New 5,5-(1,4-Phenylenebis(methyleneoxy)diisophthalic Acid Appended Zn(II) and Cd(II) MOFs as Potent Photocatalysts for Nitrophenols
Abstract
:1. Introduction
2. Results and Discussion
2.1. Molecular Structure Description
2.1.1. [Zn2(L)(H2O)(bbi)] (1)
2.1.2. [Cd2(L)(bbi)] (2)
2.2. PXRD and TGA
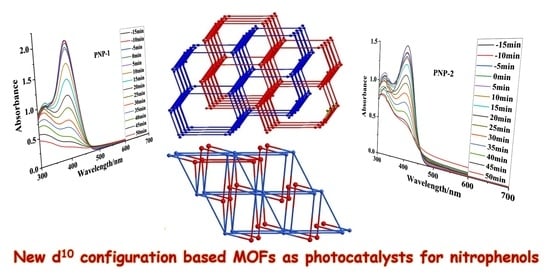
2.3. Diffuse Reflectance Spectroscopy and Photocatalytic Properties
3. Conclusions
4. Experimental Section
4.1. Materials and Methods
4.2. Synthesis of 1
4.3. Synthesis of 2
4.4. Photocatalysis
4.5. Computational Details
4.6. Hirshfeld Surface Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Chen, H.; Wang, X.X.; Li, J.; Wang, X.K. Cotton derived carbonaceous aerogels for the efficient removal of organic pollutants and heavy metal ions. J. Mater. Chem. A 2015, 3, 6073–6081. [Google Scholar] [CrossRef]
- Adnan, M.A.M.; Julkapli, N.M.; Maamor, A. Effect on different TiO2 photocatalyst supports on photodecolorization of synthetic dyes: A review. Int. J. Environ. Sci. Technol. 2019, 16, 547–566. [Google Scholar] [CrossRef]
- Gopalakrishnan, A.; Krishnan, R.; Thangavel, S.; Kim, G.V.S.J. Removal of heavy metal ions from pharma-effluents using graphene-oxide nanosorbents and study of their adsorption kinetics. J. Ind. Eng. Chem. 2015, 30, 14–19. [Google Scholar] [CrossRef]
- Carmen, S.D.; Rodrigues, O.S.G.P.; Soares, M.T.; Pinho, M.; Madeira, L.M. p-Nitrophenol degradation by heterogeneous Fenton’s oxidation over activated carbon-based catalysts. Appl. Catal. B 2017, 219, 109–122. [Google Scholar]
- Yin, H.; Kuwahara, Y.; Mori, K.; Che, M.; Yamashita, H. Plasmonic Ru/hydrogen molybdenum bronzes with tunable oxygen vacancies for light-driven reduction of p-nitrophenol. J. Mater. Chem. A 2019, 7, 3783–3789. [Google Scholar] [CrossRef]
- Xu, W.; Chen, J.; Qiu, Y.; Peng, W.; Shi, N.; Zhou, J. Highly efficient microwave catalytic oxidation degradation of 4-nitrophenol over magnetically separable NiCo2O4-Bi2O2CO3 composite without adding oxidant. Sep. Purif. Technol. 2019, 213, 426–436. [Google Scholar] [CrossRef]
- Yoo, D.K.; Ahmed, I.; Sarker, M.; Lee, H.J.; Vinu, A.; Jhung, S.H. Metal–organic frameworks containing uncoordinated nitrogen: Preparation, modification, and application in adsorption. Mater. Today 2021, 51, 566–585. [Google Scholar] [CrossRef]
- Sarker, M.; Yoo, D.K.; Lee, S.; Kim, T.-W.; Kim, C.-U.; Jhung, S.H. Conversion of Y into SSZ-13 zeolite, in the absence of extra silica, alumina and seed crystals, with N,N,N-dimethylethylcyclohexylammonium bromide, and application of the SSZ-13 zeolite in the propylene production from ethylene. Cat. Today 2021, 375, 94–100. [Google Scholar] [CrossRef]
- Lefebvre, L.; Agusti, G.; Bouzeggane, A.; Edouard, D. Adsorption of dye with carbon media supported on polyurethane open cell foam. Catal Today 2018, 301, 98–103. [Google Scholar] [CrossRef]
- López-Maldonado, E.A.; Oropeza-Guzman, M.T.; Baizaval, J.L.J.; Ochoa-Teránb, A. Coagulation–flocculation mechanisms in wastewater treatment plants through zeta potential measurements. J. Hazard. Mater 2014, 279, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Gong, J.L.; Zeng, G.M.; Deng, C.H.; Yang, H.C.; Liu, H.Y.; Huan, S.Y. Cross-linking to prepare composite graphene oxide-framework membranes with high-flux for dyes and heavy metal ions removal. Chem. Eng. J. 2017, 322, 657–666. [Google Scholar] [CrossRef]
