [Google Scholar] [CrossRef] [PubMed]
- Shishido, T.; Yamamoto, Y.; Morioka, H.; Takaki, K.; Takehira, K. Active Cu/ZnO and Cu/ZnO/Al2O3 catalysts prepared by homogeneous precipitation method in steam reforming of methanol. Appl. Catal. A Gen. 2004, 263, 249–253. [Google Scholar] [CrossRef]
- Pastor, E.; Sachs, M.; Selim, S.; Durrant, J.R.; Bakulin, A.A.; Walsh, A. Electronic defects in metal oxide photocatalysts. Nat. Rev. Mater. 2022, 7, 503–521. [Google Scholar] [CrossRef]
- Fageria, P.; Gangopadhyay, S.; Pande, S. Synthesis of ZnO/Au and ZnO/Ag nanoparticles and their photocatalytic applica-tion using UV and visible light. RSC Adv. 2014, 4, 24962–24972. [Google Scholar] [CrossRef]
- Bathla, A.; Vikrant, K.; Kukkar, D.; Kim, K.-H. Photocatalytic degradation of gaseous benzene using metal oxide nanocomposites. Adv. Colloid. Interface Sci. 2022, 305, 102696. [Google Scholar] [CrossRef]
- Demazeau, G. Solvothermal processes: Definition, key factors governing the involved chemical reactions and new trends. Z. Für Naturforschung B 2010, 65, 999–1006. [Google Scholar] [CrossRef]
- Wojnarowicz, J.; Chudoba, T.; Lojkowski, W. A review of microwave synthesis of zinc oxide nanomaterials: Reactants, process parameters and morphologies. Nanomaterials 2020, 10, 1086. [Google Scholar] [CrossRef] [PubMed]
- Pedroza-Herrera, G.; Medina-Ramirez, I.E.; Antonio Lozano-Alvarez, J.; Rodil, S. Evaluation of the photocatalytic activity of copper doped TiO2 nanoparticles for the purification and/or disinfection of industrial effluents. Catal. Today 2020, 341, 37–48. [Google Scholar] [CrossRef]
- Fu, M.; Li, Y.; Wu, S.; Lu, P.; Liu, J.; Dong, F. Sol-gel preparation and enhanced photocatalytic performance of Cu-doped ZnO nanoparticles. Appl. Surf. Sci. 2011, 258, 1587–1591. [Google Scholar] [CrossRef]
- Kuriakose, S.; Satpati, B.; Mohapatra, S. Highly efficient photocatalytic degradation of organic dyes by Cu doped ZnO nanostructures. Phys. Chem. Chem. Phys. 2015, 17, 25172–25181. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Shahid, S.; Jabin, S.; Zaman, S.; Sarwar, M.N. Synthesis and characterization and characterization of un-doped and copper doped zinc oxide nanoparticles for their optical and antibacterial studies. Dig. J. Nanomater. Biostruct. 2018, 13, 285–297. [Google Scholar]
- Rashid, M.; Ikram, M.; Haider, A.; Naz, S.; Haider, J.; Ul-Hamid, A.; Shahzadi, A.; Aqeel, M. Photocatalytic, dye degradation, and bactericidal behavior of Cu-doped ZnO na-norods and their molecular docking analysis. Dalton Trans. 2020, 49, 8314–8330. [Google Scholar] [CrossRef]
- Karthik, K.V.; Raghu, A.V.; Reddy, K.R.; Ravishankar, R.; Sangeeta, M.; Shetti, N.P.; Reddy, C.V. Green synthesis of Cu-doped ZnO nanoparticles and its application for the photocatalytic degradation of hazardous organic pollutants. Chemosphere 2022, 287, 132081. [Google Scholar] [CrossRef]
- Panchal, P.; Paul, D.R.; Sharma, A.; Choudhary, P.; Meena, P.; Nehra, S. Biogenic mediated Ag/ZnO nanocomposites for photocatalytic and antibacterial activities towards disinfection of water. J. Colloid Interface Sci. 2020, 563, 370–380. [Google Scholar] [CrossRef]
- Yarahmadi, M.; Maleki-Ghaleh, H.; Mehr, M.E.; Dargahi, Z.; Rasouli, F.; Siadati, M.H. Synthesis and characterization of Sr-doped ZnO nanoparticles for photocatalytic applications. J. Alloys Compd. 2021, 853, 157000. [Google Scholar] [CrossRef]
- Shah, N.S.; Khan, J.A.; Sayed, M.; Khan, Z.U.H.; Rizwan, A.D.; Muhammad, N.; Boczkaj, G.; Murtaza, B.; Imran, M.; Khan, H.M.; et al. Solar light driven degradation of norfloxacin using as-synthesized Bi3+ and Fe2+ co-doped ZnO with the addition of HSO5−: Toxicities and degradation pathways investigation. Chem. Eng. J. 2018, 351, 841–855. [Google Scholar] [CrossRef]