- Kalme, S.D.; Parshetti, G.K.; Jadhav, S.U.; Govindwar, S.P. Biodegradation of benzidine based dye Direct Blue-6 by Pseudomonas desmolyticum NCIM 2112. Bioresour. Technol. 2007, 98, 1405–1410. [Google Scholar] [CrossRef] [PubMed]
- Punzi, M.; Anbalagan, A.; Börner, R.A.; Svensson, B.M. Degradation of a textile azo dye using biological treatment followed by photo-Fenton oxidation: Evaluation of toxicity and microbial community structure. Chem. Eng. J. 2015, 270, 290–299. [Google Scholar] [CrossRef]
- Feng, W.; Nansheng, D.; Helin, H. Degradation mechanism of azo dye C. I. reactive red 2 by iron powder reduction and photooxidation in aqueous solutions. Chemosphere 2000, 41, 1233–1238. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, X.; Li, J.; Burda, C. Photocatalytic degradation of azo dyes by nitrogen-doped TiO2 nanocatalysts. Chemosphere 2005, 61, 11–18. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Rong, Q.; Niu, H.; Cai, Y. Platform for molecular-material dual regulation: A direct Z-scheme MOF/COF heterojunction with enhanced visible-light photocatalytic activity. Appl. Catal. B 2019, 247, 49–56. [Google Scholar] [CrossRef]
- Zhang, X.; An, D.; Feng, D.; Liang, F.; Chen, Z.; Liu, W.; Yang, Z.; Xian, M. In situ surfactant-free synthesis of ultrathin BiOCl/g-C3N4 nanosheets for enhanced visible-light photodegradation of rhodamine B. Appl. Surf. Sci. 2019, 476, 706–715. [Google Scholar] [CrossRef]
- Lu, X.; Wang, Y.; Zhang, X.; Xu, G.; Wang, D.; Lv, J.; Zheng, Z.; Wu, Y.J. NiS and MoS2 nanosheet co-modified graphitic C3N4 ternary heterostructure for high efficient visible light photodegradation of antibiotic. J Hazard. Mater. 2018, 341, 10–19. [Google Scholar] [CrossRef]
- Zhu, H.; Li, Z.; Yang, J. A novel composite hydrogel for adsorption and photocatalytic degradation of bisphenol A by visible light irradiation. Chem. Eng. J. 2018, 334, 1679–1690. [Google Scholar] [CrossRef]
- Peng, Y.; Krungleviciute, V.; Eryazici, I.; Hupp, J.T.; Farha, O.K.; Yildirim, T. Methane Storage in Metal–Organic Frameworks: Current Records, Surprise Findings, and Challenges. J. Am. Chem. Soc. 2013, 135, 11887–11894. [Google Scholar] [CrossRef]
- Ma, D.Y.; Li, Z.; Zhu, J.X.; Zhou, Y.P.; Chen, L.L.; Mai, X.F.; Liufu, M.L.; Wu, Y.B.; Li, Y.W. Inverse and highly selective separation of CO2/C2H2 on a thulium–organic framework. J. Mater. Chem. A 2020, 8, 11933–11937. [Google Scholar] [CrossRef]
- McDonald, T.M.; Mason, J.A.; Kong, X.Q.; Bloch, E.D.; Gygi, D.; Dani, A.; Crocella, V.; Giordanino, F.; Odoh, S.O.; Drisdell, W.S.; et al. Cooperative insertion of CO2 in diamine-appended metal-organic frameworks. Nature 2015, 519, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, D.; Cairns, A.J.; Liu, J.; Motkuri, R.K.; Nune, S.K.; Fernandez, C.A.; Krishna, R.; Strachan, D.M.; Thallapally, P.K. Potential of Metal–Organic Frameworks for Separation of Xenon and Krypton. Acc. Chem. Res. 2015, 48, 211–219. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, J.H.; Wang, N.Y.; Steinbach, F.; Liu, X.L.; Caro, J. Remarkably Enhanced Gas Separation by Partial Self-Conversion of a Laminated Membrane to Metal–Organic Frameworks. Angew. Chem. Int. Ed. 2015, 54, 3028–3032. [Google Scholar] [CrossRef] [PubMed]
- Guo, A.; Ban, Y.J.; Yang, K.; Yang, W. Metal-organic framework-based mixed matrix membranes: Synergetic effect of adsorption and diffusion for CO2/CH4 separation. J. Membr. Sci. 2018, 562, 76–84. [Google Scholar] [CrossRef]
- Qin, T.R.; Zhang, X.Y.; Li, D.C.; Dong, X.Y.; Qi, N.; Shang, Y.J.; Sakiyamad, H.; Afzal, M.; Alarifi, A. Temperature modulation on functional coordination polymers with tetracarboxylate linker: Syntheses, structural traits, and magnetism. J. Mol. Struct. 2023, 1291, 136074. [Google Scholar] [CrossRef]
- Qin, T.R.; Shi, Z.; Zhang, W.J.; Dong, X.Y.; An, N.; Sakiyama, H.; Muddassir, M.; Srivastava, D.; Kumar, A. 2D isostructural Ln(III)-based coordination polymer derived from Imidazole carboxylic acid: Synthesis, structure and magnetic behavior. J. Mol. Struct. 2023, 1282, 135220. [Google Scholar] [CrossRef]
- Kreno, L.E.; Leong, K.; Farga, O.K.; Allendorf, M.; Van Duyne, R.P.; Hupp, J.T. Metal–Organic Framework Materials as Chemical Sensors. Chem. Rev. 2012, 112, 1105–1125. [Google Scholar] [CrossRef] [PubMed]
- Lian, X.; Yan, B. Phosphonate MOFs Composite as Off–On Fluorescent Sensor for Detecting Purine Metabolite Uric Acid and Diagnosing Hyperuricuria. Inorg. Chem. 2017, 56, 6802–6808. [Google Scholar] [CrossRef]
- Chen, Y.; Li, J.; Yue, G.; Luo, X. Novel Ag@Nitrogen-doped Porous Carbon Composite with High Electrochemical Performance as Anode Materials for Lithium-ion Batteries. Nano-Micro Lett. 2017, 9, 32. [Google Scholar] [CrossRef]
- Seo, P.W.; Khan, N.A.; Jhung, S.H. Removal of nitroimidazole antibiotics from water by adsorption over metal–organic frameworks modified with urea or melamine. Chem. Eng. J. 2017, 315, 92–100. [Google Scholar] [CrossRef]
- Tan, G.J.; Wang, S.Y.; Yu, J.L.; Chen, J.H.; Liao, D.H.; Liu, M.; Nezamzadeh-Ejhieh, A.; Pan, Y.; Liu, J.Q. Detection mechanism and the outlook of metal-organic frameworks for the detection of hazardous substances in milk. Food. Chem. 2023, 430, 136934. [Google Scholar] [CrossRef]
- Azhar, M.R.; Abid, H.R.; Periasamy, V.; Sun, H.; Tade, M.O.; Wang, S. Adsorptive removal of antibiotic sulfonamide by UiO-66 and ZIF-67 for wastewater treatment. J. Colloid Interface Sci. 2017, 500, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Chu, C.C.; Liu, G.; Wang, Y.X. Metal–Organic Framework-Based Nanomedicine Platforms for Drug Delivery and Molecular Imaging. Small 2015, 11, 4806–4822. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.Y.; Peng, Z.X.; Peng, Y.Q.; Li, B.; Pan, Y.; Ouyang, Q.; Sakiyama, H.; Muddassir, M.; Liu, J.Q. Construction of Fe-doped ZIF-8/DOX nanocomposites for ferroptosis strategy in the treatment of breast cancer. J. Mater. Chem. B 2023, 11, 6335–6345. [Google Scholar] [CrossRef]
- Park, K.M.; Kim, H.; Murray, J.; Koo, J.; Kim, K. A facile preparation method for nanosized MOFs as a multifunctional material for cellular imaging and drug delivery. Supramol. Chem. 2017, 29, 441–445. [Google Scholar] [CrossRef]
- Rao, C.Y.; Zhou, L.Y.; Pan, Y.; Lu, C.Y.; Qin, X.Y.; Sakiyama, H.; Muddassir, M.; Liu, J.Q. The extra-large calixarene-based MOFs-derived hierarchical composites for photocatalysis of dye: Facile syntheses and contribution of carbon species. J. Alloys Compd. 2022, 897, 163178. [Google Scholar] [CrossRef]
- Jin, J.C.; Wu, J.; Liu, W.C.; Ma, A.Q.; Liu, J.Q.; Singh, A.; Kumar, A. A new Zn(II) metal–organic framework having 3D CdSO4 topology as luminescent sensor and photocatalyst for degradation of organic dyes. New J. Chem. 2018, 42, 2767–2775. [Google Scholar] [CrossRef]