- Yang, Y.Q.; Ji, W.Q.; Li, X.Y.; Zheng, Z.H.; Bi, F.K.; Yang, M.H.; Xu, J.C.; Zhang, X.D. Insights into the degradation mechanism of perfluorooctanoic acid under visible-light irradiation through fabricating flower-shaped Bi5O7I/ZnO n-n heterojunction microspheres. Chem. Eng. J. 2021, 420, 129934. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Q.; Yuan, H.; Luo, K.; Li, J.; Hu, W.; Pan, Z.; Xu, M.; Xu, S.; Levchenko, I.; et al. Comparative study of photocatalysis and gas sensing of ZnO/Ag nanocomposites syn-thesized by one- and two-step polymer-network gel processes. J. Alloys Compd. 2021, 868, 158723. [Google Scholar] [CrossRef]
- Tuncel, D.; Okte, A.N. Improved adsorption capacity and photoactivity of ZnO-ZIF-8 nanocomposites. Catal. Today 2021, 361, 191–197. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, G.R.; Liu, H.; Li, Y.B.; Jin, Z.L.; Ma, Q.X. Regular octahedron Cu-MOFs modifies Mn0.05Cd0.95S nanoparticles to form a S-scheme heterojunction for photocatalytic hydrogen evolution. Int. J. Hydrog. 2021, 46, 7230–7240. [Google Scholar] [CrossRef]
- Cassar, J.R.; Mills, E.W.; Demirci, A. Characterization of pulsed light for microbial inactivation. J. Food Eng. 2022, 334, 111152. [Google Scholar] [CrossRef]
- Jiang, G.F.; Zhu, B.J.; Sun, J.Z.; Liu, F.; Wang, Y.Q.; Zhao, C.C. Enhanced activity of ZnS (111) by N/Cu co-doping: Accelerated degradation of organic pol-lutants under visible light. J. Environ. Sci. 2023, 125, 244–257. [Google Scholar] [CrossRef]
- Lin, H.; Cheng, Q.L.; Sun, W.C.; Yang, F.X.; Ding, Y.Z.; Ma, J.W. Copper exposure effects on antibiotic degradation in swine manure vary between mes-ophilic and thermophilic conditions. Sci. Total Environ. 2022, 841, 156759. [Google Scholar] [CrossRef]
- Ahamed, M.; Akhtar, M.J.; Khan, M.A.M.; Alhadlaq, H.A. Enhanced anticancer performance of eco-friendly-prepared Mo-ZnO/RGO nanocomposites: Role of oxidative stress and apoptosis. ACS Omega 2022, 7, 7103–7115. [Google Scholar] [CrossRef]
- Yu, C.L.; Yang, K.; Xie, Y.; Fan, Q.Z.; Jimmy, C.Y.; Shu, Q.; Wang, C.Y. Novel hollow Pt-ZnO nanocomposite microspheres with hierarchical structure and enhanced photocatalytic activity and stability. Nanoscale 2013, 5, 2142–2151. [Google Scholar] [CrossRef]
- Pradeev Raj, K.; Sadaiyandi, K.; Kennedy, A.; Sagadevan, S.; Chowdhury, Z.Z.; Bin Johan, M.R.; Aziz, F.A.; Rafique, R.F.; Selvi, R.T.; Bala, R.R. Influence of Mg doping on ZnO nanoparticles for enhanced photocatalytic evaluation and antibacterial analysis. Nanoscale Res. Lett. 2018, 13, 229. [Google Scholar] [CrossRef] [PubMed]
- GB/T 5749-2006; Standards for Drinking Water Quality. National Health Commission: Beijing, China, 2006.
- Hoseinzadeh, A.; Habibi-Yangjeh, A.; Davari, M. Antifungal activity of magnetically separable Fe3O4/ZnO/AgBr nano-composites prepared by a facile microwave-assisted method. Prog. Nat. Sci. Mater. Int. 2016, 26, 334–340. [Google Scholar] [CrossRef]










| S.NO. | Materials Name | wt. (%) |
|---|---|---|
| 1 | 1% Cu-ZnO | 0.74 |
| 2 | 3% Cu-ZnO | 2.07 |
| 3 | 5% Cu-ZnO | 3.23 |
| 4 | 7% Cu-ZnO | 4.33 |
| 5 | 10% Cu-ZnO | 6.45 |
| 6 | 20% Cu-ZnO | 11.62 |
| S.NO. | Materials Name | BET Surface Area (m2/g) |
|---|---|---|
| 1 | ZnO | 52.3 |
| 2 | 1% Cu-ZnO | 54.9 |
| 3 | 3% Cu-ZnO | 51.1 |
| 4 | 5% Cu-ZnO | 50.5 |
| 5 | 7% Cu-ZnO | 49.1 |
| 6 | 10% Cu-ZnO | 59.1 |
| 7 | 20% Cu-ZnO | 59.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nan, R.; Liu, S.; Zhai, M.; Zhu, M.; Sun, X.; Chen, Y.; Pang, Q.; Zhang, J. Facile Synthesis of Cu-Doped ZnO Nanoparticles for the Enhanced Photocatalytic Disinfection of Bacteria and Fungi. Molecules 2023, 28, 7232. https://doi.org/10.3390/molecules28207232
Nan R, Liu S, Zhai M, Zhu M, Sun X, Chen Y, Pang Q, Zhang J. Facile Synthesis of Cu-Doped ZnO Nanoparticles for the Enhanced Photocatalytic Disinfection of Bacteria and Fungi. Molecules. 2023; 28(20):7232. https://doi.org/10.3390/molecules28207232
Chicago/Turabian StyleNan, Ruichun, Shurui Liu, Mengwan Zhai, Mengzhen Zhu, Xiaodong Sun, Yisong Chen, Qiangqiang Pang, and Jingtao Zhang. 2023. "Facile Synthesis of Cu-Doped ZnO Nanoparticles for the Enhanced Photocatalytic Disinfection of Bacteria and Fungi" Molecules 28, no. 20: 7232. https://doi.org/10.3390/molecules28207232