- Bala, S.; Bhattacharya, S.; Goswami, A.; Adhikary, A.; Konar, S.; Mondal, R. Designing Functional Metal–Organic Frameworks by Imparting a Hexanuclear Copper-Based Secondary Building Unit Specific Properties: Structural Correlation with Magnetic and Photocatalytic Activity. Cryst. Growth Des. 2014, 14, 6391–6398. [Google Scholar] [CrossRef]
- Yang, X.R.; Chen, Z.; Zhao, W.; Liu, C.X.; Qian, X.X.; Zhang, M.; Wei, G.Y.; Khan, E.; Ng, Y.H.; Ok, Y.S. Recent advances in photodegradation of antibiotic residues in water. Chem. Eng. J. 2021, 405, 126806. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.J.; Dang, Z.Y.; Muddassir, M.; Raza, S.; Zhong, A.G.; Wang, X.X.; Jin, J.C. A New Cd(II)-Based Coordination Polymer for Efficient Photocatalytic Removal of Organic Dyes. Molecules 2023, 28, 6848. [Google Scholar] [CrossRef]
- Chen, H.P.; Liu, P.; Liu, J.Q.; Feng, X.; Zhou, S.X. Mechanochemical in-situ incorporation of Ni on MgO/MgH2 surface for the selective O-/C-terminal catalytic hydrogenation of CO2 to CH4. J. Catal. 2021, 394, 397–405. [Google Scholar] [CrossRef]
- Dzhardimalieva, G.I.; Baimuratova, R.K.; Knerelman, E.I.; Davydova, G.I.; Kudaibergenov, S.E.; Kharissova, O.V.; Zhinzhilo, V.A.; Uflyand, I.E. Synthesis of Copper(II) Trimesinate Coordination Polymer and Its Use as a Sorbent for Organic Dyes and a Precursor for Nanostructured Material. Polymers 2020, 12, 1024. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Bala, S.; Mondal, R. Development of bio-derived nanostructured coordination polymers based on cardanol–formaldehyde polyurethanes with ‘d5’ Mn(II) and ‘d10’ Zn(II) metal nodes: Synthesis, characterization and adsorption behavior. RSC. Adv. 2016, 6, 25149–25158. [Google Scholar] [CrossRef]
- Chen, X.Y.; Peng, X.; Jiang, L.B.; Yuan, X.Z.; Fei, J.; Zhang, W. Photocatalytic removal of antibiotics by MOF-derived Ti3+- and oxygen vacancy-doped anatase/rutile TiO2 distributed in a carbon matrix. Chem. Eng. J. 2022, 427, 130945. [Google Scholar] [CrossRef]
- Li, W.Q.; Zhang, H.; Zhang, K.; Hu, W.X.; Cheng, Z.Z.; Chen, H.; Feng, X.; Peng, T.; Kou, Z.K. Monodispersed ruthenium nanoparticles interfacially bonded with defective nitrogen-and-phosphorus-doped carbon nanosheets enable pH-universal hydrogen evolution reaction. Appl. Catal. B Environ. 2022, 306, 121095. [Google Scholar] [CrossRef]
- Zhang, H.; Li, W.; Feng, X.; Zhu, L.; Fang, Q.; Li, S.; Wang, L.Y.; Li, Z.J.; Kou, Z.K. A chainmail effect of ultrathin N-doped carbon shell on Ni2P nanorod arrays for efficient hydrogen evolution reaction catalysis. J. Colloid. Interface. Sci. 2022, 607, 281. [Google Scholar] [CrossRef]
- Liu, K.G.; Rouhani, F.; Gao, X.M.; Abbasi-Azad, M.; Li, J.Z.; Hu, X.D.; Wang, W.; Hu, M.L.; Morsali, A. Bilateral photocatalytic mechanism of dye degradation by a designed ferrocene-functionalized cluster under natural sunligh. Catal. Sci. Technol. 2020, 10, 757–767. [Google Scholar] [CrossRef]
- Nasalevich, M.A.; van der Veen, M.; Kapteijn, F.; Gascon, J. Metal–organic frameworks as heterogeneous photocatalysts: Advantages and challenges. CrystEngComm 2014, 16, 4919–4926. [Google Scholar] [CrossRef]
- Qin, L.; Liang, F.L.; Li, Y.; Wu, J.N.; Wu, S.Y.G.M.Y.; Xie, S.L.; Luo, M.S.; Ma, D.Y. A 2D porous zinc-organic framework platform for loading of 5-fluorouracil. Inorganics 2022, 10, 202. [Google Scholar] [CrossRef]
- Alvaro, M.; Carbonell, E.; Ferrer, B.; Xamena, F.; Garcia, H. Semiconductor Behavior of a Metal-Organic Framework (MOF). Chem. Eur. J. 2007, 13, 5106–5112. [Google Scholar] [CrossRef]
- Saeki, A.; Koizumi, Y.; Aida, T.; Seki, S. Comprehensive approach to intrinsic charge carrier mobility in conjugated organic molecules, macromolecules, and supramolecular architectures. Acc. Chem. Res. 2012, 45, 1193–1202. [Google Scholar] [CrossRef] [PubMed]
- Givaja, G.; Amo-Ochoa, P.; Gomez-Garcia, C.J.; Zamora, F. Electrical conductive coordination polymers. Chem. Soc. Rev. 2012, 41, 115–147. [Google Scholar] [CrossRef] [PubMed]
- SzabóBárdos, E.; Cafuta, A.; Hegedűs, P.; Fónagy, O.; Kiss, G.; Babić, S.; Škorić, I.; Horváth, O. Photolytic and photocatalytic degradation of nitrofurantoin and its photohydrolytic products. J. Photoch. Photobio A 2020, 386, 112093. [Google Scholar]
- Yashas, S.R.; Shivaraju, H.P.; Sandeep, S.; Swamy, N.K.; Gurupadayya, B. Application of yttrium molybdate tethered polypyrrole nanocomposite for the photocatalytic remediation of nitrofurantoin in water. Surf. Interfaces. 2022, 32, 102102. [Google Scholar] [CrossRef]
- Singh, A.; Singh, A.K.; Liu, J.Q.; Kumar, A. Syntheses, design strategies, and photocatalytic charge dynamics of metal–organic frameworks (MOFs): A catalyzed photo-degradation approach towards organic dyes. Catal. Sci. Technol. 2021, 11, 3946–3989. [Google Scholar] [CrossRef]
- Chen, Z.; Fan, T.; Shao, M.; Yu, X.; Wu, Q.; Li, J.; Fang, W.; Yi, X. Simultaneously enhanced photon absorption and charge transport on a distorted graphitic carbon nitride toward visible light photocatalytic activity. Appl. Catal. B Environ. 2019, 242, 40–50. [Google Scholar] [CrossRef]
- Dey, S.C.; Moztahida, M.; Sarker, M.; Ashaduzzaman, M.; Shamsuddin, S.M. pH-Triggered Interfacial Interaction of Kaolinite/Chitosan Nanocomposites with Anionic Azo Dye. J. Compos. Sci. 2019, 3, 39. [Google Scholar] [CrossRef]
- Hu, L.X.; Deng, G.H.; Lu, W.C.; Pang, S.W.; Hu, X. Deposition of CdS nanoparticles on MIL-53(Fe) metal-organic framework with enhanced photocatalytic degradation of RhB under visible light irradiation. Appl. Surf. Sci. 2017, 410, 401–413. [Google Scholar] [CrossRef]
- Wu, X.Q.; Wen, G.X.; Wu, Y.P.; Dong, W.W.; Zhao, J.; Li, D.S. A novel 3D Ag(I)-MOF: Surfactant-directed syntheses and catalytic degradation of o/m/p-Nitrophenol. J. Solid. State. Chem. 2016, 242, 243–247. [Google Scholar] [CrossRef]
- Ramezanalizadeh, H.; Manteghi, F.; Production, J.C.; Zhang, C.H.; Ai, L.H.; Jiang, J. Solvothermal synthesis of MIL–53(Fe) hybrid magnetic composites for photoelectrochemical water oxidation and organic pollutant photodegradation under visible light. J. Mater. Chem. A 2015, 3, 3074–3081. [Google Scholar]
- Li, Y.Y.; Fang, Y.; Cao, Z.L.; Li, N.J.; Chen, D.Y.; Xu, Q.F.; Lu, J.M. Construction of g-C3N4/PDI@MOF heterojunctions for the highly efficient visible light-driven degradation of pharmaceutical and phenolic micropollutants. Appl. Catal. B Environ. 2019, 250, 150–162. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, L.Y.; Rao, C.Y.; Wang, G.L.; Jiang, F.; Singh, A.; Kumar, A.; Liu, J.Q. Two 3D supramolecular isomeric Zn(II)-MOFs as photocatalysts for photodegradation of methyl violet dye. Dye. Pigment. 2021, 190, 109285. [Google Scholar] [CrossRef]
- Wang, K.D.; He, X.; Dong, C.Y.; Zhong, A.G.; Liu, S.B.; Zhao, D.B. On the origin and nature of internal methyl rotation barriers: An information-theoretic approach study. Theor. Chem. Acc. 2022, 141, 68. [Google Scholar] [CrossRef]
- Cao, X.F.; Rong, C.Y.; Zhong, G.; Lu, T.; Liu, B. Molecular acidity: An accurate description with information-theoretic approach in density functional reactivity theory. J. Comput. Chem. 2018, 39, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Zhong, A.G. Dissecting the nature of halogen bonding interactions from energy decomposition and wavefunction analysis. Monatsh Chem. 2017, 48, 1259–1267. [Google Scholar]
- Zhong, A.G.; Li, R.R.; Hong, Q.; Zhang, J.; Chen, D. Understanding the Isomerization of Monosubstituted Alkanes from Energetic and Information-Theoretic Perspectives. Acta Phys.-Chim. Sin. 2018, 34, 303–313. [Google Scholar]
- Lu, L.; Wang, J.; Xie, B.; Liu, J.Q.; Yadav, R.; Singh, A.; Kumar, A. Fluorescence sensing of nitro-aromatics by Zn(II) and Cd(II) based coordination polymers having the 5-[bis(4-carboxybenzyl)-amino]isophthalic acid ligand. New J. Chem. 2017, 41, 3537–3542. [Google Scholar] [CrossRef]
- Luo, C.Y.; He, X.; Zhong, A.G.; Liu, S.B.; Zhao, D.B. What dictates alkane isomerization? A combined density functional theory and information-theoretic approach study. Theor. Chem. Acc. 2023, 142, 78. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. I. The effect of the exchange-only gradient correction. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.T.; Yang, W.T.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B Condens. Matter Mater. Phys. 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A.; Vreven, T., Jr.; Kudin, K.N.; Burant, J.C.; et al. cclib: A library for package-independent computational chemistry algorithms. J. Comp. Chem. 2008, 29, 839–845. [Google Scholar]
- Turner, M.J.; McKinnon, J.J.; Jayatilaka, D.; Spackman, M.A. Visualisation and characterisation of voids in crystalline materials. CrystEngComm 2011, 13, 1804–1813. [Google Scholar] [CrossRef]
- Wolff, S.K.; Grimwood, D.J.; McKinnon, J.J.; Turner, M.J.; Jayatilaka, D.; Spackman, M.A. Crystal Explorer (Version 3.1); University of Western Australia: Crawley, Australia, 2012. [Google Scholar]











Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bin, H.-S.; Hu, H.; Wang, J.; Lu, L.; Muddassir, M.; Srivastava, D.; Chauhan, R.; Wu, Y.; Wang, X.; Kumar, A. New 5,5-(1,4-Phenylenebis(methyleneoxy)diisophthalic Acid Appended Zn(II) and Cd(II) MOFs as Potent Photocatalysts for Nitrophenols. Molecules 2023, 28, 7180. https://doi.org/10.3390/molecules28207180
Bin H-S, Hu H, Wang J, Lu L, Muddassir M, Srivastava D, Chauhan R, Wu Y, Wang X, Kumar A. New 5,5-(1,4-Phenylenebis(methyleneoxy)diisophthalic Acid Appended Zn(II) and Cd(II) MOFs as Potent Photocatalysts for Nitrophenols. Molecules. 2023; 28(20):7180. https://doi.org/10.3390/molecules28207180
Chicago/Turabian StyleBin, Hui-Shi, Hai Hu, Jun Wang, Lu Lu, Mohd Muddassir, Devyani Srivastava, Ratna Chauhan, Yu Wu, Xiaoxiong Wang, and Abhinav Kumar. 2023. "New 5,5-(1,4-Phenylenebis(methyleneoxy)diisophthalic Acid Appended Zn(II) and Cd(II) MOFs as Potent Photocatalysts for Nitrophenols" Molecules 28, no. 20: 7180. https://doi.org/10.3390/molecules28207180